Abstract
The triglyceride-glucose (TyG) index is a simple and inexpensive new marker of insulin resistance that is being increasingly used for the clinical prediction of metabolic syndrome (MetS). Nevertheless, there are only a few comparative studies on its predictive capacity for MetS versus those using the traditional homeostasis model assessment (HOMA). We conducted a cross-sectional study using a database from the National Health and Nutrition Examination Survey (1999 March to 2020 pre-pandemic period). Using statistical methods, we compared the predictive abilities of the TyG index and HOMA (including HOMA of insulin resistance [HOMA-IR] and HOMA of beta-cell function [HOMA-β]) for MetS. A total of 34,195 participants were enrolled and divided into the MetS group (23.1%) or no MetS group (76.9%) according to the International Diabetes Federation (IDF) diagnostic criteria. After applying weighted data, the baseline characteristics of the population were described. Following the exclusion of medication influences, the final count was 31,304 participants. Receiver operating characteristic curve analysis revealed that while distinguishing between MetS and no MetS, the TyG index had an area under the curve (AUC) of 0.827 (sensitivity = 71.9%, specificity = 80.5%), and the cutoff was 8.75, slightly outperforming HOMA-IR (AUC = 0.784) and HOMA-β (AUC = 0.614) with a significance of P < 0.01. The prevalence of MetS in the total population calculated using the TyG index cutoff value was 30.9%, which was higher than that reported in the IDF diagnostic criteria. Weighted data analysis using univariate and multivariate logistic regression displayed an independent association between elevated TyG and HOMA-IR with the risk of MetS. Subgroup analysis further revealed differences in the predictive ability of the TyG index among adult populations across various genders and ethnicities, whereas such differences were not observed for children and adolescents. The TyG index is slightly better than HOMA in predicting MetS and may identify more patients with MetS; thus, its applications in a clinical setting can be appropriately increased.
Similar content being viewed by others
Introduction
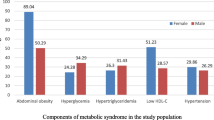
As a complex and diverse health issue, metabolic syndrome (MetS) has become the focus of public health worldwide. Symptoms including obesity, hypertension, hyperglycemia, and dyslipidemia greatly increase the risk of cardiovascular disease, diabetes, and other chronic diseases and have a profound impact on the health of individuals as well on society1. Thus, there is an urgent requirement to identify effective methods to accurately predict MetS. In this field, the ability of the triglyceride-glucose (TyG) index, a new marker of insulin resistance, has attracted much attention in recent years to evaluate MetS2,3,4. The TyG index is a simple and inexpensive metabolic index that can be calculated by combining triglyceride and glucose levels and is considered to reflect pancreatic function and insulin resistance in tissues5. In contrast, homeostasis model assessment (HOMA) is a traditional indicator used to determine insulin resistance and insulin sensitivity. HOMA mainly includes HOMA of insulin resistance (HOMA-IR), HOMA of insulin sensitivity (HOMA-IS), and HOMA of beta-cell function (HOMA-β), and is obtained by calculating the relationship between fasting insulin levels and blood glucose levels. However, HOMA has some limitations in predicting MetS such as not accurately reflecting the effect of triglycerides. Moreover, comparative studies on the predictive values of these two indicators in MetS are still relatively limited. At the time of writing this article, only Son et al. had reported that the TyG index was significantly better than HOMA-IR in a study of Korean adults6, and Dundar et al. had found that the TyG index in girls was better than HOMA-IR in a study involving Turkish children7. However, the sample sizes of these studies were small.
Therefore, we compared and analyzed the performance of the TyG index and HOMA in predicting MetS using large-scale population data from the National Health and Nutrition Examination Survey (NHANES) from 1999 to March 2020 (pre-pandemic period). There is an urgent need for a more comprehensive understanding of the advantages and limitations of these two indicators and their practical application values in clinical practice. Through this study, we hope to provide more accurate and reliable tools for the screening and management of MetS at an early stage, thus helping to reduce the incidence of MetS-related chronic diseases and improving overall health. Therefore, the results of this study may have a certain impact on future medical practice and public health policy-making.
Materials and methods
Study population
This study utilized data from NHANES in the United States. All databases are accessible through the NHANES website (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx). NHANES is a national survey conducted by the Centers for Disease Control and Prevention to assess the health and nutritional status of adults, children, and adolescents in the United States through a complex, stratified, random-sampling method, allowing estimations to be generalized to the entire United States population. We analyzed data from the past 10 cycles (1999 to March 2020 pre-pandemic period), which included a total of 107,623 participants. Among them, 71,838 participants lacking waist circumference data or WTSAF weights were excluded from the study. Additionally, 1590 participants < 10 years of age were excluded due to the absence of a clear definition for MetS diagnosis, and the remaining 34,195 participants were analyzed at baseline. Finally, 2891 participants currently taking oral hypoglycemic or triglyceride-lowering medications or those using insulin were excluded. Thus, a final cohort of 31,304 individuals was used to assess the diagnostic value of the TyG index and HOMA for MetS. The participant flowchart is depicted in Fig. 1.
Calculation formulae
The TyG index was mainly calculated using blood glucose and triglyceride levels as follows:
TyG index = Ln [fasting triglyceride (mg/dL) × fasting blood glucose (mg/dL) / 2]8; HOMA mainly included HOMA-IR, HOMA-IS, and HOMA-β, and the formulae for calculation were as follows:
HOMA-IR = fasting blood glucose (mmol/L) × fasting insulin (μU/mL)/22.5;
HOMA-IS = 1/HOMA-IR = 22.5 / fasting blood glucose (mmol/L) × fasting insulin (μU/mL);
HOMA-β = 20 × fasting insulin (μU/mL) / (fasting blood glucose [mmol/L] – 3.5)9.
Definition criteria
The following diagnostic criteria of the International Diabetes Federation (IDF) 2007 for MetS were adopted: 1. Central obesity; 2. Abnormality in any two or more of the four indicators: blood pressure, blood glucose, triglyceride, and high-density lipoprotein cholesterol. However, the criteria for adults10 and children and adolescents11 were different. The diagnostic criteria for adult MetS are shown in Table 1 and those for children and adolescents in Table 2. The percentage waist circumference for children and adolescents is shown in Supplementary Table S1. Questionnaire data for smoking, drinking, and education: As NHANES did not have questionnaires for smoking and alcohol consumption for children and adolescents, smoking and alcohol consumption data were collected only for individuals aged 20 years and older. “Smoked at least 100 cigarettes in life” was defined as smoking, and “Had at least 12 alcohol drinks per year” was defined as drinking. “Less than 9th grade” and “9th–11th grade” were defined as education below high school level; “High School Grad/GED or Equivalent” was defined as High School education level; and “Some College or AA degree” and “College Graduate or above” were defined as education above high school level. The definition of diabetes for all participants was based on the American Diabetes Association 2023 standard12 combined with questionnaires, and if they met any of the following criteria: “Have been informed of diabetes,” “Fasting blood glucose (FBG) levels ≥ 126 mg/dL” or “Glycosylated hemoglobin (HbA1c) levels ≥ 6.5%.” The definition of hypertension for all participants was based on the American Heart Association/American College of Cardiology 2017 standard13 combined with questionnaires, and if they met any of the following criteria: “Had been told about hypertension,” “Systolic blood pressure (SBP) ≥ 130 mm Hg,” or “Diastolic blood pressure (DBP) ≥ 80 mm Hg.”
Statistical analysis
R software version 4.3.1 (R Core Team, Vienna, Austria) was used for data entry and processing. Packages such as “survey,” “weights,” “matrix,” and “pROC” were mainly selected. GraphPad Prism, version 9.4.1 (GraphPad Software, San Diego, CA, USA) was used to create images. First, fasting subsample weights were selected for the weighted processing of the selected data. During the consolidation of various cycles, “4/21.2 × WTSAF4YR” was chosen as the weight for the cycles from 1999–2002, whereas for the cycles from 2003 to 2016, with its 7 cycles, “2/21.2 × WTSAF2YR” was assigned as the weight for each cycle. For the period from 2017 to March 2020 (pre-pandemic cycle), “3.2/21.2 × WTSAFPRP” was selected as the weight. N represents the included population and WN represents the number of weighted representative American population. Continuous variable data are represented as mean ± standard error (SE), and categorical variables are represented as % (SE). T-test was used for continuous variables and Chi-square test was used for classification variables. The TyG index, HOMA-IR, and HOMA-β were transformed into dichotomous variables. After data weighting, univariate and multivariate Models 1, 2, 3, and 4 were established for logistic regression analysis. Then, the TyG index and the ability of HOMA-IR and HOMA-β to diagnose MetS were analyzed and evaluated using the receiver operating characteristic (ROC) curve, and the corresponding areas under curve (AUCs), sensitivity, and specificity were calculated. Lastly, subgroup univariate analysis was conducted for adults, children, and teenagers after data weighting based on population characteristics. As HOMA-IS is the reciprocal of HOMA-IR and its diagnostic efficiency is consistent with that of HOMA-IR, further logistic regression analysis and ROC analysis were not performed. α = 0.05 was considered statistically significant.
Ethics approval and consent to participate
The NHANES data used in this study are public data and do not contain any personally identifiable information; thus, additional ethical review was not required. We have strictly abided by NHANES data usage specifications and privacy protection policies to ensure compliance and ethical data use.
Results
Baseline characteristics
The statistics of the data-completion ratio are shown in Table 3. We first divided 34,195 participants into 2 groups based on the IDF diagnostic criteria as follows: 26,304 (76.9%) in the no MetS group and 7,891 (23.1%) in the MetS group. The prevalence of MetS was 29.1% in adults and 4.8% in children and adolescents. After data were weighted for baseline analysis, the 2 groups of data were compared (Table 4). In the comparison of the TyG index and HOMA, to avoid the interference of drugs, 2,891 participants who were currently taking oral hypoglycemic and lipid-lowering drugs or using insulin were excluded. Finally, 31,304 participants were included in the analysis and were classified as 22,846 adults and 8,458 children or adolescents based on their age. Baseline analysis and intergroup comparison were conducted for each group. The baseline characteristics of adults are shown in Table 5 and those of children and adolescents in Table 6.
Predictive ability
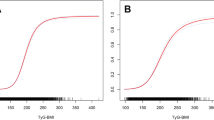
The predictive abilities of the TyG index and HOMA for the diagnosis of MetS in the included population were further evaluated with MetS as a positive diagnosis result. ROC curves were drawn and AUCs were calculated using the TyG index, HOMA-IR, and HOMA-β. The AUC of the TyG index was 0.827 (sensitivity = 71.9%, specificity = 80.5%), and the cutoff was 8.75, which was slightly higher than that of HOMA-IR (0.784) and HOMA-β (0.614), all P < 0.01 (Fig. 2). The prevalence of MetS in the population calculated using the TyG index cutoff value was 30.9%, which was higher than that reported in the IDF diagnostic criteria (23.1%). In the adult population, the AUC of the TyG index was 0.796 (sensitivity = 69.8%, specificity = 78.5%) and the cutoff was 8.80, and the prevalence of MetS in the adult population calculated using the TyG index cutoff value was 34.1%. In the child and adolescent population, the AUC of the TyG index was 0.874 (sensitivity = 76.3%, specificity = 87.3%) and the cutoff was 8.62. The prevalence of MetS in the child and adolescent population calculated using the TyG index cutoff value was 16.4%.
Logistic regression analysis
After the data were weighted, the TyG index, HOMA-IR, and HOMA-β were transformed into dichotomic variables as independent variables to construct Model 1. Univariate logistic regression analysis showed that high TyG index, HOMA-IR, and HOMA-β were correlated with the risk of MetS. Based on Model 1, the demographic variables (gender, age, and race) were adjusted to construct Model 2. Based on Model 2, the examined variables (body mass index, waist circumference, SBP, and DBP) were adjusted to construct Model 3. Based on Model 3, the laboratory variables (HbA1c, cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol levels) were adjusted to construct Model 4. Multivariate logistic regression analysis showed that, except for Model 4, elevated HOMA-β showed no association with the risk of MetS (odds ratio [OR] {95% confidence interval [CI]}) = 0.93 (0.80–1.07)]. However, in other models, elevated TyG index, HOMA-IR, and HOMA-β were all associated with the risk of MetS, as shown in Fig. 3.
Univariate and multivariate logistic regression forest plots of adjusted factors and the risk for MetS. Note: Model 1: No covariates were adjusted. Model 2: Gender, age, and race were adjusted. Model 3: Age, sex, race, BMI, WC, SBP, and DBP were adjusted. Model 4: Age, sex, race, BMI, WC, SBP, DBP, HbA1c, TC, HDL-C, and LDL-C were adjusted. Abbreviations: MetS, metabolic syndrome; TyG, triglyceride-glucose; HOMA-IR, homeostasis model assessment of insulin resistance; HOMA-β, homeostasis model assessment of beta-cell function; OR, odds ratio; CI, confidence interval, BMI, body mass index, WC, waist circumference, SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycosylated hemoglobin; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Subgroup analysis
Data were weighted for subgroup analysis and the TyG index was transformed into a binary classification variable and taken as exposure variables. Univariate analysis of the adult population revealed that the TyG index could differently predict MetS based on different genders and races (all P-interactions < 0.01). However, there was no difference in the ability of the TyG index to predict MetS among different levels of education, smoking, and drinking (all P-interactions > 0.05), as shown in Fig. 4. For the subgroup analysis of the child and adolescent population based only on gender and race, univariate analysis revealed no differences between gender (P-interaction = 0.45) and ethnicity (P-interaction = 0.29) in the ability of the TyG index to predict MetS.
Discussion
The strength of this study is that it is the largest investigation to date that has explored the predictive ability of the TyG index and HOMA for MetS within a population sample. NHANES employs a complex, stratified, and random sampling methodology to ensure accurate and reliable test results, thereby securing data authenticity and allowing for generalization to the entire United States population. Our research findings suggest a slightly superior predictive capability of the TyG index over HOMA for MetS.
Although the hyperinsulinemic euglycemic clamp technology is currently the internationally recognized gold standard to evaluate insulin resistance, its implementation requires special equipment and skilled technicians, which is expensive and time-consuming. Moreover, multiple blood samples are required for the test, which can be difficult for patients to accept. Therefore, it is only used for scientific research and cannot be applied in clinical practice on a large scale14. HOMA is now commonly used to evaluate insulin function in a clinical setting. HOMA was first proposed by Turner’s research team at Oxford University in 19859. It is a mathematical model established to reflect the interaction of glucose and insulin in different organs (including the pancreas, liver, and surrounding tissues). The model assessed insulin resistance (HOMA-IR) and islet β-cell function (HOMA-β) only by using FBG and fasting insulin values. However, in recent years, a simpler and low-cost test method, namely the TyG index, has been widely used in clinical practice to evaluate insulin resistance. The TyG index was first proposed by scholars in South America. This index can be obtained by determining routine fasting triglyceride and FBG levels in the serum. In 2008, Simental-Mendia et al. used the TyG index for the community screening of healthy participants to check for insulin resistance8. Apart from reflecting insulin resistance, studies have shown that the TyG index can also be used as an indicator to predict risks of the chronic complications of diabetes15,16, cardiovascular disease17, and cerebrovascular disease18, among others. Meanwhile, the ability of the TyG index to evaluate MetS has attracted much attention in recent years2,3,4.
Triglyceride levels are typically determined using enzymatic, chromatographic, or chemical methods. Among these, the enzymatic method is the most commonly used and relatively simple procedure. In our study, the triglyceride measurements extracted from the NHANES database were also analyzed using the enzymatic method. This method can be conducted relatively quickly and is suitable for handling large numbers of samples; thus, it is widely used in clinical practice. In contrast, the determination of insulin requires immunological methods such as enzyme-linked immunosorbent assay or radioimmunoassay, which involve more sample-processing steps and experimental procedures. These methods require antibodies to bind to the substance to be measured (such as insulin), followed by the subsequent detection of the conjugated product using a marker such as an enzyme or a radioisotope19. Sample processing is relatively complex, especially in remote primary care facilities with limited medical resources, and insulin determination may therefore be difficult. In addition, from the point of cost-effectiveness, the TyG index only requires the detection of glucose and triglyceride concentrations, which is an inexpensive process. On the other hand, HOMA requires the detection of glucose and insulin concentrations, which are associated with higher costs20. Therefore, the TyG index is comparatively easier to obtain than HOMA. Moreover, HOMA-β cannot be calculated when fasting glucose levels are less than or equal to 3.5 mmol/ L. We found that the TyG index had a slightly higher AUC value compared with HOMA based on the comparison of the AUC under the ROC curve, suggesting that the TyG index has more advantages in predicting MetS. A higher AUC means that the TyG index has a better predictive ability compared with HOMA, and the sensitivity and specificity of the TyG index are also high. Therefore, these findings suggest that the TyG index may be more suitable for the early screening and prediction of MetS than HOMA, which is, therefore, worthy of further evaluation and application in a clinical setting.
At the same time, our study also found that the cutoff value of the TyG index as a predictor of MetS was 8.75 and that there was little difference in the cutoff value between the adult population and the child and adolescent population. It is noteworthy that the prevalence of MetS calculated using the TyG index cutoff values were all higher than that diagnosed using the IDF criteria across different groups, namely, the total population (30.9% vs. 23.1%), adults (34.1% vs. 29.1%), and children and adolescents (16.4% vs. 4.8%). The difference between the total population and the adult population was within 10%, suggesting that using the TyG index may identify individuals at risk for insulin resistance or early metabolic abnormalities even if they do not meet the IDF criteria. Therefore, using the TyG index may potentially identify patients with MetS at an earlier stage, providing early intervention opportunities to prevent the development of related complications. However, in the child and adolescent population, the difference in the prevalence of MetS calculated using the IDF diagnostic criteria versus the TyG index cutoff values was about 12%, a difference that could significantly increase the workload of pediatricians. This finding suggests that the TyG index may not be appropriate for identifying MetS in children and adolescents. Therefore, additional prospective longitudinal studies are needed to explore the applicability of the TyG index in this specific population.
Using univariate and multivariate logistic regression analyses, we found that high TyG index, HOMA-IR, and HOMA-β values were significantly correlated with the risk of MetS. Even after gradually adjusting for the demographic variable and examination variable and establishing multivariable Model 2 and Model 3, respectively, high TyG index, HOMA-IR, and HOMA-β values still maintained the correlation with the risk of MetS. However, when the laboratory variables were adjusted based on Model 3 and the multivariable Model 4 was established, high HOMA-β was not associated with the risk of MetS because the 95% CI was 1, likely suggesting that HOMA-β had insufficient robustness in predicting MetS. However, in Model 4, high TyG index and HOMA-IR still maintained the correlation with the risk of MetS, providing a solid foundation for the effectiveness of the TyG index and HOMA-IR in predicting MetS.
Subgroup analysis of the TyG index to predict MetS ability showed interactions between genders and races in the adult population. These findings suggested that the applicability of the TyG index in predicting MetS is influenced by different population characteristics. Our study found that the TyG index was slightly better in predicting MetS in female adults than in male adults. There are significant differences in metabolic and physiological characteristics between males and females, including lipid-metabolism patterns, fat distribution, and hormone levels. In general, sex hormone levels vary greatly between men and women, which not only affect fat distribution but also affect metabolism. Androgens are generally associated with more fat accumulation in the abdomen, whereas estrogens may influence fat accumulation in the buttocks and thighs21. Additionally, there are differences in basal metabolic rates between men and women, and men usually have a higher basal metabolic rate22. Such metabolic and physiological differences may lead to gender differences while predicting MetS. Our study found that the ability of the TyG index to predict MetS also differed among adults from different races; however, this difference was not significant. Differences in metabolic characteristics, genetics, lifestyle, and culture among ethnic groups may contribute to differences in body fat and metabolism23. Therefore, gender and ethnic differences in the adult population may need to be considered during the practical application of the TyG index in predicting MetS to develop more personalized prevention and intervention measures.
We acknowledge certain limitations of our study. First, due to the wide time span, variations in laboratory methods or locations for measuring glucose, insulin, and triglycerides across different cycles might have potentially influenced our results. Second, being a cross-sectional study derived from observational surveys, only associations and not causes could be established.
Conclusions
The TyG index is a convenient and easy-to-calculate parameter in clinical practice. Using NHANES data, we found that TyG still had a high diagnostic value for MetS. It even slightly outperformed the traditional HOMA and the cutoff was 8.75. The prevalence of MetS in the population calculated using the TyG index cutoff was higher than that reported in the IDF diagnostic criteria. This may help identify more cases of MetS, but as the prevalence differs widely when calculating the population of children and adolescents, the TyG index that identifies MetS may not apply to this particular population. In logistic regression analysis, after adjusting for multiple variables, the TyG index still maintained a correlation with the risk of MetS. Subgroup analysis of the adult population indicated differences in the ability of the TyG index to predict MetS in different genders and races, which may need to be considered prior to practical applications. In conclusion, the TyG index may be used to predict MetS in a clinical setting, thereby serving as an important reference for the early prevention of and intervention for MetS. The findings of our study highlight the use of this important index in future clinical practice.
Data availability
All databases are accessible through the NHANES website (https://wwwn.cdc.gov/nchs/nhanes/Default.aspx).
References
Ambroselli, D. et al. New advances in metabolic syndrome, from prevention to treatment: the role of diet and food. Nutrients 15, 640. https://doi.org/10.3390/nu15030640 (2023).
Kang, S. W., Kim, S. K., Kim, Y. S. & Park, M. S. Risk prediction of the metabolic syndrome using TyG Index and SNPs: a 10-year longitudinal prospective cohort study. Mol Cell Biochem. 478, 39–45. https://doi.org/10.1007/s11010-022-04494-1 (2023).
Couto, A. N., Pohl, H. H., Bauer, M. E. & Schwanke, C. H. A. Accuracy of the triglyceride-glucose index as a surrogate marker for identifying metabolic syndrome in non-diabetic individuals. Nutrition. 109, 111978. https://doi.org/10.1016/j.nut.2023.111978 (2023).
Nabipoorashrafi, S. A. et al. The accuracy of triglyceride-glucose (TyG) index for the screening of metabolic syndrome in adults: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis. 32, 2677–2688. https://doi.org/10.1016/j.numecd.2022.07.024 (2022).
Ramdas Nayak, V. K., Satheesh, P., Shenoy, M. T. & Kalra, S. Triglyceride glucose (TyG) index: a surrogate biomarker of insulin resistance. J Pak Med Assoc. 72, 986–988. https://doi.org/10.47391/JPMA.22-63 (2022).
Son, D. H., Lee, H. S., Lee, Y. J., Lee, J. H. & Han, J. H. Comparison of triglyceride-glucose index and HOMA-IR for predicting prevalence and incidence of metabolic syndrome. Nutr Metab Cardiovasc Dis. 32, 596–604. https://doi.org/10.1016/j.numecd.2021.11.017 (2022).
Dundar, C., Terzi, O. & Arslan, H. N. Comparison of the ability of HOMA-IR, VAI, and TyG indexes to predict metabolic syndrome in children with obesity: A cross-sectional study. BMC Pediatr. 23, 74. https://doi.org/10.1186/s12887-023-03892-8 (2023).
Simental-Mendía, L. E., Rodríguez-Morán, M. & Guerrero-Romero, F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 6, 299–304. https://doi.org/10.1089/met.2008.0034 (2008).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28, 412–419. https://doi.org/10.1007/BF00280883 (1985).
International Diabetes Federation. IDF consensus worldwide definition of the metabolic syndrome. Available from: https://idf.org/media/uploads/2023/05/attachments-30.pdf. Accessed 11 Jan 2024.
International Diabetes Federation. IDF consensus definition of metabolic syndrome in children and adolescents. Available from: https://idf.org/media/uploads/2023/05/attachments-31.pdf. Accessed 11 Jan 2024.
ElSayed NA, Aleppo G, Aroda VR, et al. 2. Classification and diagnosis of diabetes: Standards of care in diabetes-2023. Diabetes Care. 46, S19–S40. https://doi.org/10.2337/dc23-S002 (2023).
Whelton, P. K., Carey, R. M., Aronow, W. S., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 71, e13–e115. https://https://doi.org/10.1161/HYP.0000000000000065 (2018).
Park, S. Y., Gautier, J. F. & Chon, S. Assessment of insulin secretion and insulin resistance in human. Diabetes Metab J. 45, 641–654. https://doi.org/10.4093/dmj.2021.0220 (2021).
Kassab, H. S., Osman, N. A. & Elrahmany, S. M. Assessment of triglyceride-glucose index and ratio in patients with type 2 diabetes and their relation to microvascular complications. Endocr Res. 48, 94–100. https://doi.org/10.1080/07435800.2023.2245909 (2023).
Ning, P., Zeng, J., Feng, Q., Chen, M. & Cao, H. Triglyceride-glucose index as a predictor of lower extremity arterial disease in patients with diabetes: A hospitalized population retrospective study. Ann Vasc Surg. 98, 173–181. https://doi.org/10.1016/j.avsg.2023.08.013 (2024).
Liu, X. et al. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: A systematic review and meta-analysis. Cardiovasc Diabetol. 21, 124. https://doi.org/10.1186/s12933-022-01546-0 (2022).
Yan, F. et al. Association between triglyceride glucose index and risk of cerebrovascular disease: systematic review and meta-analysis. Cardiovasc Diabetol. 21, 226. https://doi.org/10.1186/s12933-022-01664-9 (2022).
Shen, Y., Prinyawiwatkul, W. & Xu, Z. Insulin: A review of analytical methods. Analyst 144, 4139–4148. https://doi.org/10.1039/c9an00112c (2019).
Tahapary, D. L. et al. Challenges in the diagnosis of insulin resistance: Focusing on the role of HOMA-IR and tryglyceride/glucose index. Diabetes Metab Syndr. 16, 102581. https://doi.org/10.1016/j.dsx.2022.102581 (2022).
Muscogiuri, G. et al. Obesity: A gender-view. J Endocrinol Invest. https://doi.org/10.1007/s40618-023-02196-z (2023).
Pontzer, H. et al. Daily energy expenditure through the human life course. Science. 373, 808–812. https://doi.org/10.1126/science.abe5017 (2021).
Min, J., Goodale, H., Xue, H., Brey, R. & Wang, Y. Racial-ethnic disparities in obesity and biological, behavioral, and sociocultural influences in the United States: A systematic review. Adv Nutr. 12, 1137–1148. https://doi.org/10.1093/advances/nmaa162 (2021).
Acknowledgements
We would like to thank MogoEdit for its English editing during the preparation of this manuscript.
Funding
This project was supported by the Medical Scientific Research Project of Chengdu (No. 2021272) and the Scientific Research Fund of Chengdu Fifth People’s Hospital (No. GSPZX2022-19).
Author information
Authors and Affiliations
Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wan, H., Cao, H. & Ning, P. Superiority of the triglyceride glucose index over the homeostasis model in predicting metabolic syndrome based on NHANES data analysis. Sci Rep 14, 15499 (2024). https://doi.org/10.1038/s41598-024-66692-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-66692-9
- Springer Nature Limited