Abstract
Impaired respiratory variation of right atrial pressure (RAP) in severe pulmonary hypertension (PH) suggests difficulty tolerating increased preload during inspiration. Our study explores whether this impairment links to specific factors: right ventricular (RV) diastolic function, elevated RV afterload, systolic RV function, or RV-pulmonary arterial (PA) coupling. We retrospectively evaluated respiratory RAP variation in all participants enrolled in the EXERTION study. Impaired respiratory variation was defined as end-expiratory RAP − end-inspiratory RAP ≤ 2 mm Hg. RV function and afterload were evaluated using conductance catheterization. Impaired diastolic RV function was defined as end-diastolic elastance (Eed) ≥ median (0.19 mm Hg/mL). Seventy-five patients were included; PH was diagnosed in 57 patients and invasively excluded in 18 patients. Of the 75 patients, 31 (41%) had impaired RAP variation, which was linked with impaired RV systolic function and RV-PA coupling and increased tricuspid regurgitation and Eed as compared to patients with preserved RAP variation. In backward regression, RAP variation associated only with Eed. RAP variation but not simple RAP identified impaired diastolic RV function (area under the receiver operating characteristic curve [95% confidence interval]: 0.712 [0.592, 0.832] and 0.496 [0.358, 0.634], respectively). During exercise, patients with impaired RAP variation experienced greater RV dilatation and reduced diastolic reserve and cardiac output/index compared with patients with preserved RAP variation. Preserved RAP variation was associated with a better prognosis than impaired RAP variation based on the 2022 European Society of Cardiology/European Respiratory Society risk score (chi-square P = 0.025) and survival free from clinical worsening (91% vs 71% at 1 year and 79% vs 50% at 2 years [log-rank P = 0.020]; hazard ratio: 0.397 [95% confidence interval: 0.178, 0.884]). Subgroup analyses in patients with group 1 and group 4 PH demonstrated consistent findings with those observed in the overall study cohort. Respiratory RAP variations reflect RV diastolic function, are independent of RV-PA coupling or tricuspid regurgitation, are associated with exercise-induced haemodynamic changes, and are prognostic in PH.
Trial registration. NCT04663217.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Pulmonary hypertension (PH) is a chronic disease characterized by narrowing of pulmonary vascular lumen and subsequently increased right ventricular (RV) afterload1. Consequently, the right ventricle undergoes (mal-)adaptations driven by the burden of both pressure and volume overload2. This process results in the development of pathophysiological features including secondary tricuspid regurgitation and impaired RV diastolic and systolic function as the disease progresses2.
Impaired RV function is associated with elevated RV filling pressures, which have already been described in patients with PH as well as other diseases1. In 1873, Kussmaul published an important clinical observation: patients with compromised cardiac filling due to conditions such as constrictive pericarditis or restrictive cardiomyopathy had a paradoxical increase in jugular venous pressure during inspiration compared with healthy individuals3. In an intriguing parallel, this phenomenon extends to patients with right-sided heart failure and PH. Within this context, researchers have shown that impaired respiratory variability of right atrial pressure (RAP) in PH is an indicator of disease severity and right-sided heart function4. However, the mechanisms behind this phenomenon in patients with PH have not been fully investigated. Plausible factors include tricuspid regurgitation, RV systolic dysfunction, and RV diastolic dysfunction—each with the potential to increase RV filling pressure and to impede the variation of RAP during spontaneous inspiration. Since assessment of RV diastolic function and RV-pulmonary arterial (PA) coupling is technically challenging, studies on this topic were lacking. To address this knowledge gap comprehensively, our study utilizes gold-standard assessments of RV diastolic and systolic function via pressure–volume loop analysis to investigate the physiological mechanisms behind and functional consequences of respiratory variations in RAP.
Methods
Study design and population
All patients enrolled in the prospectively recruiting Exercise Hemodynamic, Right Ventricular Coupling and Echocardiography in Pulmonary Hypertension (EXERTION) study (ClinicalTrials.gov identifier: NCT04663217) from 2020 to 2022 were included in this post-hoc analysis. Diagnosis of PH was made by a multidisciplinary board following contemporary guidelines1. Control patients were initially referred due to suspected PH and exertional dyspnoea; PH was ruled out through invasive diagnostics (mean pulmonary artery pressure at rest ≤ 20 mm Hg). Among other criteria, severe lung disease was an important exclusion criterion due to its potential impact on RAP variation. Additional inclusion and exclusion criteria are detailed for the EXERTION study (NCT04663217). The study adheres to the principles of the Declaration of Helsinki and was approved by the local Ethics Committee of the University of Giessen Medical Faculty (approval number 117/16). Written informed consent was obtained from all participating patients.
Right heart catheterization and assessment of RAP variation during spontaneous inspiration
Right heart catheterization was performed as previously described5. Briefly, under sonographic guidance and local anaesthesia, a 7F sheath was introduced into the internal jugular vein. Measurements of pulmonary artery wedge pressure and mean pulmonary artery pressure were acquired during end-expiratory phases using a Swan-Ganz catheter. RAP was evaluated as the mean over several cardiac cycles during spontaneous respiration. Respiratory variation was computed as the disparity between end-expiratory and end-inspiratory RAP values (ΔRAP)4. A ΔRAP greater than 2 mm Hg indicated preserved respiratory variation4. Cardiac output and index were derived through the direct Fick method, when applicable, or by utilizing thermodilution1.
Pressure–volume loop assessment
Pressure–volume loops were obtained using conductance catheterization as described previously6. A 4F pressure–volume catheter (CA-41063; CD Leycom, Zoetermeer, The Netherlands) was positioned in the RV apex under sonographic guidance, and an intracardiac analyser (Inca; CD Leycom) was utilized to visualize real-time pressure–volume loops. The calculation of arterial elastance (Ea) involved determining the ratio of end-systolic pressure to stroke volume, and end-systolic elastance (Ees) was computed using the RV single-beat approach7. RV-PA coupling was defined as the Ees/Ea ratio. Diastolic stiffness (β) was determined using a custom MATLAB programme and fitting a nonlinear exponential curve through three data points on the diastolic section of the pressure–volume loops8. Additionally, we determined Eed by applying the relationship \({\text{dP}}/{\text{dV}}\, = \,\alpha \beta \, \times \,{\text{e}}^{{(\beta \, \times \,{\text{EDV}})}}\) at calculated end-diastolic volumes (EDV), with α representing a curve-fit parameter9.
Exercise protocol
Patients underwent exercise in a semi-supine position after the placement of the conductance catheter, continuing until exhaustion. We followed an incremental protocol, increasing the workload by 20 W every 2–4 min. For patients unable to begin at a 20-W workload, we adjusted the initial workload to a minimum of 5 W, with subsequent 5-W increments every 2–4 min until exhaustion. The exercise session had a maximum duration of 10–12 min. Simultaneously, we conducted echocardiography alongside symptom assessments. Pressure–volume loops were determined at two specific time points: before exercise (baseline) and at the point of maximum exertion.
Data assessment and statistical analyses
Adherence to normal distribution of all variables was assessed using the Shapiro–Wilk test. For normally distributed parameters, we present the mean ± standard deviation and employed (pairwise) t-tests for comparing means between groups. Non-normally distributed parameters are displayed as medians [Q1, Q3], and the Wilcoxon rank sum test was utilized for comparing medians across groups. Categorical parameters were compared by the chi-square test. Spearman's rho coefficient was used for correlation analyses. ∆RAP was utilized as continuous parameter for correlation analyses. The pROC package was used for receiver operating characteristic (ROC) analyses. Clinical worsening was assessed in August 2023 for all patients included in the study. Clinical worsening was defined as meeting at least one of the following criteria: (1) 6-min walk distance (6MWD) decrease ≥ 15% in two consecutive tests; (2) worsening of World Health Organization (WHO) functional class; (3) hospitalization; (4) escalation of diuretics (either dose increase or additional diuretics); (5) escalation of PH treatment; (6) lung transplantation; and (7) death. Survival analyses included Kaplan–Meier and univariate Cox regression analyses. Backward regression analysis was performed to ascertain the relationship between RAP variation and other parameters. All statistical procedures were performed using R version 4.0.4 (The R Foundation, Vienna).
Ethics approval and consent to participate
The study adheres to the principles of the Declaration of Helsinki and was approved by the local Ethics Committee of the University of Giessen Medical Faculty (approval number 117/16). Written informed consent was obtained from all participating patients.
Results
Patient characteristics
A total of 75 patients were enrolled, with 64% being female. The median age was 69 [58, 76] years, and most of the patients experienced significant exertional dyspnoea (64% were classified as WHO functional class III or IV). Of the 75 patients, 25 (33%) were diagnosed with group 1 PH, while 12 (16%) and 20 (27%) were classified as group 2 and group 4, respectively. Additionally, 18 patients (24%) had PH invasively ruled out. Table 1 shows impaired pulmonary haemodynamics in the overall study population.
The median RAP variation during spontaneous breathing was 3 [1, 5] mm Hg, with 31 patients (41%) exhibiting impaired RAP variation defined as ΔRAP ≤ 2 mm Hg. Interestingly, patients with impaired RAP variation displayed lower body mass index, higher brain natriuretic peptide levels, and impaired echocardiographic RV and right atrial (RA) function compared with patients with preserved RAP variation (Table 1). Exercise capacity (maximum oxygen uptake, 6MWD) was numerically but not statistically significantly lower in the patients with impaired RAP variation. Importantly, both RV-PA coupling (Ees/Ea) and RV diastolic function (Eed) were significantly impaired at rest in these patients. TAPSE/PASP, as a surrogate marker of RV-PA coupling, exhibited a significant impairment as well (Table 1).
Physiological basis of RAP variation
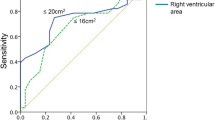
Correlation analyses were conducted to examine the relationship between RAP variation and RV diastolic function, load-independent systolic function, and RV-PA coupling. A significant correlation between ΔRAP and Eed was observed (Spearman’s rho = − 0.41, P < 0.001), whereas no correlation was evident between ΔRAP and Ees (Spearman’s rho = − 0.21, P = 0.07) or between ΔRAP and Ees/Ea (Spearman’s rho = 0.21, P = 0.08). Consequently, ΔRAP effectively identified impaired diastolic RV function (defined as Eed ≥ median [= 0.19 mm Hg/mL]) with a good area under the ROC curve (0.712 [95% confidence interval: 0.592, 0.832], Fig. 1A). By contrast, the simple assessment of RAP could not identify impaired RV diastolic function, as shown in Fig. 1B (area under the ROC curve: 0.496 [95% confidence interval: 0.358, 0.634]). Through regression analysis, we determined that only Eed, but not Ees, Ea or tricuspid regurgitation severity, showed a significant association with ΔRAP (Table 2).
Notably, during exercise, there was no difference in contractile reserve (measured as the change in Ees from rest to exercise) between the impaired and preserved RAP variation groups (0.20 [0.01, 0.35] mm Hg/mL and 0.23 [0.03, 0.44] mm Hg/mL, respectively; P = 0.42). Consistent with this finding, the ΔEes/Ea ratio was also comparable between the two groups (− 0.05 [− 0.21, 0.13] and − 0.01 [− 0.38, 0.25], respectively; P = 0.96). However, RV dilatation (measured as the change in RV EDV from rest to exercise) was more pronounced in patients with impaired RAP variation than in those with preserved RAP variation (29 [8, 40] mL vs 3 [− 7, 24] mL, P = 0.002). RV diastolic function during exertion was significantly impaired in patients with impaired RAP variation (RV end-diastolic pressure: 16 [10, 25] mm Hg vs 12 [7, 17] mm Hg, P = 0.049; Eed: 0.36 [0.28, 0.51] mm Hg/mL vs 0.27 [0.12, 0.45] mm Hg/mL, P = 0.012). Concordantly, both peak cardiac output and cardiac index were significantly reduced in the impaired RAP variation group (cardiac output: 6.4 [4.7, 8.3] L/min vs 7.7 [6.2, 10.2] L/min, P = 0.015; cardiac index: 3.2 [2.6, 4.4] L/min/m2 vs 4.2 [3.3, 4.8] L/min/m2, P = 0.031).
Prognostic significance of RAP variation
Lastly, we explored the relationship between impaired respiratory variation of RAP and clinical worsening. Overall, clinical worsening occurred in 25 patients(33%) (escalation of PH-targeted therapy in eight cases [32%], increase in diuretics in five cases [20%], hospitalization in four cases [16%], substantial decrease of 6MWD in four cases [16%], worsening of WHO functional class in two cases [8%], and death in two cases [8%]). As depicted in Fig. 2A, patients with preserved RAP variation during spontaneous breathing exhibited significantly higher survival free from clinical worsening than patients with impaired RAP variation (worsening-free survival at 1 and 2 years was 91% and 79%, respectively, in patients with preserved RAP variation and 71% and 50%, respectively, in patients with impaired RAP variation; log-rank P = 0.020). Moreover, preserved RAP variation was associated with a reduced univariate hazard ratio for clinical worsening (0.397 [95% confidence interval: 0.178, 0.884], P = 0.024). We additionally employed the 2022 European Society of Cardiology/European Respiratory Society (ESC/ERS) risk stratification scheme1 to assess indirectly the association of RAP variation with mortality in our cohort. As shown in Fig. 2B and Table 1, patients with preserved RAP variation had a more favourable risk distribution than those with impaired RAP variation.
Prognostic relevance of respiratory RAP variation. (A) Time to clinical worsening (Kaplan–Meier analysis) and (B) European Society of Cardiology/European Respiratory Society risk distribution were compared between patients with impaired (≤ 2 mm Hg) and preserved (> 2 mm Hg) RAP variation. RAP, right atrial pressure.
Subgroup analyses in group 1 and group 4 PH
Furthermore, subgroup analyses were conducted in patients with group 1 and group 4 PH. Table 3 presents the baseline characteristics of this subgroup. Among the 45 patients with group 1 and 4 PH, ∆RAP demonstrated a significant correlation with Eed (Spearman’s rho = − 0.529, p < 0.001). Consistent with hitherto mentioned findings in the overall study population, regression analysis indicated that only Eed, and not Ees, Ea, or tricuspid insufficiency, was associated with RV diastolic function. Moreover, ∆RAP identified high Eed with an AUROC of 0.731 [0.581, 0.882]. Survival analyses further revealed a significant association of impaired RAP variation with survival, as evidenced by Kaplan–Meier analysis (24-month survival: 37% vs 75%, log-rank p = 0.032) and Cox-regression analysis (HR 0.383 [0.154, 0.955], p = 0.039).
Discussion
In this study, we demonstrate that the variation of RAP during spontaneous respiration serves as a surrogate of RV diastolic function, is independent from RV-PA coupling or the severity of tricuspid regurgitation, and predicts diastolic reserve and cardiac output/index increase during exertion. Furthermore, we validate its prognostic significance concerning clinical worsening and estimated risk of mortality. The study was conducted using gold-standard conductance catheterization to assess RV diastolic function and RV-PA coupling.
Our study is the first to invasively validate the association between respiratory variation of RAP during spontaneous breathing and RV diastolic function based on the end-diastolic pressure–volume relationship. Notably, RAP variation was not independently associated with other signs of RV maladaptation, such as RV-PA uncoupling or tricuspid regurgitation severity. This observation is consistent with previous findings indicating that cardiac output/index does not differ according to RAP variation at rest4. The correlation observed between RAP variation and RV diastolic performance in our study highlights the potential of RAP variation to mirror the complex mechanisms of RV relaxation and filling during diastole. This extends our understanding beyond conventional measurements since RAP itself was not significantly associated with Eed in our study population. The simplicity of the assessment of RAP variation increases the relevance of this finding, as it can be routinely monitored during right heart catheterization.
ROC analyses indicated that RAP variation also has good discriminatory power for the differentiation of preserved and impaired RV diastolic function. Considering the invasiveness and complexity of assessment of RV diastolic function, RAP variation can serve as a simple surrogate for this parameter. Recently, we derived and validated (independently in a second cohort) the ratio of peak lateral tricuspid annulus systolic velocity to RA area index as a novel and first echocardiographic indicator of RV diastolic function10. Though numerically the area under the ROC curve for RAP variation was lower than the one described for the aforementioned echocardiographic ratio10, RAP variation gives additional information based on routine right heart catheterization.
Interestingly, there were meaningful differences between patients with impaired and preserved RAP variation during exertion. While contractile reserve was comparable between the two groups, patients with impaired RAP variation showed more prominent RV dilatation during exercise, which is known to be associated with clinical worsening11,12. Consistent with this finding, patients with impaired RAP variation also had a significantly greater increase of Eed than those with preserved RAP variation, indicating an impaired diastolic reserve in the former group, which results in significantly reduced cardiac output and cardiac index during exercise. Thus, impairment of RAP variation during spontaneous breathing is not only an indicator for RV diastolic function at rest but is also associated with exercise haemodynamics.
Al-Qadi et al.4 showed in a retrospective study the prognostic relevance of RAP variation during spontaneous breathing. In line with their observations, our study shows significant associations with clinical worsening as well as ESC/ERS risk scoring. The ESC/ERS risk stratification scheme is the current gold standard in Europe to estimate 1-year survival prognosis and was originally developed for patients with group 1 PH, but is also validated in severe interstitial lung disease-associated PH and chronic thromboembolic PH1,13,14,15,16. Since the risk scheme is highly validated, impaired RAP variation is likely to be associated with hard endpoints such as death17,18. This is consistent with previous research showing numerically higher (though statistically not significant) 1-year mortality in patients with impaired RAP variation compared with those with preserved RAP variation4.
Furthermore, studies have demonstrated that intrathoracic pressure, as measured via esophageal manometry, exerts a significant impact on pulmonary hemodynamics, particularly on static values in obese patients19. While its influence on respiratory variation is presumed to be less pronounced, additional research investigating the relevance of intrathoracic pressure on ∆RAP is warranted.
Our study is limited by its retrospective study design and lack of long-term survival data. The inclusion of only one centre in the study may limit the generalizability of the results. Nonetheless, implementation of highly sophisticated hemodynamic data and pressure–volume loops using gold-standard conductance catheterization at rest and during exercise shed light on the pathophysiological mechanism behind the impairment of RAP variation in patients with PH.
Interpretation
RAP variation in patients with PH primarily hinges on diastolic RV function rather than systolic RV function, RV-PA coupling or tricuspid regurgitation (Fig. 3). RV dilatation during exercise with impaired diastolic reserve and subsequently reduced peak cardiac output occurs more often in patients with impaired RAP variation than in those with preserved RAP variation. Lastly, impaired variation of RAP is linked with clinical worsening and risk scores indicating increased mortality.
Overview of key findings. Respiratory RAP variation in patients with PH primarily hinges on diastolic RV function. Dilatation during exercise and subsequently reduced peak cardiac output occurs more often in patients with impaired RAP variation than in those with preserved RAP variation. Lastly, impaired variation of RAP is linked with clinical worsening and a well-established surrogate of mortality. A. u., arbitrary units; Eed, end-diastolic elastance; PH, pulmonary hypertension; RAP, right atrial pressure; RV, right ventricular.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 6MWD:
-
6-Minute walk distance
- ΔRAP:
-
Disparity between end-expiratory and end-inspiratory right atrial pressure
- Ea:
-
Arterial elastance
- EDV:
-
End-diastolic volume
- Eed:
-
End-diastolic elastance
- Ees:
-
End-systolic elastance
- ESC/ERS:
-
European Society of Cardiology/European Respiratory Society
- PA:
-
Pulmonary arterial
- PH:
-
Pulmonary hypertension
- RA:
-
Right atrial
- RAP:
-
Right atrial pressure
- ROC:
-
Receiver operating characteristic
- RV:
-
Right ventricular
- WHO:
-
World Health Organization
References
Humbert, M. et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 43(38), 3618–3731 (2022).
Rako, Z. A., Kremer, N., Yogeswaran, A., Richter, M. J. & Tello, K. Adaptive versus maladaptive right ventricular remodelling. ESC Heart Fail. 10(2), 762–775 (2023).
Kussmaul, A. Ueber schwielige Mediastino-Pericarditis und den paradoxen Puls. Berl. Klin. Wochenschr. 10, 433–435 (1873).
Al-Qadi, M. O., Holbrook, J., Ford, H. J., Ceppe, A. & LeVarge, B. L. Prognostic value of respiratory variation in right atrial pressure in patients with precapillary pulmonary hypertension. Chest 164(2), 481–489 (2023).
Yogeswaran, A. et al. Evaluation of pulmonary hypertension by right heart catheterisation: Does timing matter?. Eur. Respir. J. 56(3), 1901892 (2020).
Tello, K. et al. Reserve of right ventricular-arterial coupling in the setting of chronic overload. Circ. Heart Fail. 12(1), e005512 (2019).
Tello, K. et al. More on single-beat estimation of right ventriculoarterial coupling in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 198(6), 816–818 (2018).
Vanderpool, R. R. et al. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart 101(1), 37–43 (2015).
Trip, P. et al. Clinical relevance of right ventricular diastolic stiffness in pulmonary hypertension. Eur. Respir. J. 45(6), 1603–1612 (2015).
Yogeswaran, A. et al. Echocardiographic evaluation of right ventricular diastolic function in pulmonary hypertension. ERJ Open Res. 9(5), 00226–02023 (2023).
Grunig, E. et al. Assessment and prognostic relevance of right ventricular contractile reserve in patients with severe pulmonary hypertension. Circulation 128(18), 2005–2015 (2013).
Ireland, C. G. et al. Exercise right ventricular ejection fraction predicts right ventricular contractile reserve. J. Heart Lung Transplant. 40(6), 504–512 (2021).
Galie, N. et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 37(1), 67–119 (2016).
Delcroix, M. et al. Risk assessment in medically treated chronic thromboembolic pulmonary hypertension patients. Eur. Respir. J. 52(5), 1800248 (2018).
Kylhammar, D. et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur. Heart J. 39(47), 4175–4181 (2018).
Yogeswaran, A. et al. Risk assessment in severe pulmonary hypertension due to interstitial lung disease. J. Heart Lung Transplant. 39(10), 1118–1125 (2020).
Hoeper, M. M. et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur. Respir. J. 50(2), 1700740 (2017).
Humbert, M. et al. Risk assessment in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 53(6), 1802004 (2019).
Khirfan, G. et al. Impact of esophageal pressure measurement on pulmonary hypertension diagnosis in patients with obesity. Chest 162(3), 684–692 (2022).
Acknowledgements
Editorial assistance was provided by Dr Claire Mulligan (Beacon Medical Communications, Ltd, Brighton, United Kingdom), funded by the University of Giessen.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Excellence Cluster Cardio-Pulmonary System (ECCPS) and the Collaborative Research Center (SFB) 1213—Pulmonary Hypertension and Cor Pulmonale, grant number SFB1213/1, project B08 (German Research Foundation, Bonn, Germany).
Author information
Authors and Affiliations
Contributions
Study conceptualization: AY and KT; Study design and data collection: All authors; Data analysis: AY; Drafting of the manuscript: AY and KT; Critical revision of the manuscript: All authors; Approval of manuscript for submission: All authors; Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: All authors.
Corresponding author
Ethics declarations
Competing interests
Dr. Yogeswaran reports non-financial support from the University of Giessen during the conduct of the study, and personal fees from MSD outside the submitted work. Mr. Brito da Rocha, Dr. Rako, Mr. Kaufmann, Mr. Schäfer, and Mr. Kremer report non-financial support from the University of Giessen during the conduct of the study. Dr. Ghofrani reports grants from the German Research Foundation and non-financial support from the University of Giessen during the conduct of the study, and personal fees from Bayer, Actelion, Pfizer, Merck, GSK, and Takeda, grants and personal fees from Novartis, Bayer HealthCare, and Encysive/Pfizer, and grants from Aires, the German Research Foundation, Excellence Cluster Cardiopulmonary Research, and the German Ministry for Education and Research outside the submitted work. Dr. Seeger reports grants from the German Research Foundation and non-financial support from the University of Giessen during the conduct of the study, and personal fees from Pfizer and Bayer Pharma AG outside the submitted work. Dr. Tello reports non-financial support from the University of Giessen during the conduct of the current study and speaker honoraria from Actelion and Bayer outside the submitted work.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yogeswaran, A., da Rocha, B.B., Rako, Z.A. et al. Physiological mechanisms behind respiratory variations in right atrial pressure in pulmonary hypertension. Sci Rep 14, 12547 (2024). https://doi.org/10.1038/s41598-024-61825-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-61825-6
- Springer Nature Limited