Abstract
Extracranial arteriovenous malformations (AVMs) are characterized by anomalous arterial-to-venous connections, aberrant angiogenesis, local inflammation and hypoxia, and disorganized histological architecture; however, the precise molecular perturbations leading to this phenotype remain elusive. We hypothesized that extracranial AVM tissue would demonstrate deregulation of the TGF-β/BMP signaling pathway, which may serve as a potential target in the development of molecular-based therapies for AVMs. AVM tissue was harvested during resection from 10 patients with AVMs and compared to control tissue. Blood was collected from 14 AVM patients and 10 patients without AVMs as controls. Expression of TGF-β/BMP pathway components was analyzed using RT-PCR, western blotting, and immunohistochemistry. Circulating levels of TGF-β1 were analyzed by ELISA. Paired t tests were utilized to perform statistical analysis. The mRNA levels of TGF-β1, ALK1, Endoglin (ENG), Smad6, Smad7, and Smad8 were significantly elevated in AVM tissue when compared to controls. Protein levels of TGF-β1 and Smad3 were elevated in AVM tissue while protein levels of BMP-9, ALK1, Smad1, Smad6, and Smad8 were significantly decreased in AVMs. Immunohistochemistry demonstrated increased TGF-β1 in the perivascular cells of AVMs compared to normal controls, and circulating levels of TGF-β1 were significantly higher in AVM patients. Patients with AVMs demonstrate aberrant TGF-β/BMP expression in AVM tissue and blood compared to controls. Targeting aberrantly expressed components of the TGF-β/BMP pathway in extracranial AVMs may be a viable approach in the development of novel molecular therapies, and monitoring circulating TGF-β1 levels may be a useful indicator of treatment success.
Similar content being viewed by others
Introduction
Arteriovenous malformations (AVMs) are high-flow vascular lesions composed of a network of direct arterial-to-venous connections suspected to be devoid of a normal capillary bed with impaired exchange of nutrients and oxygen. AVMs are congenital lesions affecting 1:100,000 patients1. Extracranial (peripheral) AVMs can be complex and infiltrative lesions that are often congenital and characterized by unchecked vascular expansion and soft tissue destruction. Histologically, AVMs demonstrate abnormal perivascular tissue, disorganized vascular channels and evidence of disrupted vascular remodeling. This leads to thin, leaky and friable vessels that tend to bleed, either spontaneously or during interventions2. Despite currently available surgical and endovascular techniques to treat these lesions, their recurrence rate exceeds that of most cancers3 and leads to significant functional and aesthetic morbidities4. Because of the infiltrative nature of these lesions, they are almost never “cured;” the goal of treatment is typically control of growth and bleeding5. Although the exact mortality and cost associated with AVM treatment is not known, patients typically require multiple treatment interventions throughout their lifetime. These challenges necessitate the development of topical or systemic treatment modalities that target the aberrant pro-angiogenic signaling cascades that have been demonstrated in these lesions.
The etiology of extracranial AVMs is poorly understood, although some hereditary conditions with AVM have been described. These include hereditary hemorrhagic telangiectasia (HHT) and capillary malformation-arteriovenous malformation (CM-AVM) which have been attributed to inherited mutations in RASA1, endoglin, and activin receptor-like kinase 1 (ALK1). However, most extracranial AVMs are sporadic and likely the result of somatic mutations (reviewed by Cunha et al.6). For example, somatic mutations of MAP2K1 have been reported in sporadic extracranial AVMs7, as have mosaic-activating variants in the components of RAS/MAPK signaling (KRAS, BRAF, and MAP2K1)8. While these mutations offer insights into the proliferative and pro-angiogenic nature of AVMs, they do not fully explain the dysmorphic vascular architecture of these lesions. Histologically, the AVM phenotype suggests a disordered process of vascular remodeling and differentiation, processes directly regulated by the transforming growth factor (TGF)-β/bone morphogenetic protein (BMP) superfamily9.
The highly conserved TGF-β/BMP signaling pathway is complex and involves multiple ligands, second messengers, and feedback mechanisms10,11. In endothelial cells (ECs), the secreted ligands TGF-β1 and BMP-9 initiate signaling via recruitment of two specific serine/threonine kinase heterotrimeric complexes, ALK1 and ALK512,13,14. These complexes then recruit and activate Smad signaling effectors (Smads1, 5, and 8 from ALK1 activation and Smads 2 and 3 from ALK 5 activation), which complex with Smad4 and translocate to the nucleus, where various endothelial-specific genes are then activated15. Concurrently, inhibitory Smads 6 and 7 are activated by ALK1 and ALK5 activation, serving as a negative feedback control of this pathway16. The balance of this intricate signaling cascade is critically important for normal vascular development, maintenance of vascular permeability, and vascular remodeling, all of which are abnormal in extracranial AVMs.
Disruption of the TGF-β/BMP pathway has been observed in other vascular malformations, including HHT and cerebral cavernous malformations (CCM), which can present with AVMs or vascular cavernomas6. ALK1, endoglin, and BMP-9 mutations have been described in the affected tissues of patients with HHT17,18,19 but not in sporadic extracranial AVMs. Endothelial cells carrying any of the known CCM mutations also demonstrate dysregulated TGF-β/BMP signaling20,21. Given these observations, several strategies targeting this pathway in HHT and CCM have been employed with varying degrees of success, including anti-fibrinolytics, anti-estrogens, chemotherapeutic agents, and TGF-β receptor antagonists20,22,23,24,25.
The potential of targeting the TGF-β/BMP pathway in sporadic extracranial AVMs remains unknown. The purpose of this study was to determine if extracranial AVMs exhibit aberrant TGF-β/BMP signaling and thus possibly identify a molecular pathway that could be targeted with new therapies.
Methods
Specimen collection and preparation
This study was approved by the Institutional Review Board of the University of Arkansas for Medical Sciences (#228710). Written informed consent was obtained from the participants or their guardians prior to tissue and serum collection. All described experiments were performed in accordance to the relevant guidelines and regulations set forth by the IRB. Human AVM tissues (n = 10, ages 5 months–2.5 years), normal tissues (n = 10, ages 2 months–19 years), AVM patient sera (n = 14, ages 20 months–19 years), and normal control sera (n = 10, ages 14 months–34 years) were collected from both pediatric and adult patients after induction of general anesthesia for excisions. AVM tissue was collected during AVM resection, and normal soft tissue (skin and subcutaneous tissue) was collected from otherwise healthy patients undergoing excisions of other vascular lesions such as infantile hemangioma from regions distant from their respective lesions. Patient serum was prepared from whole blood collected in BD Vacutainer SSD tubes with silica clot activator and separating polymer gel. Histological examination by a pathologist experienced with vascular anomalies confirmed the diagnosis of AVM by H&E. After surgical harvest, tissue specimens were divided and immediately put into either 10% neutral formalin for histological staining or directly frozen on dry ice and transferred to a − 80 °C freezer for western blotting or RT-PCR. Serum specimens were centrifuged for 15 min at 1000g at room temperature (RT), and supernatants were aspirated and transferred to a − 80 °C freezer for storage until analysis.
Real-time PCR
Thirty milligrams of sample tissue was used for RNA isolation with the RNeasy Plus Kit (Qiagen) according to the manufacturer’s instructions. RNA samples were reverse-transcribed into cDNA with the TaqMan Reverse Transcription Reagents Kit (Thermofisher) using random primers according to the manufacturer’s instructions. The real-time PCR amplifications were performed on an ABI 7900HT System. Thermal cycling conditions were: 1 cycle of 50 °C for 2 min, then 1 cycle of 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The total reaction volume of 10 µl contained 5 ng of cDNA template, 5 µl of 2 × PCR Master Mix (Thermofisher), and 0.5 µl of 20 × primers. RT-PCR primers for TGF-β1 (Hs00998133_m1), ALK5 (Hs00610320_m1), ALK1 (Hs00953798_m1), BMP-9 (Hs00211913_m1), SMAD1 (Hs00195432_m1), SMAD2 (Hs00183425_m1), SMAD3 (Hs00969210_m1), SMAD4 (Hs00929647_m1), SMAD5 (Hs00195437_m1), SMAD6 (Hs00178579_m1), SMAD7 (Hs00998193_m1), and SMAD8 (Hs00195441_m1) were purchased from Thermofisher Scientific. The eukaryotic 18S rRNA (Thermofisher, Hs99999901_s1) was used as the endogenous control. The comparative Ct method was used to determine relative quantification.
Western blot analysis
Total protein was extracted using T-PER tissue protein extraction reagent (Thermo Scientific) supplemented with Halt Protease Inhibitor Cocktail (Thermo Scientific) and 1 mM EDTA (Invitrogen). Protein concentrations were measured using a BCA protein assay kit (Thermo Scientific). Total protein (10–20 µg) was loaded onto NuPAGE® Novex® 4–12% Bis–Tris Protein gels (Invitrogen) for electrophoresis under reducing condition and transferred to PVDF membranes (Bio-Rad). The membranes were blocked with 5% non-fat milk in TBST (Thermo Scientific) for 1 h at RT, followed by incubation with primary antibodies at 4 °C overnight. For certain experiments, membranes were cut at specific molecular weight levels to enable the same gel to be incubated with multiple primary antibodies. Blots were then washed with TBST three times, followed by incubation with horseradish peroxidase–conjugated secondary antibodies (Santa Cruz, 1:2000) for 1 h at RT. Blots were developed with the Novex ECL Chemiluminescent Substrate Reagent Kit (Invitrogen) for 1 min in dark and exposed on Image Quant™ LAS 4000 (GE Healthcare). The primary antibodies used were TGF-β1 (Abcam ab27969, 1:2000), ALK5 (Abcam ab51871, 1:200), BMP-9 (Santa Cruz sc-514211, 1:200), ALK1 (Abcam ab68703, 1:1000), Smad1 (Cell signaling #9743, 1:1000), Smad2 (Cell signaling #5339, 1:1000), Smad3 (Cell signaling #9523, 1:1000), Smad4 (Abcam ab130242, 1:2000), Smad5 (Cell signaling #9517, 1:1000), Smad6 (Abcam ab110156, 1:1000), Smad7 (Novus 293039, 1:100), Smad8 (Santa Cruz sc-7442, 1:200), GAPDH (Cell signaling #2118, 1:10,000), and α-Tubulin (Cell signaling #2125, 1:1000). Images were captured and quantitated using Image Quant TL 7.0 software (GE Healthcare). All full length gels as well as gels that were cut prior to primary antibody incubation are included in Supplementary Material.
Immunohistochemistry
After deparaffinization and rehydration, sections were heated to 95 °C for 15 min in Citrate Buffer, pH 6.0 (Invitrogen), for antigen retrieval. Hydrogen peroxide 3% (Fisher Scientific) was used to block endogenous peroxidase activity for 15 min at RT. Sections were preincubated with normal serum for 20 min at RT, then incubated in primary antibody TGF-β1 (Abcam ab66043, 1:200) at 4 °C overnight. After washing in PBST, sections were incubated in biotinylated secondary antibody (Vector Labs, 1:200) for 30 min at RT followed by ABC reagent (Vector labs) for 30 min. Color was developed using ImmPACT™ DAB (Vector Labs). The sections were then counterstained with hematoxylin (Fisher Scientific), dehydrated, and mounted using permanent mounting media (Fisher Scientific). Slides with no primary antibody applied were used as negative controls. The staining results were validated by a blind review performed by a pathologist with extensive experience examining vascular anomalies and immunohistochemistry. Microscopy was performed and images were taken with a Biotek Cytation5 Imaging Reader (Agilent Technologies, California, USA) and Olympus BX43 light microscope with Infinity 3S camera (Olympus, Pennsylvania, USA), respectively.
Enzyme-linked immunosorbent assay
Serum concentration of TGF-β1 was determined using a Human TGF-β1 Quantikine ELISA Kit (R&D Systems, DB100B) according to the manufacturer’s instructions. Optical density was read on an iMarkTM microplate absorbance reader (Bio-Rad).
Statistical analysis
Normalized RT-PCR values, quantitation results of western blot bands, and concentrations of soluble protein on ELISA for the AVM and control groups were compared, and a paired t test was used to determine statistical significance. The statistical analysis was run on an IBM SPSS Statistics 22. P < 0.05 was considered statistically significant. Asterisks (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001) are used to indicate levels of significance in figures.
Results
mRNA expression of TGF-β/BMP pathway components is dysregulated in AVM tissue
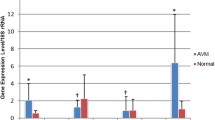
mRNA expression of TGF-β/BMP pathway components in AVM tissues (n = 10) and normal tissues (n = 10) is shown in Fig. 1. mRNA levels of TGF-β1 ligand, the serine/threonine kinase receptor ALK1, and the accessory receptor ENG were significantly elevated in AVM tissue compared with controls (P = 0.001, P = 0.016, and P = 0.001, respectively) (Fig. 1a). mRNA levels of the signaling effectors Smad6, Smad7, and Smad8 were also upregulated in AVM tissue when compared with controls (P = 0.033, P = 0.038, and P = 0.037, respectively) (Fig. 1b). mRNA levels of the TGF-β receptors TGF-βR2 and TGF-βR3, ALK5, and signaling effectors Smads1–5 were not statistically different between AVM and control tissues. BMP-9 mRNA was undetectable in both AVM and control tissues (data not shown). All target gene mRNA levels were normalized to mRNA levels of the eukaryotic 18S rRNA.
mRNA levels of TGF-β/BMP pathway components are dysregulated in AVM tissue. (a) mRNA expression of TGF-β1 ligand, the TGF-β receptors TGFβR2 and TGF-βR3, ALK1 and ALK5, and the accessory receptor endoglin (ENG) were analyzed in AVM tissue (n = 10 patients, black bars) and normal tissue (n = 10 patients, white bars). (b) mRNA expression of Smads 1–8 were analyzed in AVM tissue (n = 10 patients, black bars) and normal tissue (n = 10 patients, white bars). Relative levels of mRNAs were normalized to the expression of total 18s RNA. Error bars represent standard deviation. Paired t tests were performed to determine statistical significance (asterisks: *P < 0.05, **P < 0.01, ***P < 0.001).
Levels of TGF-β/BMP pathway component proteins are dysregulated in AVM tissue
Protein levels of TGF-β/BMP pathway components in AVM tissues (n = 10) and normal tissues (n = 10) and representative western blots are shown in Fig. 2. Protein levels of TGF-β1 ligand was significantly elevated in AVM tissues when compared to controls (P = 0.006) while levels of BMP-9 ligand protein were significantly reduced (P = 0.004) (Fig. 2a,b). Receptor ALK1 protein was significantly decreased in AVM tissue (P = 0.015) while ALK5 protein levels were similar between AVM and control tissue (Fig. 2a,b). Protein levels of TGF-β/BMP pathway signaling effectors Smad1, Smad6, and Smad8 were significantly decreased in AVM tissues (P = 0.050, P = 0.015, and P = 0.014, respectively) while Smad3 protein levels were significantly elevated in AVM tissues (P = 0.031) (Fig. 2c,d). Protein levels of Smad2, Smad5, and Smad7 were not significantly different between AVM and control tissues.
Expression of TGF-β/BMP pathway component proteins are dysregulated in AVM tissue. Protein levels of TGF-β1, ALK1, ALK5, and BMP-9 (a) and of Smad1–8 (c) were analyzed by western blot from AVM tissue samples (n = 10 patients) and normal tissue samples (n = 10 patients). Representative examples from each group are illustrated. GAPDH and α-tubulin protein expression were determined as loading controls. The total density of each protein band (n = 10 AVM and n = 10 normal) was determined using an ImageQuant developer and software, and relative expression was calculated by normalizing to loading controls (b,d). Error bars represent standard deviation. Paired t tests were performed to determine statistical significance (asterisks: *P < 0.05, **P < 0.01, ***P < 0.001).
TGF-β1 protein is elevated in AVM perivascular smooth muscle cells and in the circulation of AVM patients
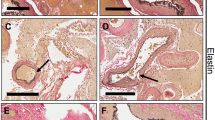
The level of TGF-β1 protein in tissues and in serum of AVM patients and normal controls is displayed in Fig. 3. Representative sections of paraffin-embedded AVM tissues (n = 2) and normal control tissues (n = 2) are illustrated in Fig. 3a–h. AVM tissues demonstrated extensive TGF-β1 staining in perivascular cells while TGF-β1 was barely detectable or absent in all normal control tissues. Circulating levels of TGF-β1 in serum collected from AVM patients (n = 14) and patients without AVMs (n = 8) are displayed in Fig. 3i. The average level of circulating TGF-β1 was significantly higher in AVM patients (10,075.3 pg/ml) when compared to normal controls (3809.7 pg/ml) (P = 0.032).
TGF-β1 protein is elevated in AVM perivascular smooth muscle cells and in the circulation of AVM patients. Paraffin-embedded slides prepared from tissue from 2 AVM patients (a,c,e,g) and 2 non-AVM patients (b,d,f,h) were stained for TGF-β1 protein and photographed under low- and high-power magnification. (i) Serum was isolated from blood samples collected from 14 AVM patients and 8 patients without AVMs, and ELISA was performed to determine the total levels of circulating TGF-β1. Error bars represent standard deviation. Paired t tests were performed to determine statistical significance (asterisks: *P < 0.05, **P < 0.01, ***P < 0.001).
Discussion
This study presents evidence that an imbalance of TGF-β/BMP signaling, a critical pathway for maintenance of vascular integrity, is present in extracranial AVM tissue. In addition, we provide evidence of increased levels of TGF-β1 ligand in both the perivascular cells of AVMs and in the circulation of AVM patients, indicating that TGF-β1 may be a useful biomarker for therapeutic monitoring.
TGF-β/BMP signaling is a complex process involving a multitude of ligands, receptors, second messengers, and transcriptional targets. Adding to the complexity of this pathway is its apparent context-dependency: in certain cell types and conditions TGF-β/BMP signaling is pro-angiogenic whereas in other contexts the pathway inhibits angiogenesis13,26. Imbalance within the various cascades stimulated by TGF-β/BMP signaling leads to alterations in vascular integrity, endothelial cell function and migration, and angiogenic drive, all of which are abnormal in extracranial AVMs. The current study demonstrates that in AVM tissue, protein and mRNA levels of key TGF-β/BMP signaling components are imbalanced when compared to normal tissue—specifically, the signaling of BMP-9 and TGF-β1 through the receptors ALK1 and ALK5, respectively.
Binding of BMP-9 to ALK1 stimulates the phosphorylation of the receptor Smads 1, 5 and 8, which then complex with Smad4 and translocate to the nucleus15. This complex regulates several genes crucial for vascular integrity, including Notch pathway genes Hes1, Hey1, Hey2, and Jag127,28 as well as the inhibitory Smad6, which regulates ALK1 expression29. Loss of ALK1 in mice leads to venous enlargement, vascular hyperbranching, and AVM formation via decreased Smad1/5/8 activity30. In our study, the protein levels of BMP-9, ALK1, Smad1, Smad6, and Smad8 were all significantly decreased in AVM tissue. Interestingly, mRNA levels of ALK1, Smad6, and Smad8 were increased in AVM tissue while mRNA levels of Smad1 remained unchanged. These data suggest that in AVM tissue, signaling through BMP-9/ALK1 is impaired, and a compensatory feedback loop increasing mRNA components of this pathway is disrupted.
Our study also demonstrates a significantly elevated level of both TGF-β1 protein and mRNA in AVM tissue. TGF-β1 binds to ALK5, which was not differentially expressed at the mRNA or protein level. The downstream effector of ALK5, Smad3, was also overexpressed at the protein level in AVM tissue compared to normal controls. Genes regulated by Smad2/3 activation include plasminogen activator inhibitor 1 (Pai-1), platelet derived growth factor b (Pdgf-b), fibronectin (Fn1), and the inhibitory Smad731. These genes play several critical roles in cell migration, clotting, senescence, wound healing, and maintenance of the extracellular matrix32,33,34,35,36,37,38,39. Taken together, our data suggest an imbalance between BMP-9/ALK1 and TGF-β1/ALK5 signaling may contribute to the multitude of impaired cellular functions present in extracranial AVMs (Fig. 4).
Schematic of dysregulated TGF-β/BMP expression in AVM endothelial cells. Levels of both circulating and intracellular TGF-β1 were elevated in AVM patients when compared to controls while intracellular BMP-9 levels were lower in AVM patients. Protein levels of components of the ALK1 → Smad1/5/8 → Smad6 pathway were decreased in AVM patients despite an increase in mRNA levels while Smads downstream of TGF-β1 → ALK5 activation demonstrated normal mRNA expression and elevated protein levels.
The etiology of extracranial AVMs remains under investigation but has been attributed to somatic mutations in several genes, including MAP2K1 (MEK1), KRAS, and BRAF40. Several studies have demonstrated cross-talk between the expression of these genes and TGF-β/BMP signaling, which may suggest an underlying mechanism of altered TGF-β/BMP signaling in extracranial AVMs. Constitutively activated MEK1 stabilizes the TβRII receptor, leading to increased signaling and decreased sensitivity to TGF-β-mediated growth suppression41,42. Oncogenic RAS can directly phosphorylate Smads 2 and 3, inhibiting their nuclear accumulation and ability to modulate downstream gene transcription43. Oncogenic BRAF mutations also activate TGF-β signaling, which, in turn, activates genes that drive endothelial-to-mesenchymal transition (EMT)44. The possible relationship between these causative somatic mutations in AVMs and imbalanced TGF-β/BMP signaling has not yet been elucidated.
Although the effect of local increased TGF-β1 production and release into circulation is unknown in AVMs, it may contribute to local inflammation, fibrosis, and smooth muscle proliferation. Overexpression of TGF-β1 in the lungs of fetal monkeys leads to pulmonary fibrosis and hyperproliferation of myofibroblasts45. During kidney ischemia, epithelial cells increase TGF-β1 secretion, which stimulates pericyte-to-mesenchymal cell transition, proliferation, and fibrosis that was reversible with anti-TGF-β1 antibody or TGF-βR inhibitor46. Increased circulating levels of TGF-β1 are associated with chronic kidney disease progression and scleroderma47,48 while TGF-β1 levels in HHT patient circulation are decreased49. In cancer, increased levels of circulating TGF-β1 are associated with increased metastasis, advanced TNM stage, and poorer prognosis50,51,52. As systemic molecular therapies are developed in the treatment of extracranial AVMs, monitoring levels of circulating TGF-β1 may be a useful indicator of treatment success, although whether this increase in circulating TGF-β1 is due to release from AVM tissue or from elsewhere in AVM patients has yet to be specifically demonstrated.
The promiscuousness of TGF-β/BMP deregulation in cancer, inflammation, fibrosis, and aberrant angiogenesis has led to the development of numerous therapeutic agents targeting this pathway (reviewed by Akhurst53 and Cunha6), including small molecule inhibitors, monoclonal antibodies, ligand traps, and anti-sense RNAs. Similar approaches have been used to target related signaling cascades in vascular anomalies. For example, sirolimus (rapamycin) has been demonstrated to be an effective agent in the treatment of lymphatic malformations by targeting the PIK3CA pathway, which is activated in the endothelial cells of these lesions54,55. ALK1 deletion activates the PI3K pathway in HHT mouse models that form AVMs, a process reversible by pharmacologic inhibition of PI3K56. FK506 (tacrolimus) increases ALK1 expression in ECs and restores TGF-β/BMP signaling in pulmonary hypertension23,57. MicroRNA-27b positively regulates TGF-β–induced endothelial-to-mesenchymal transition in ECs, which is reversible with antisense miR-2758. The effectiveness of these approaches in restoring normal TGF-β/BMP signaling in ECs isolated from extracranial AVMs should be evaluated in future studies.
Conclusion
Extracranial AVM tissue demonstrates deregulation of TGF-β/BMP pathway components, characterized by decreased protein levels of BMP-9, ALK1, and Smads 1, 6, and 8 and increased TGF-β1 and Smad3 proteins. ALK1 and Smad6–8 mRNA levels are increased in AVMs, suggesting a post-transcriptional mechanism underlying their altered expression. Patients with extracranial AVMs also have significantly higher circulating levels of TGF-β1. Targeting components of the TGF-β/BMP pathway in extracranial AVMs may offer a novel therapeutic option, and monitoring circulating TGF-β1 may be a useful indicator of treatment response.
Data availability
All available data is included in this manuscript. Full images of gels/blots are included in supplementary material. No data representing proteomics, protein sequences, DNA/RNA sequences, genetic polymorphisms, linked genotype and phenotype data, macromolecular structure, gene expression data, or crystallographic data were generated.
References
Nassiri, N., Cirillo-Penn, N. C. & Thomas, J. Evaluation and management of congenital peripheral arteriovenous malformations. J. Vasc. Surg. 62, 1667–1676. https://doi.org/10.1016/j.jvs.2015.08.052 (2015).
Wei, T. et al. Abnormal elastin and collagen deposition is present in extracranial arteriovenous malformations: A comparison to intracranial disease. Histol. Histopathol. 34, 1355–1363. https://doi.org/10.14670/HH-18-129 (2019).
Liu, A. S., Mulliken, J. B., Zurakowski, D., Fishman, S. J. & Greene, A. K. Extracranial arteriovenous malformations: Natural progression and recurrence after treatment. Plast. Reconstr. Surg. 125, 1185–1194. https://doi.org/10.1097/PRS.0b013e3181d18070 (2010).
Richter, G. T. & Suen, J. Y. Pediatric extracranial arteriovenous malformations. Curr. Opin. Otolaryngol. Head Neck Surg. 19, 455–461. https://doi.org/10.1097/MOO.0b013e32834cd57c (2011).
Timbang, M. R. & Richter, G. T. Update on extracranial arteriovenous malformations: A staged multidisciplinary approach. Semin. Pediatr. Surg. 29, 150965. https://doi.org/10.1016/j.sempedsurg.2020.150965 (2020).
Cunha, S. I., Magnusson, P. U., Dejana, E. & Lampugnani, M. G. Deregulated TGF-beta/BMP signaling in vascular malformations. Circ. Res. 121, 981–999. https://doi.org/10.1161/CIRCRESAHA.117.309930 (2017).
Couto, J. A. et al. Somatic MAP2K1 mutations are associated with extracranial arteriovenous malformation. Am. J. Hum. Genet. 100, 546–554. https://doi.org/10.1016/j.ajhg.2017.01.018 (2017).
Al-Olabi, L. et al. Mosaic RAS/MAPK variants cause sporadic vascular malformations which respond to targeted therapy. J. Clin. Investig. 128, 1496–1508. https://doi.org/10.1172/JCI98589 (2018).
Atri, D., Larrivee, B., Eichmann, A. & Simons, M. Endothelial signaling and the molecular basis of arteriovenous malformation. Cell. Mol. Life Sci. https://doi.org/10.1007/s00018-013-1475-1 (2013).
Massague, J. How cells read TGF-beta signals. Nat. Rev. Mol. Cell Biol. 1, 169–178 (2000).
Huminiecki, L. et al. Emergence, development and diversification of the TGF-beta signalling pathway within the animal kingdom. BMC Evol. Biol. 9, 28. https://doi.org/10.1186/1471-2148-9-28 (2009).
Goumans, M. J. & Ten Dijke, P. TGF-beta signaling in control of cardiovascular function. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a022210 (2018).
Goumans, M. J., Liu, Z. & ten Dijke, P. TGF-beta signaling in vascular biology and dysfunction. Cell Res. 19, 116–127. https://doi.org/10.1038/cr.2008.326 (2009).
Goumans, M. J. et al. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 21, 1743–1753. https://doi.org/10.1093/emboj/21.7.1743 (2002).
Hill, C. S. Transcriptional control by the SMADs. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a022079 (2016).
Miyazawa, K. & Miyazono, K. Regulation of TGF-beta family signaling by inhibitory Smads. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a022095 (2017).
Johnson, D. W. et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat. Genet. 13, 189–195. https://doi.org/10.1038/ng0696-189 (1996).
McAllister, K. A. et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat. Genet. 8, 345–351. https://doi.org/10.1038/ng1294-345 (1994).
Wooderchak-Donahue, W. L. et al. BMP9 mutations cause a vascular-anomaly syndrome with phenotypic overlap with hereditary hemorrhagic telangiectasia. Am. J. Hum. Genet. 93, 530–537. https://doi.org/10.1016/j.ajhg.2013.07.004 (2013).
Maddaluno, L. et al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature 498, 492–496. https://doi.org/10.1038/nature12207 (2013).
Bravi, L. et al. Sulindac metabolites decrease cerebrovascular malformations in CCM3-knockout mice. Proc. Natl. Acad. Sci. U.S.A. 112, 8421–8426. https://doi.org/10.1073/pnas.1501352112 (2015).
Fernandez, L. A. et al. Therapeutic action of tranexamic acid in hereditary haemorrhagic telangiectasia (HHT): Regulation of ALK-1/endoglin pathway in endothelial cells. Thromb. Haemost. 97, 254–262 (2007).
Albinana, V., Sanz-Rodriguez, F., Recio-Poveda, L., Bernabeu, C. & Botella, L. M. Immunosuppressor FK506 increases endoglin and activin receptor-like kinase 1 expression and modulates transforming growth factor-beta1 signaling in endothelial cells. Mol. Pharmacol. 79, 833–843. https://doi.org/10.1124/mol.110.067447 (2011).
Albinana, V., Recio-Poveda, L., Zarrabeitia, R., Bernabeu, C. & Botella, L. M. Propranolol as antiangiogenic candidate for the therapy of hereditary haemorrhagic telangiectasia. Thromb. Haemost. 108, 41–53. https://doi.org/10.1160/TH11-11-0809 (2012).
Albinana, V., Bernabeu-Herrero, M. E., Zarrabeitia, R., Bernabeu, C. & Botella, L. M. Estrogen therapy for hereditary haemorrhagic telangiectasia (HHT): Effects of raloxifene, on Endoglin and ALK1 expression in endothelial cells. Thromb. Haemost. 103, 525–534. https://doi.org/10.1160/TH09-07-0425 (2010).
Maring, J. A., van Meeteren, L. A., Goumans, M. J. & Ten Dijke, P. Interrogating TGF-beta function and regulation in endothelial cells. Methods Mol. Biol. 1344, 193–203. https://doi.org/10.1007/978-1-4939-2966-5_11 (2016).
Ricard, N. et al. BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood 119, 6162–6171. https://doi.org/10.1182/blood-2012-01-407593 (2012).
Larrivee, B. et al. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev. Cell 22, 489–500. https://doi.org/10.1016/j.devcel.2012.02.005 (2012).
Budi, E. H., Duan, D. & Derynck, R. Transforming growth factor-beta receptors and Smads: Regulatory complexity and functional versatility. Trends Cell Biol. 27, 658–672. https://doi.org/10.1016/j.tcb.2017.04.005 (2017).
Tual-Chalot, S. et al. Circulating microparticles from obstructive sleep apnea syndrome patients induce endothelin-mediated angiogenesis. Biochim. Biophys. Acta 1842, 202–207. https://doi.org/10.1016/j.bbadis.2013.11.017 (2014).
Cunha, P. P. et al. High-throughput screening uncovers miRNAs enhancing glioblastoma cell susceptibility to tyrosine kinase inhibitors. Hum. Mol. Genet. 26, 4375–4387. https://doi.org/10.1093/hmg/ddx323 (2017).
Yildirim, O. et al. Expression of platelet-derived growth factor ligand and receptor in cerebral arteriovenous and cavernous malformations. J. Clin. Neurosci. 17, 1557–1562. https://doi.org/10.1016/j.jocn.2010.04.028 (2010).
Vanlandewijck, M. et al. Functional characterization of germline mutations in PDGFB and PDGFRB in primary familial brain calcification. PLoS One 10, e0143407. https://doi.org/10.1371/journal.pone.0143407 (2015).
Rostagno, A., Williams, M. J., Baron, M., Campbell, I. D. & Gold, L. I. Further characterization of the NH2-terminal fibrin-binding site on fibronectin. J. Biol. Chem. 269, 31938–31945 (1994).
Lee, M. H., Vosburgh, E., Anderson, K. & McDonagh, J. Deficiency of plasma plasminogen activator inhibitor 1 results in hyperfibrinolytic bleeding. Blood 81, 2357–2362 (1993).
Kortlever, R. M., Higgins, P. J. & Bernards, R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat. Cell Biol. 8, 877–884. https://doi.org/10.1038/ncb1448 (2006).
Kjoller, L. et al. Plasminogen activator inhibitor-1 represses integrin- and vitronectin-mediated cell migration independently of its function as an inhibitor of plasminogen activation. Exp. Cell Res. 232, 420–429. https://doi.org/10.1006/excr.1997.3540 (1997).
Jankun, J. et al. Highly stable plasminogen activator inhibitor type one (VLHL PAI-1) protects fibrin clots from tissue plasminogen activator-mediated fibrinolysis. Int. J. Mol. Med. 20, 683–687 (2007).
Garcia-Pardo, A., Rostagno, A. & Frangione, B. Primary structure of human plasma fibronectin. Characterization of a 38 kDa domain containing the C-terminal heparin-binding site (Hep III site) and a region of molecular heterogeneity. Biochem. J. 241, 923–928. https://doi.org/10.1042/bj2410923 (1987).
El Sissy, F. N. et al. Somatic mutational landscape of extracranial arteriovenous malformations and phenotypic correlations. J. Eur. Acad. Dermatol. Venereol. https://doi.org/10.1111/jdv.18046 (2022).
Song, K., Krebs, T. L. & Danielpour, D. Novel permissive role of epidermal growth factor in transforming growth factor beta (TGF-beta) signaling and growth suppression. Mediation by stabilization of TGF-beta receptor type II. J. Biol. Chem. 281, 7765–7774. https://doi.org/10.1074/jbc.M511781200 (2006).
Chen, G., Ghosh, P. & Longo, D. L. Distinctive mechanism for sustained TGF-beta signaling and growth inhibition: MEK1 activation-dependent stabilization of type II TGF-beta receptors. Mol. Cancer Res. 9, 78–89. https://doi.org/10.1158/1541-7786.MCR-10-0216 (2011).
Kretzschmar, M., Doody, J., Timokhina, I. & Massague, J. A mechanism of repression of TGFbeta/Smad signaling by oncogenic Ras. Genes Dev. 13, 804–816. https://doi.org/10.1101/gad.13.7.804 (1999).
Anelli, V. et al. Oncogenic BRAF disrupts thyroid morphogenesis and function via twist expression. Elife https://doi.org/10.7554/eLife.20728 (2017).
Tarantal, A. F. et al. Overexpression of transforming growth factor-beta1 in fetal monkey lung results in prenatal pulmonary fibrosis. Eur. Respir. J. 36, 907–914. https://doi.org/10.1183/09031936.00011810 (2010).
Wu, C. F. et al. Transforming growth factor beta-1 stimulates profibrotic epithelial signaling to activate pericyte–myofibroblast transition in obstructive kidney fibrosis. Am. J. Pathol. 182, 118–131. https://doi.org/10.1016/j.ajpath.2012.09.009 (2013).
Lee, S. B., Kanasaki, K. & Kalluri, R. Circulating TGF-beta1 as a reliable biomarker for chronic kidney disease progression in the African-American population. Kidney Int. 76, 10–12. https://doi.org/10.1038/ki.2009.130 (2009).
Dantas, A. T. et al. Reassessing the role of the active TGF-beta1 as a biomarker in systemic sclerosis: Association of serum levels with clinical manifestations. Dis. Mark. 2016, 6064830. https://doi.org/10.1155/2016/6064830 (2016).
Letarte, M. et al. Reduced endothelial secretion and plasma levels of transforming growth factor-beta1 in patients with hereditary hemorrhagic telangiectasia type 1. Cardiovasc. Res. 68, 155–164. https://doi.org/10.1016/j.cardiores.2005.04.028 (2005).
Tsushima, H. et al. High levels of transforming growth factor beta 1 in patients with colorectal cancer: Association with disease progression. Gastroenterology 110, 375–382. https://doi.org/10.1053/gast.1996.v110.pm8566583 (1996).
Sheen-Chen, S. M., Chen, H. S., Sheen, C. W., Eng, H. L. & Chen, W. J. Serum levels of transforming growth factor beta1 in patients with breast cancer. Arch. Surg. 136, 937–940. https://doi.org/10.1001/archsurg.136.8.937 (2001).
Shariat, S. F. et al. Association of pre- and postoperative plasma levels of transforming growth factor beta(1) and interleukin 6 and its soluble receptor with prostate cancer progression. Clin. Cancer Res. 10, 1992–1999. https://doi.org/10.1158/1078-0432.ccr-0768-03 (2004).
Akhurst, R. J. & Hata, A. Targeting the TGFbeta signalling pathway in disease. Nat. Rev. Drug Discov. 11, 790–811. https://doi.org/10.1038/nrd3810 (2012).
Hammill, A. M. et al. Sirolimus for the treatment of complicated vascular anomalies in children. Pediatr. Blood Cancer 57, 1018–1024. https://doi.org/10.1002/pbc.23124 (2011).
Adams, D. M. et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics 137, e20153257. https://doi.org/10.1542/peds.2015-3257 (2016).
Ola, R. et al. PI3 kinase inhibition improves vascular malformations in mouse models of hereditary haemorrhagic telangiectasia. Nat. Commun. 7, 13650. https://doi.org/10.1038/ncomms13650 (2016).
Spiekerkoetter, E. et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J. Clin. Investig. 123, 3600–3613. https://doi.org/10.1172/JCI65592 (2013).
Suzuki, H. I. et al. Regulation of TGF-beta-mediated endothelial–mesenchymal transition by microRNA-27. J. Biochem. 161, 417–420. https://doi.org/10.1093/jb/mvx017 (2017).
Acknowledgements
The authors would like to acknowledge Dr. James Y. Suen for providing many of the surgical specimens used in this study, as well as the Arkansas Vascular Biology Program.
Funding
Arkansas Children’s Hospital ABI Investigator Initiated Research Grant.
Author information
Authors and Affiliations
Contributions
T.W. and H.Z. performed the western blot, RT-PCR, and ELISA experiments. T.W. and R.S. performed the ISH experiments. G.R. collected the patient samples, designed the study, and edited the manuscript. T.W., C.S., and G.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wei, T., Richter, G.T., Zhang, H. et al. Extracranial arteriovenous malformations demonstrate dysregulated TGF-β/BMP signaling and increased circulating TGF-β1. Sci Rep 12, 16612 (2022). https://doi.org/10.1038/s41598-022-21217-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-21217-0
- Springer Nature Limited