Abstract
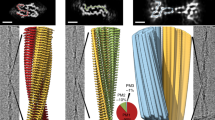
Amyloidosis of human islet amyloid polypeptide (hIAPP) is a pathological hallmark of type II diabetes (T2D), an epidemic afflicting nearly 10% of the world’s population. To visualize disease-relevant hIAPP fibrils, we extracted amyloid fibrils from islet cells of a T2D donor and amplified their quantity by seeding synthetic hIAPP. Cryo-EM studies revealed four fibril polymorphic atomic structures. Their resemblance to four unseeded hIAPP fibrils varies from nearly identical (TW3) to non-existent (TW2). The diverse repertoire of hIAPP polymorphs appears to arise from three distinct protofilament cores entwined in different combinations. The structural distinctiveness of TW1, TW2 and TW4 suggests they may be faithful replications of the pathogenic seeds. If so, the structures determined here provide the most direct view yet of hIAPP amyloid fibrils formed during T2D.



Similar content being viewed by others
Data availability
Structural data have been deposited into the Worldwide Protein Data Bank (wwPDB) and the Electron Microscopy Data Bank (EMDB) with the following accession codes: PDB 7M61, EMD-23686 (TW1); PDB 7M62, EMD-23687 (TW2); PDB 7M64, EMD-23688 (TW3); PDB 7M65, EMD-23689 (TW4). PDB accession codes for previously reported coordinates used for structural analysis in this study are: 6Y1A, 6ZRR, 6ZRQ, 6ZRF, 6VW2 for hIAPP fibrils and 6OIZ, 2M4J, 2MVX, 5KK3, 5OQV, 2NAO, 2MXU, 2BEG, 2LMN, 2MPZ, 6SHS for amyloid-β fibrils. All data are available in the paper or the Supplementary Information.
Code availability
Energetic calculations were performed using custom written software. The code is available at the MBI website (https://people.mbi.ucla.edu/sawaya/amyloidatlas/accessiblesurfacearea_v07.2d.f).
References
Eisenberg, D. & Jucker, M. The amyloid state of proteins in human diseases. Cell 148, 1188–1203 (2012).
Roberts, A. N. et al. Molecular and functional characterization of amylin, a peptide associated with type 2 diabetes mellitus. Proc. Natl Acad. Sci. USA 86, 9662–9666 (1989).
Westermark, P. Amyloid in the islets of Langerhans: thoughts and some historical aspects. Ups. J. Med. Sci. 116, 81–89 (2011).
Westermark, P. et al. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc. Natl Acad. Sci. USA 84, 3881–3885 (1987).
Cooper, G. J. et al. Amylin found in amyloid deposits in human type 2 diabetes mellitus may be a hormone that regulates glycogen metabolism in skeletal muscle. Proc. Natl Acad. Sci. USA 85, 7763–7766 (1988).
Höppener, J. W., Ahrén, B. & Lips, C. J. Islet amyloid and type 2 diabetes mellitus. N. Engl. J. Med. 343, 411–419 (2000).
Westermark, P., Wernstedt, C., Wilander, E. & Sletten, K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem. Biophys. Res. Commun. 140, 827–831 (1986).
Westermark, G. T., Gebre-Medhin, S., Steiner, D. F. & Westermark, P. Islet amyloid development in a mouse strain lacking endogenous islet amyloid polypeptide (IAPP) but expressing human IAPP. Mol. Med. 6, 998–1007 (2000).
Fernández-Alvarez, J. et al. Stable and functional regeneration of pancreatic β-cell population in nSTZ-rats treated with tungstate. Diabetologia 47, 470–477 (2004).
Pilkington, E. H. et al. Pancreatic β-cell membrane fluidity and toxicity induced by human islet amyloid polypeptide species. Sci. Rep. 6, 21274 (2016).
Mukherjee, A. et al. Induction of IAPP amyloid deposition and associated diabetic abnormalities by a prion-like mechanism. J. Exp. Med. 214, 2591–2610 (2017).
Cao, Q., Boyer, D. R., Sawaya, M. R., Ge, P. & Eisenberg, D. S. Cryo-EM structure and inhibitor design of human IAPP (amylin) fibrils. Nat. Struct. Mol. Biol. 27, 653–659 (2020).
Luca, S., Yau, W.-M., Leapman, R. & Tycko, R. Peptide conformation and supramolecular organization in amylin fibrils: constraints from solid-state NMR. Biochemistry 46, 13505–13522 (2007).
Bedrood, S. et al. Fibril structure of human islet amyloid polypeptide. J. Biol. Chem. 287, 5235–5241 (2012).
Röder, C. et al. Cryo-EM structure of islet amyloid polypeptide fibrils reveals similarities with amyloid-β fibrils. Nat. Struct. Mol. Biol. 27, 660–667 (2020).
Gallardo, R. et al. Fibril structures of diabetes-related amylin variants reveal a basis for surface-templated assembly. Nat. Struct. Mol. Biol. 27, 1048–1056 (2020).
Zhang, W. et al. Heparin-induced tau filaments are polymorphic and differ from those in Alzheimer’s and Pick’s diseases. eLife 8, e43584 (2019).
Schweighauser, M. et al. Structures of α-synuclein filaments from multiple system atrophy. Nature 585, 464–469 (2020).
Scheres, S. H. W. Amyloid structure determination in RELION-3.1. Acta Crystallogr. D Struct. Biol. 76, 94–101 (2020).
Chen, M.-S. et al. Characterizing the assembly behaviors of human amylin: a perspective derived from C-terminal variants. Chem. Commun. 49, 1799–1801 (2013).
Sakagashira, S. et al. Missense mutation of amylin gene (S20G) in Japanese NIDDM patients. Diabetes 45, 1279–1281 (1996).
Meier, D. T. et al. The S20G substitution in hIAPP is more amyloidogenic and cytotoxic than wild-type hIAPP in mouse islets. Diabetologia 59, 2166–2171 (2016).
Sakagashira, S. et al. S20G mutant amylin exhibits increased in vitro amyloidogenicity and increased intracellular cytotoxicity compared to wild-type amylin. Am. J. Pathol. 157, 2101–2109 (2000).
Cao, P. et al. Sensitivity of amyloid formation by human islet amyloid polypeptide to mutations at residue 20. J. Mol. Biol. 421, 282–295 (2012).
Janson, J. et al. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 53, 474–481 (2004).
Miklossy, J. et al. Beta amyloid and hyperphosphorylated tau deposits in the pancreas in type 2 diabetes. Neurobiol. Aging 31, 1503–1515 (2010).
Peila, R., Rodriguez, B. L., Launer, L. J. & Honolulu-Asia Aging Study. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes 51, 1256–1262 (2002).
Oskarsson, M. E. et al. In vivo seeding and cross-seeding of localized amyloidosis: a molecular link between type 2 diabetes and Alzheimer disease. Am. J. Pathol. 185, 834–846 (2015).
Moreno-Gonzalez, I. et al. Molecular interaction between type 2 diabetes and Alzheimer’s disease through cross-seeding of protein misfolding. Mol. Psychiatry 22, 1327–1334 (2017).
O’Nuallain, B., Williams, A. D., Westermark, P. & Wetzel, R. Seeding specificity in amyloid growth induced by heterologous fibrils. J. Biol. Chem. 279, 17490–17499 (2004).
Krotee, P. et al. Common fibrillar spines of amyloid-β and human islet amyloid polypeptide revealed by microelectron diffraction and structure-based inhibitors. J. Biol. Chem. 293, 2888–2902 (2018).
Andreetto, E. et al. A hot-segment-based approach for the design of cross-amyloid interaction surface mimics as inhibitors of amyloid self-assembly. Angew. Chem. Int. Ed. 54, 13095–13100 (2015).
Lövestam, S. et al. Seeded assembly in vitro does not replicate the structures of α-synuclein filaments from multiple system atrophy. FEBS Open Bio 11, 999–1013 (2021).
Fujiwara, H. et al. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 4, 160–164 (2002).
Sorrentino, Z. A. & Giasson, B. I. The emerging role of α-synuclein truncation in aggregation and disease. J. Biol. Chem. 295, 10224–10244 (2020).
Asthana, S., Mallick, B., Alexandrescu, A. T. & Jha, S. IAPP in type II diabetes: basic research on structure, molecular interactions and disease mechanisms suggests potential intervention strategies. Biochim. Biophys. Acta Biomembr. 1860, 1765–1782 (2018).
Jurgens, C. A. et al. β-cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am. J. Pathol. 178, 2632–2640 (2011).
Krotee, P. et al. Atomic structures of fibrillar segments of hIAPP suggest tightly mated β-sheets are important for cytotoxicity. eLife 6, e19273 (2017).
Falcon, B. et al. Tau filaments from multiple cases of sporadic and inherited Alzheimer’s disease adopt a common fold. Acta Neuropathol. 136, 699–708 (2018).
Falcon, B. et al. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature 568, 420–423 (2019).
Zhang, W. et al. Novel tau filament fold in corticobasal degeneration. Nature 580, 283–287 (2020).
Boyer, D. R. et al. Structures of fibrils formed by α-synuclein hereditary disease mutant H50Q reveal new polymorphs. Nat. Struct. Mol. Biol. 26, 1044–1052 (2019).
Johnson, K. H. et al. Feline insular amyloid: immunohistochemical and immunochemical evidence that the amyloid is insulin-related. Vet. Pathol. 22, 463–468 (1985).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Grant, T. & Grigorieff, N. Automatic estimation and correction of anisotropic magnification distortion in electron microscopes. J. Struct. Biol. 192, 204–208 (2015).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Grant, T. & Grigorieff, N. Measuring the optimal exposure for single particle cryo-EM using a 2.6-Å reconstruction of rotavirus VP6. eLife 4, e06980 (2015).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Wagner, T. et al. Two particle-picking procedures for filamentous proteins: SPHIRE-crYOLO filament mode and SPHIRE-STRIPER. Acta Crystallogr. D Struct. Biol. 76, 613–620 (2020).
He, S. & Scheres, S. H. W. Helical reconstruction in RELION. J. Struct. Biol. 198, 163–176 (2017).
Scheres, S. H. W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Terwilliger, T. C., Sobolev, O. V., Afonine, P. V. & Adams, P. D. Automated map sharpening by maximization of detail and connectivity. Acta Crystallogr. D Struct. Biol. 74, 545–559 (2018).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr. 66, 486–501 (2010).
Afonine, P. V. et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D Struct. Biol. 74, 531–544 (2018).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 (2010).
The PyMOL Molecular Graphics System, Version 1.8 (Schrödinger, 2015).
Warmack, R. A. et al. Structure of amyloid-β (20-34) with Alzheimer’s-associated isomerization at Asp23 reveals a distinct protofilament interface. Nat. Commun. 10, 3357 (2019).
Lu, J.-X. et al. Molecular structure of β-amyloid fibrils in Alzheimer’s disease brain tissue. Cell 154, 1257–1268 (2013).
Schutz, A. K. et al. Atomic-resolution three-dimensional structure of amyloid β fibrils bearing the Osaka mutation. Angew. Chem. Int. Ed. 54, 331–335 (2015).
Colvin, M. T. et al. Atomic resolution structure of monomorphic Aβ42 amyloid fibrils. J. Am. Chem. Soc. 138, 9663–9674 (2016).
Gremer, L. et al. Fibril structure of amyloid-β(1–42) by cryo-electron microscopy. Science 358, 116–119 (2017).
Walti, M. A. et al. Atomic-resolution structure of a disease-relevant Aβ(1–42) amyloid fibril. Proc. Natl Acad. Sci. USA 113, E4976–E4984 (2016).
Xiao, Y. et al. Aβ(1–42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer’s disease. Nat. Struct. Mol. Biol. 22, 499–505 (2015).
Luhrs, T. et al. 3D structure of Alzheimer’s amyloid-β(1–42) fibrils. Proc. Natl Acad. Sci. USA 102, 17342–17347 (2005).
Paravastu, A. K., Leapman, R. D., Yau, W.-M. & Tycko, R. Molecular structural basis for polymorphism in Alzheimer’s β-amyloid fibrils. Proc. Natl Acad. Sci. USA 105, 18349–18354 (2008).
Sgourakis, N. G., Yau, W.-M. & Qiang, W. Modeling an in-register, parallel ‘iowa’ aβ fibril structure using solid-state NMR data from labeled samples with rosetta. Structure 23, 216–227 (2015).
Kollmer, M. et al. Cryo-EM structure and polymorphism of Aβ amyloid fibrils purified from Alzheimer’s brain tissue. Nat. Commun. 10, 4760 (2019).
Acknowledgements
We thank the Southern California Islet Cell Resources Center for providing human islets for this study. We thank X. Zhao at the HHMI Janelia Cryo-EM Facility for help with microscope operation and data collection. We acknowledge support from NIH AG 054022, NIH AG061847 and DOE DE-FC02-02ER63421. D.R.B. was supported by the National Science Foundation Graduate Research Fellowship Program.
Author information
Authors and Affiliations
Contributions
Q.C. designed experiments and performed data analysis. F.K. prepared islet cells from donors. Q.C. and L.S. performed Congo red staining of islet cells. Q.C., L.S. and B.A.N. performed fibril extraction from islet cells. Q.C. and R.A. performed immunoprecipitation in fibril extraction. R.A. and J.L. performed western blot and MTT assays. K.A.M. helped with western blots. Q.C. prepared hIAPP fibrils and cryo-EM grids. Q.C. and D.R.B. collected cryo-EM data. Q.C. performed cryo-EM data processing and model building, J.L. assisted with particle picking. Q.C. and M.R.S. performed solvation energy calculations. All authors analyzed the results and wrote the manuscript. D.S.E. supervised and guided the project.
Corresponding author
Ethics declarations
Competing interests
D.S.E. is an advisor and equity shareholder in ADRx, Inc. The remaining authors declare no competing interests.
Additional information
Peer review information Nature Structural & Molecular Biology thanks Sjors Scheres and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Supplemental information on extraction of patient islet cells and seeding of fibril growth.
a, Congo Red staining of slices of islet cells from various T2D donors (see Supplementary Table 1). b, Dot blot of fractions from extracted patient islet cells probed by anti-hIAPP (top) and anti-amyloid fibrils OC (bottom) antibodies. c, Negatively stained images of S1 and P fractions in (b). d, Dot blot of fractions from immunoprecipitation of the S1 fraction probed by anti-hIAPP (top), OC (middle) antibodies and no primary antibody (bottom) as a base line. e, Negatively stained images of flow through, elute−2 and elute−3 fractions in (b). The EM image of the elute−1 fraction is shown in Fig. 1b. f, ThT aggregation curves of fresh prepared synthetic hIAPP peptide incubated alone (black) or with elute-1 (red), elute-2 (blue) or elute-3 (green) fraction in (d) as fibril growth seeds. Data are shown as mean ± s.d., n=3 independent experiments. Note the elute-1 fraction from immunoprecipitation shows notable seeding ability as its curve shows shortened lag time and stronger ThT readings compared to hIAPP alone, whereas the elute-2 and elute-3 fractions show no ability in altering the hIAPP aggregation curve. Please see methods for the detailed definitions of fractions in panels b–f.
Extended Data Fig. 2 Cryo-EM data processing.
a, Representative micrographs of 8 identifiable morphologies during data processing (TW1-TW4 and NT1-NT4, scale bar 500 Å). b, Representative 2D classes of NT1-NT4. c, Central slices of final 3D reconstructions of TW1-TW4. d-e, FSC curves between two half-maps (e) and the cryo-EM reconstruction and refined atomic model (f). In half-maps FSC, FSC curves (black) are fitted (red) with the model function 1/(1+exp((x-A)/B)), with A=0.2328 and B=0.01517 for TW1, A=0.2347 and B=0.01234 for TW2, A=0.2255 and B=0.01427 for TW3, and A=0.2252 and B=0.009377 for TW4.
Extended Data Fig. 3 Different views of the cryo-EM maps with five layers shown.
For each morphology, the top view shows clear separation of β-strands, and the tilted views on the middle and bottom show clear separation of the layers of β-sheets along the fibril axis.
Extended Data Fig. 4 Structural comparisons of hIAPP fibrils.
a, Superposition of chain A and B of TW1 (left) and TW4 (right). b, Superposition of TW3, 6Y1A and 6ZRF, note that these three structures are very similar to each other. c, Superposition of chain A of TW1, TW3 and TW4 (top) and of TW1 chain A and 6ZRR chain C (bottom) at CF1 region. d, Superposition of TW1 chain A and TW2 (left), or TW1 chain B and TW2 (right). For superimposition details of panels a, b and d see Supplementary Table 2.
Extended Data Fig. 5 Detailed analysis of hIAPP fibril structures.
a, Above in black: the amino acid sequence of hIAPP; below: the residues visible in different hIAPP fibril structures. b, Plausible conformations of flexible N-termini of TW2 (purple), TW3 (green) and TW4 (red and orange) suggested by weak densities. The superposition of Thr6 to Ala13 region of TW2 and TW4 chain A is shown as an insert. c, The interactions around Tyr37 for TW1 (chain A, blue; chain B, cyan), TW2 (purple), TW3 (green), and TW4 (chain A, red; chain B, orange). d, The detailed view of an unexplained density around Gly33. The red dot represents the center of the unexplained density, and the length of each dash line is: i, 4.0 Å; ii, 2.9 Å; iii, 3.7 Å; iv, 5.2 Å. e, Different conformations of chain A of TW1 (marine) and TW4 (red, left) or chain B of TW1 (cyan) and TW4 (orange, right) outside the CF1 or CF2 region, respectively. TW1 and TW2 are superimposed at the CF1 (chain A) or CF2 (chain B) region. f, The interfaces between protofilaments A and B for TW1 (marine and cyan), TW2 (purple), TW3 (green), TW4 (red and orange), and 6ZRQ (gray), as well as between protofilaments B (top) and C (bottom) for 6ZRR (grey). Ab, area buried. In panels c, e, and f, hydrogen bonds with distances between 2.3 and 3.2 Å are shown as black dashed lines. For superimposition details for panel b see Supplementary Table 2.
Extended Data Fig. 6 Explanation of swapped version of 6VW2.
Original version (left) and swapped version (right) of SUMO-tagged recombinant hIAPP fibril structure. Two symmetrically related chains are colored black and grey. CF2 was shown as surface and colored red.
Extended Data Fig. 7 Distribution of different polymorphs in reported hIAPP cryo-EM datasets.
hIAPP polymorphs are colored by (a) core folds or (b) homotypic vs. heterotypic pairings. From panel a, we found CF2 is more abundant in S20G dataset compared to wildtype ones with the exception of 6VW2 dataset. In S20G dataset, 6ZRQ contributes to 76% of the solvable fibrils and is purely composed of protofilaments with CF2; 6ZRR contributes to the other 24% and contains two protofilaments with CF2 and one protofilament with CF1. In contrast, wild-type fibrils, 6ZRF and 6Y1A are the only solvable species in their datasets and they contain only CF1 in their protofilaments; in the wild-type fibrils in this study, we also observe more protofilaments with CF1 than that with CF2. TW1 and TW4 have equal amount of protofilaments with CF1 and CF2, but TW3 contains only protofilaments with CF1. From panel b, we note when formed in vitro without patient seeds, we observed homo-dimer forms of fibrils in most datasets (Cao et al., Röder et al., and the wile-type of Gallardo et al.) and only in one dataset did we find a small portion of heterotypic species (S20G of Gallardo et al., in which 6ZRR contributes to 24% of the solvable population compared to 76% of 6ZRQ that is homo-dimer form). Whereas in patient-extract-seeded fibrils we see higher populations of heterotypic species (TW1 and TW4 contribute to 40% out of 65% of solvable population).
Extended Data Fig. 8 Solvation energy maps of reported cryo-EM hIAPP fibril structures.
Residues are colored from unfavorable (blue, 2.5 kcal/mol) to favorable stabilization energy (red, −2.5 kcal/mol).
Extended Data Fig. 9 Western blot of S1 fraction of fibril extraction and MTT assays of seeded hIAPP fibrils.
a, Western blot of S1 fraction (originally characterized in Fig. S1b) probed by antibodies that target hIAPP, amyloid-β, tau (K18), and α-synuclein. The only antibody to recognize and label the S1 fraction is anti-hIAPP, suggesting that the S1 fraction consists primarily of hIAPP. Moreover, the molecular weight of the band corresponds to full-length hIAPP. When probed with amyloid-β, tau, and α-synuclein antibodies, bands appear only in positive control lanes (labeled as “+”, see Methods for detail). b, Rin5F cells were treated with different concentrations of patient-fibril-seeded hIAPP fibrils, and significantly less MTT dye reduction was observed compared to cells without adding fibrils (****p<0.0001 using one-way ANOVA test, data are shown as mean ± s.d., n=3 independent experiments).
Extended Data Fig. 10 Main chain tracing of TW2 and TW4.
Low resolution 3D reconstructions of TW2 (a) and TW4 (b) displayed to illustrate the main chain tracing.
Supplementary information
Supplementary Information
Supplementary Text 1–7, references and Tables 1–3.
Rights and permissions
About this article
Cite this article
Cao, Q., Boyer, D.R., Sawaya, M.R. et al. Cryo-EM structures of hIAPP fibrils seeded by patient-extracted fibrils reveal new polymorphs and conserved fibril cores. Nat Struct Mol Biol 28, 724–730 (2021). https://doi.org/10.1038/s41594-021-00646-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-021-00646-x
- Springer Nature America, Inc.
This article is cited by
-
Misfolded protein oligomers: mechanisms of formation, cytotoxic effects, and pharmacological approaches against protein misfolding diseases
Molecular Neurodegeneration (2024)
-
Impact of distinct FG nucleoporin repeats on Nup98 self-association
Nature Communications (2024)
-
Structural polymorphism of amyloid fibrils in ATTR amyloidosis revealed by cryo-electron microscopy
Nature Communications (2024)
-
The islet tissue plasminogen activator/plasmin system is upregulated with human islet amyloid polypeptide aggregation and protects beta cells from aggregation-induced toxicity
Diabetologia (2024)
-
Network of hotspot interactions cluster tau amyloid folds
Nature Communications (2023)