Abstract
Phylogenomics of bats suggests that their echolocation either evolved separately in the bat suborders Yinpterochiroptera and Yangochiroptera, or had a single origin in bat ancestors and was later lost in some yinpterochiropterans1,2,3,4,5,6. Hearing for echolocation behaviour depends on the inner ear, of which the spiral ganglion is an essential structure. Here we report the observation of highly derived structures of the spiral ganglion in yangochiropteran bats: a trans-otic ganglion with a wall-less Rosenthal’s canal. This neuroanatomical arrangement permits a larger ganglion with more neurons, higher innervation density of neurons and denser clustering of cochlear nerve fascicles7,8,9,10,11,12,13. This differs from the plesiomorphic neuroanatomy of Yinpterochiroptera and non-chiropteran mammals. The osteological correlates of these derived ganglion features can now be traced into bat phylogeny, providing direct evidence of how Yangochiroptera differentiated from Yinpterochiroptera in spiral ganglion neuroanatomy. These features are highly variable across major clades and between species of Yangochiroptera, and in morphospace, exhibit much greater disparity in Yangochiroptera than Yinpterochiroptera. These highly variable ganglion features may be a neuroanatomical evolutionary driver for their diverse echolocating strategies4,14,15,16,17 and are associated with the explosive diversification of yangochiropterans, which include most bat families, genera and species.
Similar content being viewed by others
Main
Traditional classifications grouped bats lacking laryngeal echolocation in the Megachiroptera and placed echolocators in the Microchiroptera. However, phylogenomic analyses strongly favour the division of echolocating bats between the suborders Yinpterochiropera and Yangochiroptera. Hearing is indispensable for bat echolocation, and for this crucial function the neurons in the spiral ganglion connect the hair cells in the cochlea to the brain. Computed tomography (CT) and histological investigations across 39 species of 19 families of bats (Figs. 1–3, Extended Data Fig. 1, Supplementary Tables 1–4, Methods) revealed structures of the spiral ganglion and its bony canal, providing direct evidence from the inner ear that Yangochiroptera developed a unique neuroanatomy of this ganglion, consistent with the evolutionary inference from phylogenomic hypotheses1,2,3,4,5,6,18 (Fig. 2, Extended Data Figs. 1, 2, Supplementary Information). These ganglion canal characters and their disparity patterns can be mapped onto the bat phylogeny via morphometrics (Fig. 2, Extended Data Fig. 2).
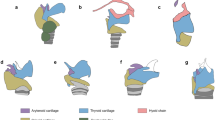
a, Hipposideros caffer (Yinpterochiroptera, Hipposideridae; left), skull (middle) and inner ear (right). b, Schematic of the cis-otic ganglion with the foraminal wall of Rosenthal’s canal (far left) and the ‘uncoiled’ model of the foraminal wall along the cochlea for Yinpterochiroptera (second from left). CT visualizations of cochleas of Hypsignathus monstrosus (Pteropodidae) (top right) and Rhinolophus blasii (Rhinolophidae) (bottom right). The cochlear nerve fascicles enter through foramina of the tractus foraminosus of the internal auditory meatus; the ganglion is enclosed by Rosenthal’s canal embedded in the bony modiolus of the cochlea (thus cis-otic). These are therian plesiomorphies, and characterize all Yinpterochiroptera. c, Left, the fenestral pattern of Rosenthal’s canal wall in some Yangochiroptera, and enlarged fenestrae through a thinner wall from the basal three-quarter turn to the apex; the basal half turn of cochlea has foramina, as in all other therian mammals. Right, CT visualization of Tadarida brasiliensis (Molossidae). In the fenestral pattern, the ganglion is still in the modiolus of the cochlea (cis-otic), but the thinner canal wall has much larger openings than foramina—an intermediate character state between the ancestral foraminal wall and the most derived wall-less and trans-otic pattern for Yangochiroptera. d, Left, the wall-less pattern and trans-otic ganglion of most Yangochiroptera; Rosenthal’s canal is wall-less and confluent with the internal auditory meatus (IAM) and its ganglion is placed in the IAM, from the apical turn of the cochlea to the basal half turn that has foraminal walls. Right, CT visualization from Miniopterus inflatus (Miniopteridae) shows that the partition between IAM and Rosenthal’s canal is absent, and the ganglion is displaced into the internal auditory meatus and outside the cochlear space (trans-otic). This pattern is present in all Yangochiroptera except Noctilio, and is the most derived neuroanatomical configuration of all therian mammals (Extended Data Figs. 1–10).
a, Rates of evolution among the major patterns of canal wall and ganglion placement, assessed by the proportion of wall-less and/or fenestral wall to total cochlear length (100, full foraminal wall between the base and apex; 0, wall-less between basal half turn and apex). Families of bats examined by this study are listed in Extended Data Fig. 1, Supplementary Tables 1, 2. b, Morphological disparity of bat ears. The percentage of ossified wall of Rosenthal’s canal as a percentage of cochlear length (horizontal axis) is plotted against the number of coiled turns of the cochlea to the nearest quarter turn. All yinpterochiropteran bats have a fully enclosed (cis-otic) ganglion in the walled canal over the entire cochlear length regardless of number of turns, as do outgroups. By contrast, in all Yangochiroptera, with the exception of Noctilio, apical turns lack a canal wall and the ganglion is trans-otic in the internal auditory meatus. The three superfamilies of Yangochiroptera occupy different regions of the cochlear turns by ossification morphospace: Emballonuroidea (green), Noctilionoidea (yellow) and Vespertilionoidea (red). Characterization of Rosenthal’s canal wall of the examined bats is summarized in Extended Data Fig. 1 and described in Supplementary Information.
a, Schematic model of the wall-less configuration in which the ganglion space is open to the IAM. b, The extent of wall-less pattern between the apex and half cochlear turn, shown in an uncoiled cochlear model. c, d, Wall-less Rosenthal’s canal is confluent with IAM along the whole cochlea, and the open ganglion (visualized in yellow) is visible in cross section and on the inner surface of the IAM. c, Visualized trans-otic ganglion exposed in the IAM (medial view of pars cochlearis), and cross-sectional view of the whole cochlea. d, The ganglion’s position in the pars cochlearis, and in magnified local area at one full cochlear turn, showing the trans-otic placement of the ganglion in the IAM space. e, Histological section of the spiral ganglion in trans-otic placement in the IAM space and absence of the tracts foraminous. Top, local area at a half cochlear turn. Middle, cross-section of the whole cochlea. Bottom, local area at one full cochlear turn. f, CT slices to corroborate the absence of tractus foraminosus and Rosenthal’s canal wall, and the placement of the ganglion in IAM. This is the most derived condition, present in all yangochiropteran bats except Noctilio (details in Extended Data Figs. 1, 8–10 and Supplementary Information).
In all mammals including bats (Fig. 1), the cochlear spiral ganglion contains bipolar neurons that transmit auditory information from the mechanosensory hair cells in the organ of Corti via their axons in cochlear cranial nerve to the cochlear nuclei in the brain19,20,21. Previous studies have concentrated on characteristics of basilar membrane, hair cell density and the peripheral (dendrite) connections of ganglion neurons7,8,9,10. Here we focus on the central (axon) connections of ganglion neurons, also known as cochlear nerve fascicles, and their neuroanatomy (Fig. 1, Supplementary Information). These ganglion correlates are associated with the intricate bony structures of the cochlea in therian mammals: neuron bodies of spiral ganglion are enclosed by the ganglion canal (also known as Rosenthal’s canal)—the peripheral processes (dendrites or radial fibers) of the neurons are embedded in the primary bony lamina. The central processes (axons) of ganglion neurons, also known as nerve fascicles, traverse through the tiny foramina in Rosenthal’s canal wall and connect the spiral ganglion to the trunk of cochlear nerve and onward to the brain (Fig. 1b, Extended Data Figs. 3–6, Supplementary Information).
In Yinpterochiroptera and in non-chiropteran therian mammals (Extended Data Fig. 1, Methods), Rosenthal’s canal has a thick wall perforated by dense foramina, separating the ganglion from the internal auditory meatus and enclosing it in the bony cochlea (Figs. 1, 2, Extended Data Figs. 1–6). We call this configuration of Rosenthal’s canal the foraminal wall and call the enclosed placement of ganglion cis-otic (Fig. 1, Extended Data Fig. 3). Originating in Mesozoic therian mammals, this arrangement is highly conserved in all marsupial and placental mammals22,23. These are ancestral features of bats and characterize all Yinpterochiroptera (Fig. 1, Extended Data Figs. 1, 4–6).
Derived cochlear ganglion neuroanatomy
Here we describe two novel configurations of the ganglion and its canal in Yangochiroptera: the wall-less Rosenthal’s canal with trans-otic ganglion placement and the fenestral wall of Rosenthal’s canal with cis-otic ganglion placement (Figs. 1, 3, Extended Data Figs. 6–10). The wall-less pattern of Rosenthal’s canal is characterized by the absence of bony partition between the spiral ganglion space and the internal auditory meatus such that the space of Rosenthal’s canal is continuous with the internal auditory meatus (Figs. 1, 3). The ganglion transgresses from the lumen of Rosenthal’s canal to the wider, open space of the internal auditory meatus. This placement is ectopical, relative to the cis-otic placement of all other therian mammals. The ganglion itself is in direct contact with the cochlear nerve trunk in the internal auditory meatus (Fig. 3). This wall-less pattern is present in the apical cochlear turn in 24 out of 26 species of Yangochiroptera examined here (Extended Data Fig. 1). Bats of Myzopodidae, Furipteridae, Thyropteridae, Natalidae, Miniopteridae and Vespertilionidae (all families of Yangochiroptera) have the most extensive wall-less canals; this state is present in the first (basal) and second turns, as well as in the apical turn. The wall-less Rosenthal’s canal is the most derived pattern of all therian mammals, and represents an extreme evolutionary transformation of the mammalian cochlear ganglion. Its characters are new synapomorphies of the Yangochiroptera (Fig. 1d, Extended Data Figs. 9, 10, Supplementary Information). We note that these characteristics are lost in Noctilio albiventris and Noctilio leporinus, which have a plesiomorphic foraminal wall. As Noctilio is deeply nested in the noctilionoid clade rooted phylogenetically by Myzopodidae, Furipteridae and Thyropteridae, all of which have the wall-less pattern throughout (Extended Data Fig. 2), we interpret this as a recent reversal in Noctilio.
The fenestral pattern of the ganglion canal is characterized by large openings of the canal wall that are about 200 μm to 300 μm across (Fig. 1, Extended Data Figs. 7, 8), about 8 to 10 times the size of the foramina in the plesiomorphic wall pattern. The fenestral wall is also thinner than the foraminal wall, with a thickness of 20% to 25% of the foraminal wall. The larger fenestra allow for corresponding variation in the bundle size of cochlear nerve fascicles. The spiral ganglion is enclosed by the primary bony lamina (Fig. 1), similar to its cis-otic ganglion placement in the foraminal canal.
Disparity of ganglion canal in bats
This newly recognized fenestral pattern is present in members of all three yangochiropteran superfamilies: Coleura afra of Emballonuridae, Emballonuroidea (Extended Data Fig. 7), Sturnira lilium and Artibeus jamaicensis of Phyllostomidae, Noctilionoidea24,25 (Extended Data Fig. 1), and Tadarida brasiliensis and Molossus rufus of Molossidae, Vespertilionoidea (Extended Data Fig. 8). However, the fenestral pattern is limited locally to the first (basal) and the second cochlear turns in these bats (Extended Data Fig. 1), and it invariably gives way to the wall-less pattern in the third (apical turn) of the same individual. Therefore, in species with the fenestral pattern, the fenestral and wall-less patterns always co-occur, and these configurations are polymorphic states of the Rosenthal’s canal—they are continuously variable states, transitioning into one another along the ganglion canal of same specimen. In local areas of cochlea, the fenestral wall is structurally intermediate between the extreme and highly transformed wall-less pattern (Fig. 2, Extended Data Fig. 1, Methods) and the foraminal wall of Yinpterochiroptera. The latter is a highly conserved symplesiomorphy of all therians (Extended Data Figs. 7, 8).
By our ancestral state reconstruction of discrete characters among the 39 examined bat species, the wall-less pattern in apical turn is the inferred ancestral state of Rosenthal’s canal for Yangochiroptera. The fenestral pattern in the basal and second turns is present in several species scattered across five families, suggesting the fenestral pattern may be secondarily derived in clades in which this feature occurs (Fig. 2, Extended Data Fig. 2), as is the case with the reversal to foraminal wall in Noctilio.
The wall-less and fenestral patterns involve different degrees of reduction in canal wall ossification, which therefore shows much higher variation in Yangochiroptera than the highly conserved foraminal wall of Yinpterochiroptera (Fig. 2a). Further, the Yangochiroptera and Yinpterochiroptera exhibit contrasting patterns of evolutionary disparity in morphospace (Fig. 2, Extended Data Fig. 2), as measured by degree of wall reduction (newly discovered by our investigation) and the spiral cochlear turns, a general metric for quantifying cochlear morphology of therian mammals6,26,27,28.
Yangochiropterans show high variability in progressive reduction of the ganglion canal wall along the cochlea, and also high variability in number of cochlear turns. The prominent disparity of Rosenthal’s canal in Yangochiroptera as a whole is foremost influenced by the remarkable disparity in species of Noctilionoidea. An extreme outlier of this wide disparity is Myzopoda, characterized by 3.75 cochlear turns (very high for bats) and by a wall ossified for less than 10% along the cochlear length (more than 90% of Rosenthal’s canal is wall-less) (Fig. 2, yellow polygon). The other extremes are species of Noctilio (known for its specialized foraging), which resemble yinpterochiropterans in having an ossified wall along the entire cochlea, overlapping that group in morphospace (Fig. 2b). Two other superfamilies—Vespertilionoidea and Emballonuroidea—occupy distinct regions of morphospace (Fig. 2b, red and green polygons, respectively). Species of the family Molossidae have the most complex neuroanatomy, with all three (wall-less, fenestral and foraminal) patterns along the ganglion canal, as seen in Molossus and Tadarida (Extended Data Figs. 1, 8).
Prominent disparities of the Rosenthal’s canal in Yangochiroptera are apparent across multiple phylogenetic hierarchies: between superfamilies, families and even within species in multiple states as the wall-less and fenestral wall can co-exist in the same bat. The structural variation in ossification (from foraminal to fenestral to wall-less) within species and the numerous transitions of these characters recovered over the group’s history (Fig. 2a) are the most distinctive patterns of disparity in evolution of Yangochiroptera.
By contrast, Yinpterochiroptera show far more limited evolutionary disparity: there is little variability in ossified ganglion canal wall, despite high variability in cochlear turns within the clade. The wider range of cochlear turns of Yinpterochiroptera is consistent with observations on other cochlear structures of this clade6,26,27. The group’s disparity pattern is plesiomorphic because it is fundamentally the same as in non-chiropteran outgroups (Fig. 2b).
Many neuroanatomical variables of the mammalian ganglion and its nerves are directly correlated with the wall configuration of Rosenthal’s canal. These include the total neuron count, the overall girth, the innervation density of the ganglion29 and clustering of nerve fascicles (bundles of neuronal axons), all of which are variable11,12,13,29. For bats, it has also been established that variations of these neuroanatomical characteristics are relevant for cochlear correspondence of the best hearing frequencies along the length of the spiral ganglion7,8,9,10,30,31.
The size (girth) of the bony ganglion canal is a major determinant of overall ganglion size7,11,12,13,30 and the local neuron density of the ganglion10,11. The small size of foraminal openings imposes constraints on the clustering of cochlear nerve fascicles, while the fenestral openings can relax such constraints13 (Fig. 1, Extended Data Fig. 3). The structurally most transformed wall-less pattern removes all constraints of the ganglion canal, enabling clustering of the ganglion axons without restriction, and at the same time enlarging the ganglion space for greater numbers of neurons (Supplementary Table 5). Overall, the derived fenestral and wall-less patterns facilitate variation at multiple levels of neuroanatomy, in sharp contrast to the foraminal canal wall, which tightly restricts the variation of the ganglion in all other therians, including the Yinpterochiroptera. Our observations here (Figs. 1, 2, Extended Data Fig. 3) are also consistent with recent evidence that ossification of cochlear structure follows different developmental trajectories in the two suborders6.
Evolutionary implications
The neuroanatomical synapomorphies and their marked disparity pattern show a broad phylogenetic concordance with the broad range of echolocation strategies in Yangochiroptera (Fig. 2a, Extended Data Fig. 1). Most yangochiropterans echolocate with short pulses of broadband frequency-modulated (FM) sound between long intervals of silence. Calls with short, widely spaced pulses are known as low duty cycle (LDC) echolocation4,14,15. Two yangochiropterans—Pteronotus parnellii and Pteronotus mesoamericanus—deviate from this pattern and differ from other Pteronotus species, using calls of narrowband or constant frequency (CF) in longer pulses, known as high duty cycle (HDC) echolocation with Doppler-shift compensation. Their calls are convergent on those of most rhinolophoid bats (Yinpterochiroptera). As most species of Mormoopidae use LDC calls, the HDC echolocation of P. parnellii and P. mesoamericanus was acquired during the diversification of Pteronotus.
Of the 26 yangochiropteran species examined here (Fig. 2, Extended Data Fig. 1), 24 species use FM echolocation with short pulses4,14 (that is, LDC). Of these, a large majority (22 species in 12 families) have the derived wall-less pattern in the apical cochlear turn. All examined members of three of the four families of Vespertilionoidea and three families of Noctilionoidea have the wall-less pattern in all three cochlear turns (Fig. 2, Extended Data Fig. 1). Several noctilionoid bats have a polymorphic mosaic of wall-less and fenestral patterns in local parts of the cochlea (Extended Data Fig. 1).
Our phylogenetic analyses show that these neuroanatomical apomorphies evolved in a common ancestor of yangochiropterans, and are synapomorphies of Yangochiroptera (Fig. 2, Extended Data Fig. 2). As they are the characteristics possessed only by Yangochiroptera (at least ancestrally) and by no yinpterochiropteran, we hypothesize that they have contributed to the diverse echolocating strategies of these bats32. There is a broad (although incomplete) concordance of these ganglion-related apomorphies and frequency-modulated echolocation and LDC calling in a majority of yangochiropterans.
It was proposed that the earliest echolocators of extant Chiroptera used calls with short, broadband and multiharmonic sound7,32. These putatively plesiomorphic behavioural patterns are exemplified by Megadermatidae and Rhinopomatidae (yinpterochiropterans with foraminal walls; Extended Data Figs. 1, 6), and to some extent also by Nycteridae (yangochiropteran with fenestral wall3,7,32). By comparison, HDC echolocation is a clearly derived pattern used by other yinpterochiropteran families (Rhinolophidae, Hipposideridae and Rhinonycteridae), involving a well-defined auditory fovea, a striking apomorphy of these rhinolophoid echolocators despite a plesiomorphic Rosenthal’s canal and cis-otic ganglion.
The novel phylogenetic and morphospace patterns (Figs. 1–3, Extended Data Figs. 1, 2) clearly show that yangochiropterans diverged by transformation of spiral ganglion neuroanatomy from yinpterochiropterans, supporting either a parallel or a convergent evolution of echolocation. Currently, there is not enough information to test whether the lack of laryngeal echolocation in Pteropodidae represents a secondary loss (Supplementary Information). Fossil evidence suggests that the earliest known bats (outside extant Chiroptera) probably had some capacity for echolocation3,6,27,33,34. The osteological correlates of the ganglion and their distribution among extant bat families (Figs. 1–3, Extended Data Figs. 1, 2) can be used now to map evolutionary patterns onto extinct bats, and can be tested with fossils. Fossil bats closer to Yangochiroptera than Yinpterochiroptera would be expected to have wall-less or fenestral walls. Fossil bats on the stem to crown Chiroptera would probably have foraminal walls.
Bats are spectacular examples of adaptive radiation35. Recent morphometrical analyses of the cranial shape and mandibular structure suggest that the adaptive changes in skulls are driven less by dietary diversification than by echolocation36,37,38,39. Echolocation is the primary sensory function for most bats4,40. The differences in echolocation are relevant for different foraging modes and therefore influence the diversification of the main clades of bats35,36,37,38,39,41,42,43,44. We hypothesize that the highly transformed and evolutionarily highly variable ganglion canal structures are such neuroanatomical correlates for the larger cochlear ganglia and more varied nerve fascicles in Yangochiroptera, enhancing the capability of peripheral signal processing by the ganglion for a wide range of echolocating strategies7,8,9,10,30,31,32 (Extended Data Fig. 7, 8, Supplementary Table 5).
In summary, we report a major evolutionary apomorphy in the ganglion neuroanatomy for Yangochiroptera—the Rosenthal’s canal becomes wall-less, which allows the ganglion to transgress, partially or entirely, to the internal auditory meatus. This reduces the spatial constraint on the ganglion, allowing increased neuron density (Fig. 1, Extended Data Fig. 1). The wall-less pattern and transitional fenestral pattern in Yangochiroptera are variable between superfamilies, between bat species, and can be polymorphic in individual bats. These variations drive a much wider morphospace distribution of the neuroanatomy in Yangochiroptera than in Yinpterochiroptera, manifested in their striking divergence in evolutionary disparity (Fig. 2b, Extended Data Fig. 2). Finally, we hypothesize that the apomorphic ganglion neuroanatomy and its disparity evolved in tandem with the wider range of echolocating strategies used by Yangochiroptera (Fig. 2, Extended Data Fig. 1). The freedom from constraint afforded by the wall-less condition and trans-otic ganglion is clearly associated with the explosive diversification in echolocators of Yangochiroptera, which today account for 70% of the families, 90% of the genera and 82% of the species of all echolocating bats.
Methods
CT scans of inner ear cochleas of bats and outgroups
Intact skulls of 39 bat species of 19 bat families, and isolated petrosals of five non-chiropteran outgroups were scanned, all on GE Phoenix CT scanners (240/180 kv dual X-ray tubes): 38 bat species scanned at UChicago (https://luo-lab.uchicago.edu/paleoCT.html). One bat (yangochiropteran Mystacina robusta) and two outgroup species (Setifer setosus and Mus musculus) were scanned at AMNH (https://www.amnh.org/research/microscopy-and-imaging-facility) and one outgroup species (Didelphis virginiana) was scanned at University of Bonn (Germany). All bat species were scanned at ≤15 µm; resolution for each scanned specimen is detailed in Supplementary Table 3.
Histological confirmation
To confirm CT scan with soft tissue histological sections on the spiral ganglion placement and its canal wall patterns, we obtained histological sections of inner ears of five bat species that were also CT scanned (Supplementary Table 1). These exemplar species are: foraminal wall pattern, Epomophorus wahlbergi and Hipposideros caffer (Extended Data Fig. 4); fenestral wall pattern, Coleura afra (Extended Data Fig. 6); Noctilio albiventris (exemplar for diverse Noctilionoidea); and wall-less pattern of Rosenthal’s canal, Miniopterus inflatus (Fig. 3). Inner ear of bats were decalcified in EDTA solution and then were sliced into sections at 8 µm or 12 µm thickness. Tissues on histological sections were then contrast-stained with haematoxylin and eosin. Histology work was performed on microtomes in Institute for Genomic Biology, University of Illinois Urbana-Champaign, and at AMNH. Histological confirmation of the ganglion placement and Rosenthal canal wall pattern on additional 15 bat species are based on published histological photographs (or drawings) of previous studies of bat cochleas (sources listed in Supplementary Table 3).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
Skull specimens of bats and mammalian outgroups examined by CT scanning in this study are in the collection of Field Museum of Natural History, the teaching collections of UChicago, and the mammalogy collection of American Museum of Natural History. The specimen list is presented in Supplementary Table 2. CT scanning resolutions for all scanned specimens are listed in Supplementary Table 2. Metric measurements of CT visualization of the Rosenthal’s Canal and cochlear canal turns of these specimens are presented in Supplementary Table 3. Photographs of histological sections are presented in the figures and extended data figures.
Code availability
The metric measurements of the Rosenthal’s canal and cochlear turns were obtained using the Mimics visualization package (www.materialise.com). Methods of morphological disparity analysis and ancestral state reconstruction are presented in the Supplementary Information.
References
Teeling, E. C. et al. Molecular evidence regarding the origin of echolocation and flight in bats. Nature 403, 188–192 (2000).
Teeling, E. C. et al. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 307, 580–584 (2005).
Maltby, A., Jones, K. E. & Jones, G. in Handbook of Mammalian Vocalization—An Integrative Neuroscience Approach (ed Brudzynski, S. M.) 37–50 (Elsevier, 2010).
Jones, G., Teeling, E. C. & Rossiter, S. J. From the ultrasonic to the infrared: molecular evolution and the sensory biology of bats. Front. Physiol. 4, 117 (2013).
Jebb, D. et al. Six reference-quality genomes reveal evolution of bat adaptations. Nature 583, 578–584 (2020).
Nojiri, T. et al. Embryonic evidence uncovers convergent origins of laryngeal echolocation in bats. Curr. Biol. 31, 1353–1365.e3 (2021).
Bruns, V., Fielder, J. & Kraus, H. J. Structural diversity of the inner ear of bats. Myotis 21/22, 52–61 (1984).
Ramprashad, F., Money, K. E., Landolt, J. P. & Laufer, J. A neuroanatomical study of the cochlea of the little brown bat (Myotis lucifugus). J. Comp. Neurol. 178, 347–363 (1978).
Vater, M. in Animal Sonar: Processes and Performance (eds Nachtigall, P. E. & Moore, P. W. B.) 225–241 (Springer, 1988).
Henson, O. W. & Henson, M. M. in Animal Sonar: Processes and Performance (eds Nachtigall, P. E. & Moore, P. W. B.) 301–305 (Springer, 1988).
Echteler, S. M. & Nofsinger, Y. C. Development of ganglion cell topography in the postnatal cochlea. J. Comp. Neurol. 425, 436–446 (2000).
Johnson, S. B., Schmitz, H. M. & Santi, P. A. TSLIM imaging and a morphometric analysis of the mouse spiral ganglion. Hear. Res. 278, 34–42 (2011).
Gacek, R. R. Clustering is a feature of the spiral ganglion in the basal turn. ORL 74, 22–27 (2012).
Fenton, M. B. Describing the echolocation calls and behavior of bats. Acta Chiropterologica 1, 127–136 (1999).
Fenton, M. B., Faure, P. A. & Ratcliffe, J. M. Evolution of high duty cycle echolocation in bats. J. Exp. Biol. 215, 2935–2944 (2012).
Lazure, L. & Fenton, M. B. High duty cycle echolocation and prey detection by bats. J. Exp. Biol. 214, 1131–1137 (2011).
Neuweiler, G. Evolutionary aspects of bat echolocation. J. Comp. Physiol. A 189, 245–256 (2003).
Teeling, E., Dool, S. & Springer, M. in Evolutionary History of Bats: Fossils, Molecules and Morphology (eds Gunnell, G. F. & Simmons, N. B.) 1–22 (Cambridge Univ. Press, 2012).
Dabdoub, A. & Fritzsch, B. in The Primary Auditory Neurons of the Mammalian Cochlea. Springer Handbook of Auditory Research (eds Dabdoub, A. et al.) 1–10 (Springer, 2016).
Goodrich, L. V. in The Primary Auditory Neurons of the Mammalian Cochlea. Springer Handbook of Auditory Research (eds Dabdoub, A. et al.) 11–48 (Springer, 2016).
Yang, T., Kersigo, J., Jahan, I., Pan, N. & Fritzsch, B. The molecular basis of making spiral ganglion neurons and connecting them to hair cells of the organ of Corti. Hear. Res. 278, 21–33 (2011).
Luo, Z.-X., Ruf, I. & Martin, T. The petrosal and inner ear of the Late Jurassic cladotherian mammal Dryolestes leiriensis and implications for ear evolution in therian mammals. Zool. J. Linn. Soc. 166, 433–463 (2012).
Luo, Z.-X. & Manley, G. A. in The Senses—A Comprehensive Reference Vol. 2 2nd edn (eds Fritzsch, B. & Grothe, B.) 207–252 (Elsevier, 2020).
Vater, M. & Siefer, W. The cochlea of Tadarida brasiliensis: specialized functional organization in a generalized bat. Hear. Res. 91, 178–195 (1995).
Carter, R. T. & Adams, R. A. Ontogeny of the larynx and flight ability in Jamaican fruit bats (Phyllostomidae) with considerations for the evolution of echolocation. Anatomical Rec. 297, 1270–1277 (2014).
Davies, K. T., Maryanto, I. & Rossiter, S. J. Evolutionary origins of ultrasonic hearing and laryngeal echolocation in bats inferred from morphological analyses of the inner ear. Front. Zool. 10, 2 (2013).
Simmons, N. B., Seymour, K. L., Habersetzer, J. & Gunnell, G. F. Primitive Early Eocene bat from Wyoming and the evolution of flight and echolocation. Nature 451, 818–821 (2008).
Ekdale, E. G. Comparative anatomy of the bony labyrinth (inner ear) of placental mammals. PLoS ONE 8, e66624 (2013).
Spoedlin, H. Anatomy of cochlear innervation. Am. J. Otolaryngology 6, 453–467 (1985).
Kössl, M. & Vater, M. The cochlear frequency map of the mustache bat, Pteronotus parnellii. J. Comp. Physiol. A 157, 687–697 (1985).
Vater, M. in Ontogeny, Functional Ecology, and Evolution of Bats (eds Adams, R. A. & Pedersen, S. C.) 137–173 (Cambridge Univ. Press, 2000).
Simmons, J. A. & Stein, R. A. Acoustic imaging in bat sonar: echolocation signals and the evolution of echolocation. J. Comp. Physiol. A 135, 61–84 (1980).
Simmons, N. B., Seymour, K. L., Habersetzer, J. & Gunnell, G. F. Inferring echolocation in ancient bats. Nature 466, E8 (2010).
Veselka, N. et al. A bony connection signals laryngeal echolocation in bats. Nature 463, 939–942 (2010).
Shi, J. J. & Rabosky, D. L. Speciation dynamics during the global radiation of extant bats. Evolution 69, 1528–1545 (2015).
Jacobs, D. & Bastian, A. High duty cycle echolocation may constrain the evolution of diversity within horseshoe bats (Family: Rhinolophidae). Diversity 10, 85 (2018).
Arbour, J. H., Curtis, A. A. & Santana, S. E. Signatures of echolocation and dietary ecology in the adaptive evolution of skull shape in bats. Nat. Commun. 10, 2036–13 (2019).
Hedrick, B. P. et al. Morphological diversification under high integration in a hyper diverse mammal clade. J. Mamm. Evol. 27, 563–575 (2020).
Rojas, D., Warsi, O. M. & Dávalos, L. M. Bats (Chiroptera: Noctilionoidea) challenge a recent origin of extant Neotropical diversity. Syst. Biol. 65, 432–448 (2016).
Thiagavel, J. et al. Auditory opportunity and visual constraint enabled the evolution of echolocation in bats. Nat. Commun. 9, 98 (2018).
Schnitzler, H.-U. & Kalko, E. K. V. Echolocation by insect-eating bats. Bio Science 51, 557–569 (2001).
Denzinger, A. & Schnitzler, H.-J. Bat guilds, a concept to classify the highly diverse foraging and echolocation behaviors of microchiropteran bats. Front. Physiol. 4, 1 (2013).
Schnitzler, H.-U., & Denzinger, A. Auditory fovea and Doppler shift compensation: adaptations for flutter detection in echolocating bats using CF-FM signals. J. Comp. Physiol. A 197, 541–559 (2011).
Neuweiler, G. Auditory adaptations for prey capture in echolocating bats. Physiol. Rev. 70, 615–641 (1990).
Revell, L. J. phytools: an R package for phylogenetic comparative biology (and other things): phytools: R package. Methods Ecol. Evol. 3, 217–223 (2012).
Paradis, E. & Schliep, K. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 35, 526–528 (2019).
R Core Team. R: A Language and Environment for Statistical Computing. http://www.R-project.org/ (R Foundation for Statistical Computing, 2013).
Hsiao, C. J., Jen, P. H.-S. & Wu C. H. The cochlear size of bats and rodents derived from MRI images and histology. NeuroReport 26, 478–482 (2015).
Acknowledgements
This research was supported by UChicago Metcalf Fellowship and Graduate Fellowships from NSF and AMNH (to R.B.S.), by Postdoctoral Fellowships from NSF and University of Illinois (to D.J.U.), Field Museum Brown Mammal Research Fund and grant from JRS Biodiversity Foundation (to B.D.P.) and UChicago Biological Sciences Division and NSF fundings (to Z.-X.L.). We thank J. Schultz, K. Sears, N. Simmons, G. Manley, R. MacPhee and J. Flynn for discussion; K. Sears and E. Rodriguez for access to histological sectioning facilities. Full acknowledgements are presented in the Supplementary Information.
Author information
Authors and Affiliations
Contributions
Conceptualization: R.B.S., Z.-X.L. and B.D.P. Data contribution: all authors. CT scanning: A.I.N. and R.B.S. CT visualization and morphometrical analysis: R.B.S. Histological sections: R.B.S. and D.J.U. Providing access to museum collections and histological specimens: B.D.P. Interpretation of anatomical data: R.B.S., Z.-X.L., B.D.P. and A.I.N. Compilation of Supplementary Information: R.B.S., Z.-X.L. and B.D.P. Graphics: R.B.S., A.I.N. and Z.-X.L. Writing: R.B.S., Z.-X.L. and BDP, and approved by all authors. The project was coordinated by Z.-X.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Nature thanks Brock Fenton, Amanda Lauer and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Phylogenetic evolution and systematic character distribution of the wall patterns of Rosenthal’s canal for placement of the spiral ganglion among bats and outgroups, showing phylogeny and classification.
A (left, cladogram): phylogeny of Chiroptera (tree topology based on Ref. 35.-Shi and Rabosky, 2015) Superfamilies are indicated by numbers: 1- Rhinolophoidea, 2- Emballonuroidea, 3 - Noctilionoidea, 4 - Vespertilionoidea. B.-D. (right columns), wall patterns of Rosenthal’s canal (RC) from the basal ½ turn to the third (apical) turn of the entire cochlea (explanation: between the fenestra vestibuli and basal ½ turn landmark, the spiral ganglion canal has small foramina in all therian mammals, and no variation in the local area). The research here focuses on the structural and phylogenetic variations in ganglion canal wall between the basal ½ turn and the apex). B. RC wall pattern at the basal ½ cochlear turn; C. RC wall pattern at the 1.5 cochlear turn; D. RC wall pattern at the 2.5 cochlear turn. For taxa with less than 2.5 turns, the patterns in the remainder of coil from turn 1.5 onward is represented. The three major wall patterns (lower left panel) are explained in Fig. 1 and Extended Data Fig. 2. Structures of the cochlear canal and the spiral ganglion canal are based on CT scanning (Supplementary Tables 1–3; n = 45). Of these, 15 bats and two outgroups are examined both by CT scans and then corroborated by original histological sections of this study, or from published histological literature (Supplementary Table 1). Symbol † - two taxa (Homo sapiens and Crocidura russala) that are based on published cochlear structure from literature. E. Echolocation duty cycle for bats in this study (LDC= Low Duty Cycle, HDC = High Duty Cycle). Foraging habitats and foraging modes of bats. Abbreviations for foraging habitats for echolocation: E –around the edges of open space; Nc – narrow space with cluttered background; O - open and uncluttered foraging space (Ref. 42.- Denzinger and Schnitzler 2013); TC - tongue-clicking echolocation. Abbreviations for foraging modes: A – aerial foraging of insects; Fr – frugivorous; Fl –foraging for fluttering insects; G- gleaning for prey; Tw – trawling over water.
Extended Data Fig. 2 Ancestral state reconstruction for the wall configurations of Rosenthal’s canal of bats and therian mammal outgroups. Each taxon is assigned a diagnostic character in one of the three major canal wall patterns.
The assignments were based on one of the two alternative representation schemes. A. The wall structure of the ganglion canal of each taxon is represented by the most derived ganglion wall pattern for taxa with multiple patterns. B. The most extensive canal wall pattern (character state), ranked by the highest percentage to the total cochlear length, is assigned to be the diagnostic character of the taxon. The ancestral state reconstruction at the main nodes of the cladogram is estimated by utilizing the phytools (Ref. 45 - Revell 2012) and ape (Ref. 46 - Paradis and Schliep, 2019) programs in R (Ref. 47 - R core team, 2013). The foraminal wall with cis-otic ganglion (Blue - primitive); the fenestral wall and cis-otic ganglion (Green - intermediate); the wall-less canal and trans-otic ganglion (Red – most derived). Tree topology is adapted from Shi and Rabosky (2015) (Ref. 35.) and Teeling et al. (2016). C. Morphological disparity of the inner ear ganglion by the enclosure of the cochlear ganglion (measured via percent ossification, see main text) and cochlear turns. Symbols and plot are identical to Fig. 2, but adjusted to show alternate groupings of Yangochiroptera (red) and Yinpterochiroptera + outgroups (blue). Source data in Supplementary Table 3.
Extended Data Fig. 3 Three major neuroanatomical configurations of the spiral ganglion and Rosenthal’s canal in bats and therian outgroups.
A. Schematic configuration of Rosenthal’s canal, its tractus foraminosus (= foraminal wall), and their structural relationships to the cochlear nerve fiber fascicles and the cis-otic ganglion placement. B. Schematic model of the bony canal for the cis-otic ganglion and the foramina on the surface of internal auditory meatus (nerve structures omitted for clarity). B1. Felis catus (mammal outgroup; domestic cat): the foraminal canal wall structure as seen on the surface of the internal auditory meatus, visualized by CT. B2. Hypsignathus monstrosus (Yinpterochiroptera, Pteropodidae) – the hammer-headed bat: the foraminal RC wall in the internal auditory meatus. B3. Rhinolophus blasii (Yinpterochiroptera, Rhinolophidae) Blasius’s horseshoe bat: the foraminal RC wall in the internal auditory meatus. The foraminal configuration (B1 - B3) is typical of therian mammals and plesiomorphic for Chiroptera as a group and for Yinpterochiroptera. C and C1. Tadarida brasiliensis (Yangochiroptera, Molossidae) – the Brazilian free-tailed bat: the fenestral configuration with large openings (tractus fenestralis) in the thinner wall with the cis-otic placement of the spiral ganglion. The large fenestra typically are present in the basal and near the ½ cochlear turns in bats having this pattern. The fenestral pattern is more derived than the plesiomorphic foraminal pattern, and it is an intermediate character state between the foraminal pattern and the most derived wall-less pattern. D and D1. Miniopterus inflatus (Yangochiroptera, Miniopteridae) – the greater long-fingered bat: the wall-less pattern of Rosenthal’s canal, typically between the apex and the basal ½ cochlear turn, allows the trans-otic placement of the spiral ganglion, making ganglion space confluent with the space of the internal auditory meatus in the absence of RC wall. With the exception of the species of Noctilio, all Yangochiroptera show this pattern in the apical turn of the cochlea (Extended Data Fig. 1). Structurally, this is the most extreme neuroanatomical pattern of the ganglion and its canal. The confluence of spiral ganglion space and the internal auditory meatus eliminates the constraint of many small foramina for the cochlear nerve fascicles to connect to the spiral ganglion and helps to shorten the ganglion’s connection to cochlear nerve trunk. It also provides more space to accommodate greater numbers of ganglion neurons in higher density.
Extended Data Fig. 4 Therian outgroup (Felis catus) and yinpterochiropteran bats (Epomophorus wahlbergi and Rousettus aegyptiacus): foraminal wall of the Rosenthal’s canal (RC) for cis-otic ganglion placement.
A. Schematic illustration of the cochlear nerve fiber fascicles and the cis-otic spiral ganglion, and their anatomical relationships to osteological structures. A1. The tractus foraminosus (foraminal wall) extends from the base to the apex of the cochlea, shown as dense foramina in the wall of internal auditory meatus in a schematic uncoiled cochlea. Yinpterochiropteran bats (Epomophorus – non-echolocating and Rousettus – tongue-clicking echolocation) have the plesiomorphic foraminal wall and cis-otic ganglion placement, as in non-chiropteran therian mammals (represented by Felis catus, Carnivora, Laurasiatheria). B. Epomophorus wahlbergi: Histological section through modiolar section of the whole cochlea. B1. Histological details at basal ½ cochlear turn. C. Epomophorus - CT visualization of location of the ganglion in a transparent cochlea, with CT slice through to visualize the relationship of the ganglion. C1. Cut-away CT model the modiolar section of the cochlea to show the tractus foraminosus and ganglion (yellow) in Rosenthal’s canal, relative to the cut-away internal auditory meatus. D. Epomophorus CT slice through the modiolar section of the cochlea to illustrate the osteological structures of the ganglion and cochlear nerve fibers (corresponding to histological section of B). D1. Details of the cochlea and Rosenthal’s canal at the 1/2 cochlear turn. E. Rousettus aegyptiacus – CT slice across the modiolar plane of the cochlea to show the foraminal wall pattern of Rosenthal’s Canal in the entire internal auditory meatus. E1. Details of ossified foraminal wall of the Rosenthal’s canal at basal ½ turn of Rousettus. F. Felis catus (representative of laurasiatherian outgroup) - Cut-away CT visualization model of foramina on the ossified wall of the internal auditory meatus, and the inclusion of the ganglion (yellow) in Rosenthal’s canal. F1. Felis – CT slice through modiolar plane to show details of Rosenthal’s canal and its foraminal wall, relative to the internal auditory meatus. G. CT slice through the entire cochlea at the modiolar section. G1. Felis – enlarged details of Rosenthal’s canal and its structures at the basal 1.2 cochlear turn. The tractus foraminosus in the internal auditory meatus, the foraminal wall of the ganglion canal, and the internal placement of cis-otic ganglion are the ancestral pattern of therians, and their Mesozoic dryolestoid relatives (Ref. 22.- Luo et al. 2012). These are plesiomorphic for laurasiatherian placentals, including Felis catus and all yinpterochiropteran bats.
Extended Data Fig. 5 Histology and CT scans of neuroanatomy of Rosenthal’s canal for the cis-otic spiral ganglion in Hipposideros caffer (Yinpterochiroptera, Hipposideridae).
Sundevall’s roundleaf bat is a laryngeal echolocating bat. A. Diagram of the foraminal wall of Rosenthal’s canal for cis-otic placement of the ganglion, showing the tractus foraminosus (foraminal wall pattern) for the fascicles of cochlear nerve to connect with the ganglion in the Rosenthal’s canal; A1. Distribution of foramina along the length of cochlea. B. Histological section at the modiolus, to show internal auditory meatus, the canaliculi of tractus foraminosus in the entire cochlea; B1, Histological structures of the spiral ganglion in Rosenthal’s canal, the cochlear nerve fascicles through canaliculi of tractus foraminosus, and the radial fibers of the ganglion through the habenula perforata in the primary bony lamina, at the 1½ cochlear turn. C. and C1. CT scan slice through the modiolar section, corresponding to histology section of B and B1: to show the ganglion space in Rosenthal’s canal, the tractus foraminosus in canal wall, and the habenula perforata in primary bony lamina for radial ganglion fibers at the 1½ cochlear turn. D and D1. CT slice through transparent model, and cut-away section corresponding to histological section, to show the foraminal pattern on the surface of the internal auditory meatus near the 1½ to 2 cochlear turns. E. Cut-away CT visualization of the whole cochlea at the modiolar section. E1. Intact cochlea in the medial (endocranial) view of the internal auditory meatus to show the foraminal wall pattern.
Extended Data Fig. 6 Foraminal Rosenthal’s canal and cis-otic placement for two yinpterochiropteran LDC echolocators, Rhinopoma hardwickii and Lyroderma lyra.
A. Anatomy of key neural and osteological structures. A1. Distribution of foramina for cochlear nerve fascicles from the base to the apex of the cochlea. B. Rhinopoma – uCT sections through the modiolar section of the whole cochlea. B1. Detail of Rosenthal’s canal at the first ½ turn of the cochlea. C. Transparent inner ear model with the spiral ganglion (yellow) visible. C1. and C2. Cut-away section of the inner ear model to visualize the location of the spiral ganglion in relation to Rosenthal’s canal. D. Details of the inner ear model in the medial (endocranial) view to visualize the extent of ossification within the modiolar region. E. Lyroderma lyra (“Megaderma lyra”)- transparent inner ear model with the spiral ganglion (yellow) visible. E1. and E2. Cut-away section of the inner ear model to visualize the location of the spiral ganglion in relation to Rosenthal’s canal. F uCT sections through the modialor section of the whole cochlea. F1. Detail of Rosenthal’s canal at the first ½ turn of the cochlea. G. Details of the inner ear model in the medial (endocranial) view in order to visualize the extent of ossification within the modiolar region.
Extended Data Fig. 7 Histology and CT scans of neuroanatomy of Rosenthal’s canal for fenestral wall of Rosenthal’s canal for the Cis-otic Spiral Ganglion in Coleura afra (Yangochiroptera, Emballonuridae).
The African sheath-tailed bat Coleura afra is a laryngeal echolocating bat of Yangochiroptera. A. Diagram of the fenestral wall (tractus fenestralis) of Rosenthal’s canal (RC); the large fenestrae of RC wall are well developed beyond the basal ¾ turn, and the RC becomes wall-less in the apical ½ turn. The basal-most part of the cochlea retains the standard mammalian pattern of small foramina. B. Histology section through the modiolar plane and the internal auditory meatus to show the cis-otic placement of the ganglion, the tractus fenestralis that forms the canal wall. B1. Histological details of the fenestral openings of the 1½ cochlear turn. C. CT scan slice corresponding to the histological section of the whole cochlea. C1. CT visualization of the ganglion space in Rosenthal’s canal and the fenestral openings in canal wall at the 1½ cochlear turn. D. Transparent cochlea and D1. Cut-away section corresponding to histological sections (B and B1): the fenestras in the internal auditory meatus near the 1 to 1½ cochlear turns, and wall-less pattern of the apical turn. E. Intact cochlea in the medial (endocranial) view of the internal auditory meatus to show the fenestral wall pattern. E1. Cut-away CT visualization of the whole cochlea at the modiolar section.
Extended Data Fig. 8 Fenestral wall of Rosenthal’s canal and cis-otic placement of ganglion in Tadarida brasiliensis (Yangochiroptera, Molossidae) by CT scans.
The Brazilian free-tailed bat Tadarida brasiliensis is a laryngeal echolocating bat. A. Diagram of the fenestral wall of Rosenthal’s canal. A1. The canal wall shows large fenestrae between the basal ½ turn and 1½ turn, but it becomes wall-less in the apical ½ turn. B. and C. Outline illustration and CT scan slice across the modiolar section of the fenestral pattern up to ½ turn and the wall-less pattern in apical ½ turn. C1. Details of the large fenestral opening (tractus fenestralis) between the RC canal and the internal auditory meatus. D and D1. Transparent CT visualization and cut-away section of local details of fenestrae in the internal auditory meatus. E. Intact cochlea in the medial (endocranial) view of the internal auditory meatus to show the fenestral wall; and E1. Modiolar section to show the continuous variation of fenestral pattern (up to 1½ turn) and the wall less pattern (the apical ½ turn) along the length of the cochlea.
Extended Data Fig. 9 Yangochiropteran vesper bats Pipistrellus abramus (Vespertilionidae) and Myotis lucifugus (Vespertilionidae): wall-less Rosenthal’s canal with trans-otic placement of spiral ganglion.
The Japanese pipistrelle bat (P. abramus) and the Little brown bat (M. lucifugus) are laryngeal echolocating bats. Their neuroanatomical RC and ganglionic patterns are the most derived characters among mammals, only known in yangochiropterans. A. Schematic diagram to show the absence of Rosenthal’s canal wall and the confluence of the ganglion with cochlear nerve in internal auditory meatus (IAM). A1. Rosenthal’s canal has no wall between the basal ½ turn and apex, although the basal most ½ turn has foramina. B and B1. Histological section of Pipistrellus showing the spiral ganglion is placed in IAM, starting from near the base of the cochlea (Ref. 48 - Hsiao et al. 2015: fig. 2. Histological section reproduced with permission/license from Copyright Clearance Center: www.rightfind.com). C. and C1. CT slice through the modiolar section to show that Rosenthal’s canal wall is entirely absent from the basal ½ to the apex; and the presumptive position of the ganglion is in IAM. D. Transparent model of cochlea to visualize the position of the ganglion. D1. Solid CT model of cochlea cut away at the modiolar section visualize the wall-less condition of Rosenthal’s canal space and the placement of spiral ganglion in IAM. E. Myotis - Transparent cochlea to visualize the spiral ganglion space (yellow). E1. Cut-away surface to visualize that the ganglion space is open and confluent with the IAM. E2. Cut-away model to show the open and confluent condition of Rosenthal‘s canal of the entire internal auditory meatus. E3. Solid cochlea model of the internal auditory meatus to show the exposure of the spiral ganglion. F. Myotis - CT slice through the modiolar section. F1. Detailed osteological structures at 1½ turn. F2. Detailed osteological structure at the cochlear base. F3. Detailed osteological structures at 1½ turn. C. Transparent model of the cochlea. Abbreviations: bm – basilar membrane; IAM – internal auditory meatus; RC – Rosenthal’s canal; sg – spiral ganglion; sm – scala media; st – scala tympani; sv – scala vestibuli; tcm – tectorial membrane; TF – tractus foraminosus; vm – vestibular membrane.
Extended Data Fig. 10 Wall-less Rosenthal‘s canal for trans-otic placement of the spiral ganglion of Pteronotus parnellii (Yangochiroptera, Mormoopidae) - Parnell’s mustached bat, a laryngeal echolocating bat.
A. Schematic model of osteological structures. A1. Uncoiled schematic cochlea to visualize the wall-less part of Rosenthal’s Canal between the basal ½ turn and the apical turn. B. Whole mount prepared nerve structures in the basal cochlear turn: cochlear nerve trunk, cochlear nerve fiber fascicles, spiral ganglion, and ganglion radial fibers to hair cells (image redrawn from Henson and Henson 1988: fig. 1) (Ref. 10). C. CT slice corresponds to whole-mount cochlear nerve dissected (B) and visualizes the foraminal wall of Rosenthal‘s canal in the basal ½ turn, and the absence of the wall of Rosenthal‘s canal beyond basal ½ turn. The absence of Rosenthal’s canal wall at ½ turn is corroborated by histology (Ref. 30 - Kössl and Vater 1985: fig. 1). D. Transparent inner ear model with location of the spiral ganglion (yellow). D1. Modiolar section of cochlea: Spiral ganglion is positioned in the internal auditory meatus between the basal ½ turn and the apex. D2. Details in modiolar section to visualize that the space of the ganglion is open and confluent with the IAM. E. CT visualization of the internal auditory meatus.
Supplementary information
Supplementary Information
This file contains Supplementary Information Parts 1–12, including a Figure, Tables 1–6 and references.
Rights and permissions
About this article
Cite this article
Sulser, R.B., Patterson, B.D., Urban, D.J. et al. Evolution of inner ear neuroanatomy of bats and implications for echolocation. Nature 602, 449–454 (2022). https://doi.org/10.1038/s41586-021-04335-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-04335-z
- Springer Nature Limited
This article is cited by
-
Correlated evolution between body size and echolocation in bats (order Chiroptera)
BMC Ecology and Evolution (2024)
-
Molecular adaptations underlying high-frequency hearing in the brain of CF bats species
BMC Genomics (2024)
-
Development of the hyolaryngeal architecture in horseshoe bats: insights into the evolution of the pulse generation for laryngeal echolocation
EvoDevo (2024)
-
The vocal apparatus: An understudied tool to reconstruct the evolutionary history of echolocation in bats?
Journal of Mammalian Evolution (2023)
-
Ear anatomy traces a family tree for bats
Nature (2022)