Abstract
The radiation-based sterile insect technique (SIT) has successfully suppressed field populations of several insect pest species, but its effect on mosquito vector control has been limited. The related incompatible insect technique (IIT)—which uses sterilization caused by the maternally inherited endosymbiotic bacteria Wolbachia—is a promising alternative, but can be undermined by accidental release of females infected with the same Wolbachia strain as the released males. Here we show that combining incompatible and sterile insect techniques (IIT–SIT) enables near elimination of field populations of the world’s most invasive mosquito species, Aedes albopictus. Millions of factory-reared adult males with an artificial triple-Wolbachia infection were released, with prior pupal irradiation of the released mosquitoes to prevent unintentionally released triply infected females from successfully reproducing in the field. This successful field trial demonstrates the feasibility of area-wide application of combined IIT–SIT for mosquito vector control.
Similar content being viewed by others
Main
SIT, in which artificially reared radiation-sterilized males are released into the field to mate with wild females—thereby preventing them from producing viable offspring—has successfully suppressed populations of several insect pests of agricultural and veterinary importance1. However, despite various trials, SIT has not been widely used against mosquitoes because of the difficulty of irradiating males without reducing their mating competitiveness and survival2,3,4. A promising alternative approach is the related IIT5, in which released males are infected with the maternally inherited endosymbiotic bacteria Wolbachia, resulting in sterile matings with field females that are not infected with the same Wolbachia strain, a phenomenon known as cytoplasmic incompatibility6,7. An advantage of IIT is that Wolbachia-based sterilization has little or no effect on male mating competitiveness and survival8,9,10. Historically, in a small-scale pilot field trial IIT successfully eradicated the primary filariasis vector Culex quinquefasciatus5—although another trial showed limited success11—but the approach has not been deployed operationally, primarily because the accidental release of fertile females risks causing population replacement, whereby individuals infected with the same Wolbachia strain as released males replace the wild-type field population, preventing future population suppression (as matings between released males and field females are no longer incompatible)11,12,13. Consequently, previous studies14,15,16,17 have proposed combining IIT and SIT so that any residual females that are not removed from the released males are sterilized using low-dose irradiation without affecting the males’ mating competitiveness or survival. There has been a resurgence of interest in IIT18,19,20 in the past decade, partly because of the development of the ability to artificially transfer Wolbachia strains between mosquitoes21,22, and the first small-scale field release of artificially Wolbachia-infected mosquitoes for IIT was recently reported18. Concurrently, the combined IIT–SIT approach has also been under renewed consideration and development23,24,25,26, but has not yet been deployed.
The globally invasive mosquito A. albopictus is an important vector of arboviruses—including dengue and Zika viruses—that is particularly challenging to control using traditional approaches27,28. Unlike some other mosquito vectors, A. albopictus is superinfected with two native Wolbachia strains (wAlbA and wAlbB), complicating the development of Wolbachia-based control strategies. Various Wolbachia strains have previously been artificially introduced into A. albopictus29,30,31,32, including wPip in mosquitoes cured of their native double wAlbA/wAlbB infection33, but these endosymbiont–host associations are either unsuitable for IIT—as they are pathogenic or do not inhibit arboviruses—or their appropriateness has not been fully determined. Here we report the generation and characterization of an A. albopictus line (termed HC) with an artificial triple-Wolbachia infection, and demonstrate its use in an open-release field trial of the combined IIT–SIT approach. We show that the mass release of millions of factory-reared incompatible adult HC males over a two-year period enabled near-elimination of wild-type A. albopictus field populations, without their replacement by released HC mosquitoes.
Generation and characterization of HC
For use in IIT, Wolbachia must induce high levels of cytoplasmic incompatibility to effectively sterilize wild females, and have high maternal transmission to enable efficient mass production of only infected males for release as well as low fitness costs to ensure that released males can mate competitively with respect to wild males. In addition, as a responsible safety precaution, any released mosquitoes should have a lower vector competence for human pathogens than the target field population34. Accordingly, we created an endosymbiont infection appropriate for IIT by transferring wPip from its native mosquito host Culex pipiens into the A. albopictus HOU line by embryonic microinjection21, generating the mosquito line HC, which possesses a triple-Wolbachia infection (the artificially transinfected wPip as well as the original native wAlbA and wAlbB strains)9 (Extended Data Fig. 1). HC females were subsequently outcrossed with males of a wild-type mosquito line (GUA) with the native double wAlbA and wAlbB infection, initially collected from the area of our field trial, to create comparable nuclear genetic backgrounds in both mosquito lines, as well as with wild mosquitoes in our study region9.
Laboratory reciprocal-cross experiments demonstrated that wPip in HC causes complete unidirectional cytoplasmic incompatibility with GUA—of 7,578 eggs resulting from crosses between HC males and GUA females, none hatched, whereas HC females rescued cytoplasmic incompatibility when mated with either GUA or HC males (Fig. 1a). The total density of Wolbachia was higher in the ovaries of HC compared to GUA (Fig. 1b, c), and was stably maintained by 100% maternal transmission across subsequent generations. In laboratory cage populations, wPip also had no apparent effects on the fitness of HC males and females9,24,25, and only very minor effects on the mating competitiveness of HC males (Fig. 1d). In addition, wPip significantly reduced the vector competence of HC females for both horizontal and vertical transmission of dengue and Zika viruses (Extended Data Fig. 2 and Supplementary Information), similar to that of some other mosquito–Wolbachia associations35,36,37,38. These results demonstrate that the A. albopictus HC line has the characteristics required for use in IIT control programs.
a, Reciprocal crosses between HC and GUA lines. Letters above columns indicate significant differences between groups (mean ± s.e.m.; n = 5 for each cross, ANOVA and Tukey’s multiple comparisons test, F(3, 16) = 513.5, P < 0.0001). b, Fluorescence in situ hybridization (FISH), showing Wolbachia distribution and density in ovaries. Scale bar, 100 µm. c, Real-time quantitative PCR (RT-qPCR) analysis of the relative number of Wolbachia wsp gene copies (mean ± s.e.m.; n = 7 pools of two ovary pairs for each group, two-sided Mann–Whitney test, P = 0.006). d, Egg hatch rate in laboratory cage populations with different GUA female:GUA male:HC male ratios. Two-sided binomial test: n = 3,681, P = 0.0002 (1:1:1); n = 4,083, P < 0.0001 (1:1:5); n = 2,392, P = 0.0009 (1:1:10). e, Invasion of wPip in laboratory GUA populations after a single release of different numbers of HC females at generation 0. For release of 6% and 12% HC females, a single simultaneous inundative release of HC males at a 4:1 ratio with GUA males was also used, to mimic accidental female release during IIT. NC, negative control. f, g, Combined IIT–SIT in semi-field cages: egg hatch rate (f) and adult female population sizes (g). Target GUA populations were established in six replicate cages for 12 weeks before the release (indicated by the dashed red lines) of HC males, with HC females to mimic female contamination. Black triangles represent mathematical model outputs (mean goodness of fit: egg hatch rate R2 ≈ 0.9325; number of females captured R2 ≈ 0.8417).
Laboratory cage experiments showed that wPip invades wild-type GUA populations following release of only HC females, and that this population replacement may be facilitated by simultaneous inundative release of HC males (Fig. 1e and Supplementary Information). These observations indicate that the accidental release of HC females during an IIT program could result in the introduction of wPip into the target field population, and that this risk increases as HC males are released. Therefore, we tested the effectiveness of low-dose irradiation24,25 for the prevention of unintended population replacement during population suppression and/or elimination by simulating, in semi-field cage populations of GUA, accidental HC female release during an IIT intervention using the release of HC males (Fig. 1f, g and Supplementary Information). During these experiments, sufficient HC females were released to mimic a 2.0% contamination rate of the released males, and the number of released HC males was chosen to result in an initial 5:1 ratio of released HC to GUA males. The level of pupal irradiation used was previously shown to completely sterilize HC females24 without affecting male mating competitiveness24,25. Successful eradication of wild-type GUA mosquitoes occurred in the semi-field cages, without population replacement by the released wPip-infected HC mosquitoes (Fig. 1f, g). Mathematical modelling accurately described and predicted target population dynamics in the semi-field cage experiments, and supported the notion that a 5:1 ‘over-flooding’ ratio of HC to wild-type males is sufficient for effective population suppression and/or elimination (Fig. 1f, g, Extended Data Fig. 3 and Supplementary Information).
Field trial of combined IIT–SIT using HC
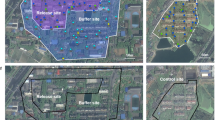
The preceding experimental and theoretical observations indicated that combined IIT–SIT using HC has the potential to eradicate A. albopictus field populations, as well as prevent unintended population replacement caused by accidental release of HC females. Therefore, we optimized mass rearing of HC for large-scale production39,40 and, with approval from the Chinese Ministry of Agriculture, undertook an open-release field trial in residential areas of two isolated riverine islands in Guangzhou, the city with the highest dengue transmission rate in China, and where A. albopictus is the only vector (Fig. 2a, b).
a, b, Satellite images of control and release sites 1 (a) and 2 (b) in Guangzhou city (map data: Google, DigitalGlobe). c, Release schedule. d, e, Effect of HC male release on A. albopictus larval stages in release sites 1 (d) and 2 (e). Vertical green dashed lines indicate onset of HC release. Red dashed line in d indicates that only IIT was used in release site 1 in 2015. Red solid lines in d and e indicate period of combined IIT–SIT in both release sites in 2016 and 2017. Two-sided Mann–Whitney test. Pre-release period: site 1 2014, n = 22, P = 0.164; site 2 2015, n = 26, P = 0.0805. Release period: IIT only: site 1 2015, n = 34, P < 0.0001; 12 March–21 May, n = 11, P = 0.0032. Release period: combined IIT–SIT: site 1 (2016, n = 37, P < 0.0001; 2017, n = 34, P < 0.0001); site 2 (2016, n = 32, P < 0.0001; 2017, n = 35, P < 0.0001).
In the year before HC release, baseline data were collected weekly in both release and control sites using ovitraps during the local mosquito breeding season between March and November (site 1 in 2014 and site 2 in 2015) (Fig. 2c). Overall, A. albopictus were highly abundant during this period, with no significant differences and strong temporal correlations in egg numbers and hatch rates between control and release sites (Fig. 2d, e and Extended Data Fig. 4a–f), validating the appropriateness of the control sites selected.
During the intervention period, adult male HC mosquitoes were released at multiple locations within each site, three times per week during the mosquito breeding season, for either three (site 1) or two (site 2) consecutive years (Fig. 2c), and A. albopictus populations were monitored weekly with ovitraps and adult-collecting BG-Sentinel traps (Extended Data Fig. 5).
In 2015, at site 1 only, at the beginning of the field trial, a limited number of HC mosquitoes were released because only manual checks were carried out for contaminant females (that is, IIT only, without irradiation). As cytoplasmic incompatibility causes embryonic death when HC males mate with wild-type females, the number of eggs hatching in each ovitrap was recorded. Initially, HC male release resulted in 55% population suppression, based on the number of eggs hatching per ovitrap (from 12 March 2015 to 21 May 2015), but this effect diminished as the mosquito season peaked (late May to early June, Fig. 2d), consistent with a low ratio of HC to wild-type males (see below). Consequently, the site 1 area was reduced from 16 June to increase the density of released males, after which the population-suppression effect increased. Overall, there was a yearly mosquito population reduction in release sites compared to control sites of 62% and 65% as measured at the larval and adult female stages, respectively (Figs. 2d, 3a). These observations demonstrate that IIT alone can suppress field populations, but only if sufficient numbers of male mosquitoes are released.
a, b, Relative density of adult females collected weekly in control and release sites 1 (a) and 2 (b). The red dashed line in a indicates period of IIT only in 2015. Red solid lines in a and b indicate the period of combined IIT–SIT in 2016 and 2017. Two-sided Mann–Whitney test. Site 1 2015, n = 34, P < 0.0001; site 1 2016, n = 37, P < 0.0001; site 1 2017, n = 37, P < 0.0001; site 2 2016, n = 37, P < 0.0001; site 2 2017, n = 38, P < 0.0001. c, d, Spatial dynamics of adult suppression at release sites 1 (c) and 2 (d) during the dengue transmission season in Guangzhou in 2017.
In 2016 and 2017, pupal irradiation to sterilize contaminant females (that is, combined IIT–SIT) replaced manual checks, enabling the production and release of larger numbers of mosquitoes (Fig. 4c) and allowing high-density releases throughout sites 1 and 2. In both release sites, the numbers of eggs and adults collected markedly declined (Fig. 2d, e, 3a, b, Extended Data Fig. 6a–f). In 2016 and 2017, we observed a yearly reduction of more than 94% in the average number of hatched eggs per ovitrap in release sites compared to control sites, and no viable eggs for up to 13 weeks (Fig. 2d, e). Similarly, there were yearly reductions of 83% to 94% in the average number of wild-type adult females caught per trap, with none detected for up to 6 weeks (Fig. 3a, b). Furthermore, declines in egg hatching coincided with declines in numbers of collected eggs and adults, consistent with cytoplasmic incompatibility rather than other factors driving the loss of wild-type A. albopictus.
a, Number of HC males released weekly and observed ratios of HC to wild-type males in the field at release site 1. Blue dashed line indicates target overflooding ratio of 5:1. b, Comparison of observed and expected weekly egg hatch rates at release site 1. Two-sided paired t-test after arcsine transformation. 2015, n = 27, P = 0.6522; 2016, n = 31, P < 0.0001; 2017, n = 33, P < 0.0001. c, Total number of HC males produced weekly and female contamination rate at adult stage in mass rearing facility. d, Comparison of monthly positive female rate detected in HC males in mass rearing facility (laboratory quality control) and that observed in adults collected from the field. Two-sided paired t-test after arcsine transformation: Laboratory (n = 19) versus site 1 (n = 18), P < 0.0001; versus site 2 (n = 16), P = 0.0012. Pearson correlation: site 1, r = 0.110, n = 18, P = 0.664; site 2, r = 0.839, n = 16, P < 0.0001.
The spatial dynamics of population suppression were analysed across different zones within the release sites (Fig. 3c, d). Consistently, the highest levels of population suppression were observed in more-isolated areas surrounded by vegetation and with limited transport links (zones 12–19 in site 1 and zones 2 and 3 in site 2), whereas less isolated zones nearer transportation routes with frequent traffic were relatively resistant to population suppression (in site 1, zones 10 and 11 had an ongoing bridge construction and zones 20–22 were adjacent to a shipping harbour, and there was considerable motor traffic in zones 7 and 8 of site 2), which suggests that human activities facilitate mosquito immigration into release sites and compromise the efficiency of A. albopictus elimination.
As the ratio of released HC to wild-type males is critical for population suppression, we measured this by detecting wPip in field-collected A. albopictus males (Fig. 4a). As indicated by our mathematical modelling and semi-field cage studies, we set a 5:1 ratio of HC to wild-type males as the target over-flooding ratio. As expected, in 2015, when fewer HC males were released and population suppression was relatively weak, the average male release ratio was 4.4:1 HC to wild-type males. However, in 2016 and 2017, when there was an increase in HC male release and population suppression was strong, yearly averaged ratios varied between 8.7 and 15.8.
The relative mating performance of HC to wild-type males in the field was also inferred from egg hatch rates (Fig. 4b). In 2015, when the released HC mosquitoes were not irradiated, expected and observed egg hatch rates were not significantly different, indicating that non-irradiated HC and wild-type males had similar mating competitiveness (as found in laboratory studies25) (Fig. 1d). However, in 2016 and 2017, when the released HC mosquitoes were irradiated the observed egg hatch was between 1.5 and 2.1-fold higher than expected, which suggests that the relative competitive mating ability of irradiated HC to wild-type males was 0.5 to 0.7—consistent with other laboratory-based cage experiments (Extended Data Table 1). Nevertheless, the reduced mating competitiveness of HC males as a result of irradiation was apparently offset by the increased number of mosquitoes released.
As accidental HC female release could lead to unintended population replacement, thereby preventing further population suppression (particularly as population suppression proceeds and the ratio of wPip-positive females relative to wild-type field females increases), the number of adult female wPip-positive mosquitoes was carefully monitored both before and after their release (Fig. 4c, d). A mean of 0.24% ± 0.03% (s.e.m.) contaminant HC females were released in 2016 and 2017 (Fig. 4d), which is below the 2% level simulated in the semi-field cage experiments that successfully prevented population replacement (Fig. 1f, g). Relative to the number of HC males, the proportion of wPip-positive females in release sites was generally higher than the pre-release contamination rate (Fig. 4d), possibly reflecting sex-specific differences in mortality and/or trap collection18,41. The pre-release contamination rate correlated with the wPip-positive field rate in site 2 but not in site 1 (Fig. 4d), suggesting that—at least in the former—the wPip-positive adult females that were caught were those that were released and did not originate from reproduction in the field. If a viable breeding wPip-infected field population had established, the numbers of A. albopictus would be expected to increase after an initial period of population suppression, with a concomitant decline in the observed level of cytoplasmic incompatibility (as compatible matings with wPip-infected females would have increased). However, there was no evidence of an increase over time in either absolute or relative numbers of eggs and adult females collected, regardless of whether all or only wPip-positive mosquitoes were considered (Fig. 3a, b, Extended Data Figs. 6b, e, 7). Notably, there was also no evidence of an increase in the proportion of eggs hatching (Fig. 2d, e), which would be expected if compatible matings were becoming more frequent. Additionally, larvae hatched from collected eggs were also wPip-negative in nearly all ovitraps (Extended Data Fig. 8a–c). Overall, 16 ovitraps with wPip-positive larvae were detected on 14 separate spatially and/or temporally isolated occasions among 1,844 ovitrap weeks (Extended Data Fig. 8a–c), indicating that very few accidentally released HC females had offspring in the field. Whether this might drive population replacement in the long run is uncertain, as the viability of the wPip-positive larvae is not known. Nevertheless, irradiation provides protection against accidental female release, especially compared to manual checking, as wPip-positive larvae did not increase despite a more-than-tenfold increase in the number of mosquitoes being released. In addition, irradiated HC males also induced HC female sterility (Extended Data Fig. 9), further reducing the risk of population replacement.
Successful population suppression resulted in a significant increase in community support for the field trials. Interviews carried out before the mosquito releases indicated that 13.0% of residents were supportive, with 76.4% and 10.6% being neutral and negative, respectively (Fig. 5a). However, after successful population suppression, there was a marked shift in attitudes, with a majority of the residents interviewed being supportive (54.3%)—probably owing to reduced mosquito nuisance biting27,42. Human landing catches verified the efficiency of A. albopictus population suppression, indicating its epidemiological importance for vector-borne disease transmission. The mosquito-biting rate by wild-type A. albopictus significantly decreased by 96.6% and 88.7%, respectively, in release sites 1 and 2, compared to their respective control sites (Fig. 5b).
a, Pie chart showing community support for the field trial in release site 1 before (13.0%, n = 123 interviews) and after (54.3%, n = 431) mosquito releases (χ2 = 71.29, P < 0.0001). b, Mosquito human landing catches in release and control sites 1 and 2, July to November 2017. Mean ± s.e.m.; n = 4 independent biological replicates for both control and release sites; two-sided paired t-test. Site 1, t = 6.988, 3 degrees of freedom, P = 0.006; site 2, t = 3.566, 3 degrees of freedom, P = 0.0376.
In conclusion, combined IIT–SIT nearly eliminated two field populations of A. albopictus over a two-year period. The few mosquitoes remaining were probably migrants from outside the study area, as indicated by population genetic analyses43 and their presence in areas with good transport links, whereas isolated areas were mosquito-free. The possibility of population replacement emphasizes the importance of releasing mosquitoes that cannot increase pathogen transmission. As shown here, wPip markedly reduces arbovirus transmission by wild-type A. albopictus with their native double wAlbA and wAlbB infection, so unintended population replacement could even be beneficial in the short- and long-term, by initially collapsing vector populations and rendering any newly established populations incompetent for pathogen transmission. However, the aim of population suppression is preferable and has greater public acceptance, as it enables reduction of nuisance biting and pathogen transmission, and long-term mosquito eradication in the absence of immigration. The combined IIT–SIT approach is environmentally friendly and cost-effective (see Supplementary Information), enabling vector control in complex and inaccessible urban habitats in which implementation of standard vector control is difficult27,28, as released males actively seek wild females, and allows release of much higher numbers of male mosquitoes in comparison to IIT alone, while simultaneously protecting against accidental female release. Area-wide application of this approach will necessitate the development of novel technologies, especially with regard to scaling-up production capacity and enabling efficient mass release of mosquitoes.
Methods
No statistical methods were used to predetermine sample size. The experiments were not randomized. The investigators were not blinded to allocation during experiments and outcome assessment.
Maintenance of mosquito lines
The four A. albopictus lines (HOU, HC, GUA and GT) and Culex pipiens molestus used in this study were maintained on 10% sugar solution at 27 ± 1 °C and 80 ± 10% relative humidity (RH), with a 12:12 h light:dark photoperiod, according to standard rearing procedures. For routine colony maintenance and experimental studies, including viral infection assays, female mosquitoes were blood-fed on mice according to protocols approved by the Michigan State University Institutional Animal Care and Use Committees (03/14-036-00), and the Ethics Committee on Laboratory Animal Care of the Zhongshan School of Medicine, Sun Yat-sen University (No. 2014-003 and No. 2017-041).
Transinfection and the generation of the HC and GT lines
The natively superinfected HOU line of A. albopictus, and C. pipiens molestus natively infected with wPip were used as a recipient and donor, respectively, for transinfection according to the previously described approach21. Cytoplasm was withdrawn from donor embryos through embryonic microinjection, and immediately injected into the posterior of 60–90-min-old recipient embryos using an IM300 microinjector (Narishige Scientific). After injection, embryos were incubated at 80% RH and 27 °C for approximately 1 h and transferred to wet filter paper. Embryos were then allowed to develop for 5–7 days before being hatched. Adult females (G0) that survived embryo microinjection were isolated as pupae and mated with HOU males. Following blood-feeding and oviposition, G0 females were assayed for wPip infection using PCR primers specific for the WO phage orf7 gene of wPip44. G1 females from the infected G0 female were then sib-mated, blood-fed, isolated and allowed to oviposit, followed by PCR assay for the wPip infection. From the wPip-infected G1 females, one line (designated HC) with a stable association was chosen for further studies, including confirmation of infection with wAlbA and wAlbB using strain-specific primers for PCR diagnosis. The primers for wAlbA have previously been reported45. The primers for wAlbB were: wsp, forward 5′-ACGTTGGTGGTGCAACATTTG-3′; reverse 5′-TAACGAGCACCAGCATAAAGC-3′. Males of a wild-type mosquito line GUA, with the native double wAlbA and wAlbB infection (initially collected from the field in Guangzhou, China), were subsequently outcrossed with HC females for seven generations to create comparable nuclear genetic backgrounds in both mosquito lines for subsequent experiments and the field release of HC9. To generate the aposymbiotic line GT, the HC line was fed with 10% sucrose containing 1 mg/ml tetracycline solution for five consecutive generations. Removal of Wolbachia from the mosquito was confirmed by PCR in the subsequent generations.
Experimental crosses to determine cytoplasmic incompatibility
Cytoplasmic incompatibility assays were conducted as previously described29. In brief, ten virgin females were mated with ten virgin males, with five replicates for each cross. Mated females were blood-fed weekly using mice. Oviposition sites were constantly available to females, and oviposition paper was changed weekly for three weeks. After egg maturation for 5–7 days on wet filter paper, eggs were immersed in water. Two days later, hatched eggs were counted to determine the hatch rate.
Wolbachia visualization in ovaries
Wolbachia were visualized in the ovaries by FISH, as previously described with slight modifications21. Dissected ovaries from females (about 10 days old) were fixed in 4% formaldehyde for 15 min. The ovaries were washed consecutively in methanol, acetone and finally PBST, and then incubated overnight at 37 °C in hybridization solution (Dig Easy Hyb Granules, Roche) containing 200 ng of Wolbachia-specific 5′-FITC-labelled 16S rDNA oligonucleotide probes W1 and W2 (Bioneer)46. Following hybridization, samples were washed with PBST and stained with DAPI (Roche) for 5 min. Samples were then mounted on a glass slide with neutral resin and a cover slip, before viewing with an Olympus IX70 fluorescence microscope.
Virus culture and titration
Zika virus (ZIKV) strain Z16006 was isolated from a patient in February 2016 by Guangdong Provincial Center for Disease Control and Prevention (CDC). Both ZIKV and DENV-2 (New Guinea C strain) were cultured in C6/36 cells before infecting mosquitoes according to standard procedures35. ZIKV was passaged for only three generations, after initially seeding cultures at a multiplicity of infection of ~1 virus particle per cell. Infected cells were grown in DMEM supplemented with 10% FBS, and incubated for 6 days at 35 °C and 5% CO2. Cells were subsequently collected, thawed and frozen to facilitate release of the virus particles. ZIKV used for mosquito oral infection was titrated on BHK cells in 96-well plates at half-maximal tissue culture infection dose, and DENV was titrated using the plaque assay method as previously reported47. C6/36 and BHK cell lines were purchased from the ATCC. None of these cell lines was found in the database of commonly misidentified cell lines maintained by ICLAC and NCBI Biosample. All these cell lines were authenticated by ATCC and did not have mycoplasma contamination.
Nucleic acid extraction and RNA reverse transcription
Total RNA was extracted from whole mosquitoes, their tissues or saliva samples, as well as virus cell culture supernatant, using RNAiso (Takara) according to the manufacturer’s protocol. Extracted RNA was dissolved in RNase-free water, DNase-treated and then immediately reverse-transcribed using HiScript Q RT SuperMix for qPCR (Vazyme). cDNA was stored at −20 °C for subsequent RT-qPCR analyses. To measure Wolbachia genome copy number, ovaries were dissected from female mosquitoes, and total DNA was extracted from pools of two ovary pairs using the phenol–chloroform method, then dissolved in ddH2O, and stored at −20 °C for subsequent PCR analysis.
Virus and Wolbachia quantification
The genome copy numbers of ZIKV and Wolbachia were measured using RT-qPCR as previously described37,48. Plasmids containing target gene fragments of ZIKV NS1, wsp or rps6 were cloned, quantified using a NanoDrop 2000 (Thermo), and then used for serial dilutions (from 10−1 to 10−7) to construct the standard curve49. RT-qPCR was performed using SYBR Premix Ex Taq (Takara) on a Roche 480 instrument using the following conditions: 95 °C for 30 s, then 40 cycles of 90 °C for 5 s, and finally 60 °C for 20 s, followed by melting curve analysis. ZIKV and Wolbachia copies in mosquito tissues were normalized using the mosquito rps6 gene. The genome copy numbers of Wolbachia were measured using previously reported50 primers (440F, 691R), and the primers used to measure ZIKV in RT-qPCR were newly designed and had the following sequences: NS1: forward 5′-GAGACGAGATGCGGTACAGG-3′, and reverse 5′-GGGGGAGTCAGGATGGTACT-3′; rps6: forward 5′-CGTCGTCAGGAACGTATTCG-3′, and reverse 5′-TCTTGGCAGCCTTGACAGC-3′.
Vector competence assay using oral infection
Mosquitoes were infected with ZIKV or DENV-2 through blood-feeding as previously described47. In brief, freshly propagated ZIKV or DENV-2 supernatant was mixed 1:1 with human blood, and then the mixture was added into glass feeders covered with pig intestine as a membrane. Glass feeders were connected to a water bath circulating system (Fisher) to keep the blood at 37 °C. Mosquitoes were allowed to feed on the mixture for 30–45 min. Only engorged mosquitoes were collected and maintained in standard rearing conditions, and were kept in a double cage system to prevent escape. ZIKV replication was determined by viral genome copy numbers in mosquito abdomens at 7 days post infection (dpi), and dissemination was measured by ZIKV infection status in one mosquito hind leg at 14 dpi. Total RNA of the dissected tissues was extracted, reverse-transcribed and quantified by RT-qPCR. To study ZIKV and DENV-2 horizontal transmission potential, at 14 dpi, saliva of each mosquito was collected by the forced salivation technique with modifications51. In brief, mosquitoes were anaesthetized with CO2, and their legs and wings were removed. The mosquito proboscis was inserted into a 10-µl pipette tip containing 6 µl FBS for 30 min at room temperature. A plaque assay was used to determine DENV-2 infection level in saliva. To quantify ZIKV genome copies in the saliva, RNA of 16 saliva samples from each mosquito line were extracted, reverse-transcribed and quantified by RT-qPCR. To determine the infectivity of the viruses from orally infected mosquitoes, 24 saliva samples from each mosquito line (in total 48 saliva samples) were immediately separately injected into 4–5-day-old adult female GUA mosquitoes. Each mosquito was injected with ~1 μl supernatant, and each individual saliva sample was injected into 4–6 mosquitoes. After seven days incubation under standard rearing conditions, the injected mosquitoes for each saliva sample were killed and their bodies were pooled, homogenized, and tested by RT-qPCR for ZIKV detection. Samples with positive results indicated that infectious ZIKV particles were present in the saliva that was originally used to inject the mosquitoes, suggesting that the corresponding mosquitoes from whom the saliva was originally taken had the potential to transmit ZIKV37.
Mosquito transmission assay using ZIKV-infected suckling mice
After propagation in C6/36 cells, ZIKV supernatant, with 107.4 viral genome copies per ml, was used to inject female GUA mosquitoes via thorax inoculation using a Nanoject II microinjector (Drummond). Ten days post-infection, 10 mosquitoes were allowed to bite 4 1-day-old suckling Kunming mice (KM) with each mouse receiving 3–4 bites. Mice were sex-matched and randomized for the experiment. During the 48–72 h post-biting during which the viraemia developed, each suckling mouse was used to feed and infect 6–8 day-old GUA and HC mosquitoes. For each mouse, HC was allowed to feed for 1 h and then removed, immediately followed by GUA for the same period of time. In total 4–6 engorged mosquitoes were collected from each suckling mouse. The engorged mosquitoes were kept under standard rearing conditions for 7 days, as described above. Then, total RNA was extracted from each mosquito whole body, followed by PCR with reverse transcription (RT–PCR) to check their ZIKV infection status.
ZIKV vertical-transmission assay
Thirty 3–4-day-old female GUA and HC mosquitoes were infected with ZIKV by intrathoracic inoculation using ZIKV culture supernatant, with 107.2 viral genome copies per ml. At 7, 14 and 21 dpi, mosquitoes were blood-fed, and eggs were collected 3 days after each blood meal. After egg hatch, fourth-instar larvae from each gonotrophic cycle were collected, and five larvae were pooled for RNA extraction. ZIKV infection status was evaluated by RT–PCR. For the first and second gonotrophic cycles, 10 pools were collected; for the third gonotrophic cycle, 15 pools were collected.
Laboratory cage male mating competitiveness assays
Four adult cages were prepared with fifty GUA males and fifty females. Varying numbers of HC males (0, 50, 250 or 500) were released into the cages, so that the ratio of GUA females:GUA males:HC males was 1:1:0, 1:1:1, 1:1:5 or 1:1:10. Mosquitoes were allowed to mate for two days. The mosquitoes were then blood-fed for approximately 20 min. Two days after blood-feeding, egg cups were inserted into the cages for collecting eggs. Eggs were collected for two nights, and the egg hatch rate was then determined as described in the cytoplasmic incompatibility cross experiment. The egg hatch rate was compared to the expected hatch rate assuming: (i) random mating and equal mating competitiveness between HC and GUA males, and (ii) complete unidirectional cytoplasmic incompatibility between HC males and GUA females.
Population replacement in laboratory cages
The population cage experimental design was as previously described22,52. Each population cage started with 50 GUA females and 50 GUA male adults. Three days after cage establishment, cages were provided with mice for blood-feeding, followed by the release of blood-fed HC females into the cages. The number (3, 7, 13 and 33) of HC females introduced into each cage was varied to produce an initial female infection frequency of 6% 12%, 20% and 40%, respectively. No additional HC females were released. To promote population replacement in the release cages with 6% and 12% initial female infection frequency, a single release of 200 HC males was used at the start of the experiment to induce cytoplasmic incompatibility and suppress viable progeny production by GUA females. An uninfected control GUA population cage was set up without any additional introductions of mosquitoes. Oviposition sites were provided in population cages two days post-blood meal. Eggs were collected for two consecutive nights, matured for an additional 5–7 days, and then hatched. All hatched larvae were reared to adults, and 50 females and 50 males were randomly selected to establish the next generation. After eggs were collected at each generation, approximately 10–20 females were randomly selected in each cage and examined for wPip infection by PCR to determine female infection frequency.
Semi-field cage population suppression experiments
Each of the six semi-field cages (1.75 × 1.75 × 1.75 m, 5.36 m3, Live Monarch) were set up with: (i) 1 plastic container filled with 300 ml deionized water and lined with filter paper for oviposition; (ii) 1 plastic cylinder filled with 200 ml deionized water for holding larvae, which was covered with a plastic board to prevent oviposition (the plastic board was removed every day to release newly emerged adults and then replaced); and (iii) 2 plastic cups each with a piece of filter paper and filled with 80 ml sugar solution (10%) (the sugar feeders were changed twice a week). The environmental conditions were 25.0 ± 0.5 °C, and 36.0 ± 6.0% RH, measured by means of 3 data loggers (HoBo) located on the top of 3 randomly selected cages.
To establish GUA populations in the treatment and control cages, 200 third-instar GUA larvae were transferred to the plastic cylinder containers in each cage weekly, from week 0 to week 4. From week 3, females were fed on a sausage filled with pig blood (60–70 ml per sausage) placed on the top of the cages 3 times a week. From weeks 5 to 12, the GUA populations were maintained by returning 150 third-instar larval offspring into each cage every week. Eggs laid in each cage were collected twice a week, counted, dried, stored in a plastic bag in the climate-controlled room (25 ± 1.0 °C, 60 ± 10% RH), and then hatched after being left to mature for 7–12 days. The hatch rates of eggs from each cage were recorded each week. To monitor the population dynamics inside the experimental cages, adults were randomly collected using aspirators placed in the centre of each cage for 10 min each week. After immobilization at 4 °C, the captured adults were counted and sexed, and then returned to their respective cages.
Starting from week 12, 375 HC male adults were released weekly into the treatment cages, representing a 5:1 (375 HC males: 75 GUA males) initial release ratio of HC to GUA males based on the assumption that 75 fertile GUA males would eclose from the 150 larvae introduced weekly into the target populations. To mimic a 2% female contamination rate, which could happen during mass rearing owing to lack of perfect sex-separation, eight HC female adults were released together, each time, with the HC males.
Before release, both HC male and female pupae were irradiated at 28 Gy, which is a dose known to effectively sterilize females but to not negatively affect male mating performance24. HC pupae were collected and placed in the centre of a plastic plate, which was placed in the middle of the irradiation cylinder. Irradiation was performed with 4.2 s transit time at the dose rate 2.144 Gy/s by a Gammacell irradiator 220 (Atomic Energy of Canada). Irradiated pupae were placed into a plastic cage in the climate-controlled room for emergence. The release frequency was twice a week with a 48-h interval. HC males were 3–4 or 5–6 days old during the first and second releases, respectively. Each time, either 188 or 187 HC males were released into each treatment cage, resulting in a total of 375 HC males per week per cage.
Starting from week 13 (week 1 post-release), the number of GUA larvae returned to each treatment cage was adjusted to reflect the effect of HC male releases on the mosquito population. To maintain a stable population, 150 larvae were returned to each control cage every week. The number of larvae returned to each HC treatment cage was calculated to reflect the level of population suppression as determined by egg hatch rate in the treatment cage in relation to the control cage in the previous week. For example, if the egg hatch rate in week 15 was 80% and 50% in a control and treatment cages, respectively, then 94 larvae (150 × 0.5/0.8) were returned to the treatment cage in week 17.
To assess whether combined IIT–SIT can prevent population replacement caused by HC female contamination, from week 19 (week 7 post-release), the larvae in excess of those that had been returned to the experimental cages were randomly sampled each week to examine wPip infection. Each time, up to 300 larvae were tested for wPip infection (all larvae were examined if there were <300 larvae). Larvae were tested in groups of 10 or 20 pooled larvae if numbers fell between 30 and 300, and larvae were tested individually if there were less than 30.
Mass-production and irradiation of HC males
Mass-production of HC males included five steps: adult rearing, larvae rearing, sex separation, X-ray irradiation and packaging according to a protocol described previously with slight modifications39,40,53. Approximately 3,000 female pupae and 1,000 male pupae (3:1 ratio of female to male) were placed into an adult cage (stainless steel, 30 × 30 × 30 cm) for eclosion40. Adults were continuously provided with 10% sugar solution. Sheep blood, provided weekly by a local abattoir, was mixed with ATP (500 mg ATP in 100 ml blood) and then used to feed females at 5–6 and 9–10 days old. Two days after blood-feeding, mosquitoes were provided oviposition sites to collect eggs for two days. After their eggs were collected twice, mosquitoes were euthanized by putting adult cages in a freezer at −20 °C overnight. Eggs were matured for one week before hatching in water. After hatching, 6,600 larvae were added to each tray (length × width × height = 58 cm × 38 cm × 4 cm) filled with water at a depth of 1.5 cm39. Larvae were fed daily with food containing 60% liver powder, 30% shrimp powder and 10% yeast for six days. No larval food was added when larvae started to develop into pupae at day 7. At day 8, pupae mixed with a few larvae were collected and then went through a Fay–Morlan sorter to separate male pupae from female pupae and larvae54. Before 2016, approximately 1,000 male pupae were transferred to each plastic ‘release’ bucket (17-cm diameter × 17-cm height), which contained water (1-cm depth) at the bottom and had a lid with a large hole covered on top by mesh gauze to allow for air exchange and prevent the escape of adult mosquitoes as they eclosed. Cotton soaked in 10% sugar solution was placed on top of the gauze, when pupae started to eclose into adults 24 h after transfer into buckets. After one day, water was removed through the gauze by turning the bucket upside down. After the mosquitoes were immobilized at 5 °C, a manual visual check was used to individually remove any residual females mixed in with the adult males. The quality-controlled males were then transported to the field for release. Starting from 2016, the male pupae collected through mechanical sorters were exposed to irradiation at 45 Gy for 1,000 s to sterilize any residual females using an X-ray irradiator (Wolbaki) developed specifically to treat mosquito pupae. Approximately 65,000 to 75,000 pupae were placed together in a canister (diameter 7.5 cm × height 7.5 cm), with 2 canisters being simultaneously irradiated. After treatment, pupae were packaged into buckets for release, as described above, but without the manual check of adults for contaminant females.
Control of female contamination in released HC males
As a key quality control in the laboratory during mass rearing, the female contamination rate (FCR) was monitored at both pupal and adult stages. Each batch of sex-sorted pupae was checked by randomly selecting 4,000 of the pupae, and manually sexing each individual by microscopic examination of their terminalia55. The batch of pupae sampled qualified for release if the FCR was below 1%. If the FCR was over 1%, mechanical sex separation and manual screening of the batch of pupae was repeated until the FCR was less than 1%. This resulted in an average rate of <0.5% contaminant females present in the pupae, which—before 2016—was further reduced by manually removing females after they eclosed into adults (as described above). In 2016 and onwards, following irradiation and packaging, we randomly selected 10% of the release buckets for checking adults to record the FCR, which was less than 0.3% in both 2016 and 2017. To further monitor the risk of female contamination in inducing population replacement in the field, female adults were also collected weekly from the release sites and assayed for wPip infection by PCR. The female-positive rates from the laboratory and field were compared to test for a correlation between these variables. In addition, larvae hatched from ovitraps collected weekly from the release sites were used to assay wPip infection, to monitor whether contaminant females had produced offspring in the field.
Description of study areas
With an approximately 3.3 km2 area, Shazai island (22° 51′ 31.99″ N, 113° 32′ 40.51″ E) is located in the Nansha District, Guangzhou City, China. There is a human population of 1,865 individuals across 505 houses in its only residential area (25 ha), which was selected as release site 1 (Fig. 2a). An area with similar size and ecological conditions in Xiaohu island (22° 50′ 49.07″ N, 113° 31′ 37.54″ E), separated from Shazai by a bridge, was selected as its control site. Located in the Panyu District, Guangzhou, Dadaosha island (22° 54′ 56.39′′ N, 113° 25′ 43.84′′ E) is approximately 10.9 km2 in area. One of its residential areas (7.5 ha) with 350 people across 158 houses was selected as release site 2, and 2 nearby control sites were located either on the same island or Guanlong island (22° 54′ 53.03′′ N, 113° 26′ 48.63′′ E) (Fig. 2b). Separated by rivers, these study areas are relatively isolated, with evidence of passive mosquito dispersal along human transportation networks, either through terrestrial or marine vehicles. The local dengue transmission in Guangzhou occurs from the middle of August to the end of November with A. albopictus as the only vector. As a typical subtropical area, Guangzhou has a mosquito season from March to November, with a peak from June to September and almost no mosquitoes detected from December to February.
Pre-release monitoring of release and control sites
Prior to the release of HC males, A. albopictus populations were monitored weekly using approximately 100 ovitraps (Tianpai) for release site 1 and its associated control site between June and November 2014, and 24 or 25 ovitraps for release site 2 and one of its control sites (site 2a) between May and November 2015. With the control site 2b added in 2016, data from control sites 2a and 2b were combined for their comparison to release site 2. The ovitraps contained a piece of filter paper (10 × 6 cm) for collecting eggs, and 50 ml water previously infused with bamboo leaves for attracting females to oviposit. Every week, ovitraps were placed in the field for 7 days, and then collected and placed in an incubator (Yiheng) for 6 days at 27 ± 1 °C, 80 ± 10% RH, and a photoperiod of 12:12 h (light: dark). The number of ovitraps that contained eggs was recorded, and the number of hatched eggs was determined by visual examination using a stereomicroscope (Olympus). The proportion of positive ovitraps was calculated as the number of ovitraps containing eggs divided by the total number of ovitraps used, and the egg hatch rate per trap was calculated as the number of hatched eggs divided by the total number of eggs collected.
Field release of HC mosquitoes
Mosquitoes were transported from the mass-rearing factory to the release sites by a van three times per week (Tuesday, Thursday and Saturday), and on the next day released in the morning between 07:00 and 10:00. The mass-rearing factory was approximately 1 h driving time from the release sites. Cotton soaked with 10% sugar solution was continuously supplied to the adults before release. Mosquitoes were released every 50 m with approximately 100 and 40 fixed release spots in release sites 1 and 2, respectively, preferably near vegetation (for example, trees or underbrush). During release, buckets were opened by removing the mesh and all mosquitoes immediately flew away. The number of buckets for release in each spot was adjusted empirically based on the recently determined HC-to-wild-type male ratio in each zone, with the goal of reaching the 5:1 target ratio. On average, between 1.5 and 2.6 million HC males were released weekly, making for a total of 52.7 and 92.6 million males released overall at site 1 in 2016 and 2017, respectively. In release site 2, on average between 600,000 and 890,000 males were released weekly, such that a total of 19.7 and 32.1 million males released overall in 2016 and 2017, respectively.
Monitoring population suppression
A. albopictus populations in all control and release sites were monitored weekly, throughout the period of HC male release, using ovitraps and BG-Sentinel traps (Biogents). Release sites 1 and 2 were divided into 22 and 8 zones, respectively, to precisely monitor mosquito density and dynamics (Fig. 3c, d). Mosquito releases occurred in all the zones, except that a rolling-carpet approach was used, owing to initial restrictions in male production, to limit releases to 6 zones in the north end of release site 1 on 16 June 2015, and then gradually expanded to the neighbouring zones until all zones were covered from 11 July 2015 to 20 October 2015. To monitor the mosquito population, on average 5 ovitraps and 2 BG traps were used per zone, with a total of 110 ovitraps and 44 BG traps distributed in release site 1, and 40 ovitraps and 16 BG traps in release site 2 (the locations are shown on the map in Extended Data Fig. 5). There were 100 ovitraps and 30 BG traps distributed in control site 1, and 30 ovitraps and 12 BG traps distributed in each of the two control sites for release site 2. All the ovitraps and BG traps were labelled with specific numbers, corresponding to their locations, to enable sample locations of collected mosquitoes to be tracked.
Seven days after being left in the field, ovitraps were collected and brought back to the laboratory. The total number of ovitraps collected, those with eggs present and the number of eggs in each positive ovitrap were recorded. The positive ovitraps were incubated for six days in an incubator as described above. The hatched eggs were counted and recorded under a dissecting microscope. The average number of hatched eggs per ovitrap, in both release and control sites, was determined each week, and used to measure population suppression at immature (egg and larval) stages.
BG traps were continuously run for 24 h every Monday, 48 h after the last release, in both release and control sites. The captured adults were sent to the laboratory, and were put into a freezer for at least 30 min at −20 °C before further characterization. Then, A. albopictus were identified, sexed and the number of males and females in each trap was counted and recorded. All collected females were assayed for wPip infection by PCR. After wPip-positive females were removed, the average number of females in both release and control sites per BG trap was determined each week, and used to measure population suppression at the adult stage.
To determine the ratio of released HC to wild-type males, collected males were tested for wPip infection using PCR. The following criteria were used to determine the size of the tested samples: (i) all the collected A. albopictus males were individually assayed for wPip infection if the total number of males collected in a release site was less than 300, or the number of males in an individual trap was less than 20; (ii) 50% of collected males were assayed when the total number of collected males was between 300 to 1000; (iii) 33.3% of collected males were assayed when the total number of collected males was more than 1,000. For each week, the ratio of released HC to wild-type males was then calculated by dividing the number of wPip-positive males by the number of wPip-negative males.
Although the proportion of egg-positive ovitraps and total number of eggs per trap are useful and important measures of overall relative population size, they do not distinguish between the dead and viable eggs present in the ovitrap, and thus are incapable of indicating the direct reduction in the number of viable eggs caused by cytoplasmic incompatibility matings. Consequently, the number of hatched eggs was the main parameter used to compare the level of population suppression at the larval stage between release and control sites. However, it should be noted that hatched eggs could be produced by wild females that migrate into the area after mating outside it, as well as by resident females emerging within the site that mate with wild males.
Population suppression was also estimated by changes in the number of adult females collected in BG traps. This will produce an overestimate of the effective population size contributing to future generations because many of the females will have been mated by released HC males and therefore contribute no offspring to the next generation. Adult counts included only wPip-negative females.
We expressed population suppression at release sites relative to control sites (that is, in terms of the per cent reduction in numbers of hatched eggs and adult females collected at the release sites relative to the numbers observed at control sites) (Fig. 2d, e, 3a, b, Extended Data Figs. 4, 6). We used the averaged observations from its two control sites for release site 2 and used only one site as a control at site 1.
Mating competitiveness and expected hatch rate
To estimate mating competitiveness of the released HC males in the field, the expected egg hatch rate (He) in the release site was determined based on the weekly ratio (N) of HC to wild-type males, and the egg hatch rate (Hc) in the control site in the same week, assuming complete unidirectional cytoplasmic incompatibility between HC males and wild-type females, equal mating competitiveness between HC and wild-type males, and random mating. As a result, the following equation was used to calculate the expected egg hatch rate, He = Hc × [1/(N + 1)]. The observed egg hatch rate was then compared to the expected hatch rate in the same week, with the difference between these rates taken to reflect relative mating competitiveness.
PCR assay of wPip infection
Each individual adult or pool of larvae from an ovitrap were homogenized in DNA extraction buffer (Daan Gene). After a brief centrifugation, samples were incubated at 99 °C for 10 min, and the supernatant was then used as template for PCR assay. A 20 μl RT-qPCR reaction consisted of 2 μl DNA template, 15 μl A buffer (containing primers, fluorescent probe and dNTPs) and 3 μl B buffer (containing DNA polymerase). In brief, RT-qPCR reaction was performed according to the procedures of Wolbachia wPip Detection Kit (Wolbaki). The specific-primers used for the assay were designed for phage WO of wPip and consisted of: orf7F: 5′-GTTTGTGCAGCTAATAG-3′; and orf7R: 5′-GTCTGCAAGGCCTATTTCTACTG-3′; and the sequence for the fluorescent probe was 5′-CTTTCAATTGAAAAGATTCGATCAAC-3′. The RT-qPCR conditions comprised of 15 min at 95 °C, followed by 40 cycles of 15 s at 95 °C, 45 s at 55 °C, 30 s at 72 °C, and finally 30 s in 1 °C steps from 60 to 95 °C to generate the melting curve for confirmation that the fluorescence detected was for the specific PCR product.
If wPip-positive larvae were observed, the sample was further screened by standard PCR using primers specific to the ribosomal protein S6 (rps6) gene of C. quinquefasciatus, the only possible mosquito species with wPip co-occurring in the field sites, to exclude any false positives resulting from the collection of Culex larvae in the ovitraps. The specific-primers used for the assay were designed for rps6 gene and consisted of: rps6F: 5′-TGCCGCGTCGTCTTGAATC-3′; and rps6R: 5′-GTATTGACCTCGTCGCGCTT-3′. The 20 μl PCR reaction consisted of 2 μl DNA template, 10 μl PCR Master Mix (Dongsheng), 1 μl of each primer (10 μM) and 6 μl ddH2O. The PCR conditions comprised of 5 min at 98 °C, followed by 40 cycles of 30 s at 98 °C, 5 s at 55 °C, 30 s at 72 °C, and then 10 min at 72 °C for the final extension. PCR products were electrophoresed on a 1.5% agarose gel, which contained 1 μg/ml ethidium bromide. If a product size of approximately 350 bp was obtained, the sample was considered to contain wPip derived from Culex mosquitoes.
Community engagement
Before the open field releases of HC commenced, meetings and seminars involving various stakeholders (for example, public health officials, scientific experts and the general public) were held to introduce the principle, efficacy and biosafety of A. albopictus population suppression through release of HC males to induce incompatible matings. We then received a permit authorizing field trials from the Ministry of Agriculture of the People's Republic of China and declarations of support from the different administrative levels of local government (that is, district, town and village). A series of community engagement activities were launched, including organizing meetings with village representatives, visiting households and distributing basic information on mosquitoes and mosquito-borne diseases, as well as the aims and methods of our project. During the course of these activities, we answered questions or concerns raised by the residents. A questionnaire survey led by village officials was also undertaken in each household. Signed informed consent was obtained from residents who had agreed to HC release, granting us permission to perform necessary activities around their residences, including releasing mosquitoes and placing monitoring tools (that is, traps) near their houses. Among 506 households in Shazai, 455 households were contacted and informed consent, to release HC mosquitoes in or around their property, was obtained from 453 (99.6%) households. In Dadaosha, informed consent was obtained from all the 141 contacted households. No mosquitoes were released at households (or around their neighbouring residences) that did not consent to the release of HC mosquitoes.
During the release period, we maintained communication with the different administrative levels of local government, updating them on the status of the project, and informing them of preliminary and ongoing results as they became available. In the release sites, we maintained a close relationship with the residents through performing house-to-house surveys twice per week, in which their feedback was sought regarding the mosquito releases. Regular information on the level of mosquito population suppression was provided to those households if concerns were raised. In addition, we kept the public informed and updated with the progress of the project through posters, newsletters, radio broadcasts, print and TV news media, and the mobile phone app WeChat. All these activities ensured that the residents understood, were satisfied with and supported the continued release of mosquitoes.
Community surveys were conducted in release site 1 to investigate if residents shifted their opinion about the field trial before and after release. Residents were randomly selected and interviewed to determine whether they either supported, rejected, or were neutral about the release of HC males. Specific reasons for their views were also sought.
Mosquito human-landing assay
Human-landing catches were performed at both release sites and their associated control sites, according to a protocol approved by the Ethics Committee on Medical Research of the Zhongshan School of Medicine, Sun Yat-sen University. Sixteen localities were selected in each of Shazai and Xiaohu, and ten localities were selected in each of the Dadaosha release and control sites. All the selected localities were close to houses, in shaded and sheltered areas (that is, locations where A. albopictus is most likely to be found), and near the release locations. The experiments were conducted between 09:00 and 11:00 or between 16:00 and 18:00. Researchers worked in pairs, by standing at localities for 15 min, and collecting mosquitoes from the other person. The same pairs of researchers monitored mosquitoes in both release and control sites to reduce variation in the attractiveness of different individuals to mosquitoes. During the collection periods, mosquitoes that landed or flew around the volunteers were manually captured by mosquito aspirators. All captured mosquitoes were marked with time, date and location of collection, and sent back to the laboratory for species and sex identification, and further investigation. The procedure was performed four times, in both Shazai and Dadaosha and their associated control sites, from July to November 2017. The mosquito biting index was calculated as the average number of A. albopictus females caught per person in 15 min.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (v.5.00). ANOVA and Tukey’s multiple comparisons test were used to compare egg hatching in cytoplasmic incompatibility cross experiment. Differences in mosquito infective rates were analysed using Fisher’s exact test. Pearson’s correlations were used to test for an association in mosquito numbers between release and control sites before suppression. Mann–Whitney tests were undertaken to compare Wolbachia density in ovaries, the number of wPip-positive females within different release years, and mosquito density between release and control sites, including the proportion of egg-positive ovitraps, the average total number of eggs per ovitrap, the number and proportion of eggs hatching per ovitrap, as well as total number of female adults per trap per 24 h. To compare within each year these measures between respective control and release sites, we first calculated their average values for all traps per week, and then compared separately for each year these weekly averages between the control and release sites using Mann–Whitney tests. Binomial test was used to compare the expected and observed egg hatch in mating competitiveness assay in laboratory cage populations, and paired t-tests after arcsine transformation were used to compare observed and expected egg hatch rates in the field, and female contamination rate in the laboratory and field. χ2 test was computed to compare community support and paired t-test was used to compare mosquito biting before and after the release period.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this paper.
Data availability
Source Data for the main and Extended Data figures are provided in the online version of this paper. Any other relevant data are available from the corresponding authors upon reasonable request.
References
Dyck, V. A., Hendrichs, J. & Robinson, A. S. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management (Springer Netherlands, 2005).
Dame, D. A., Curtis, C. F., Benedict, M. Q., Robinson, A. S. & Knols, B. G. Historical applications of induced sterilisation in field populations of mosquitoes. Malar. J. 8 (Suppl 2), S2 (2009).
Helinski, M. E., Parker, A. G. & Knols, B. G. Radiation biology of mosquitoes. Malar. J. 8 (Suppl 2), S6 (2009).
Lees, R. S., Gilles, J. R., Hendrichs, J., Vreysen, M. J. & Bourtzis, K. Back to the future: the sterile insect technique against mosquito disease vectors. Curr. Opin. Insect Sci. 10, 156–162 (2015).
Laven, H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature 216, 383–384 (1967).
LePage, D. P. et al. Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature 543, 243–247 (2017).
Yen, J. H. & Barr, A. R. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens L. Nature 232, 657–658 (1971).
Chambers, E. W., Hapairai, L., Peel, B. A., Bossin, H. & Dobson, S. L. Male mating competitiveness of a Wolbachia-introgressed Aedes polynesiensis strain under semi-field conditions. PLoS Negl. Trop. Dis. 5, e1271 (2011).
Zhang, D., Zheng, X., Xi, Z., Bourtzis, K. & Gilles, J. R. Combining the sterile insect technique with the incompatible insect technique: I impact of Wolbachia infection on the fitness of triple- and double-infected strains of Aedes albopictus. PLoS ONE 10, e0121126 (2015).
Atyame, C. M. et al. Comparison of irradiation and Wolbachia based approaches for sterile-male strategies targeting Aedes albopictus. PLoS ONE 11, e0146834 (2016).
Curtis, C. F. et al. A field trial on control of Culex quinquefasciatus by release of males of a strain integrating cytoplasmic incompatibility and a translocation. Entomol. Exp. Appl. 31, 181–190 (1982).
Dobson, S. L., Fox, C. W. & Jiggins, F. M. The effect of Wolbachia-induced cytoplasmic incompatibility on host population size in natural and manipulated systems. Proc. R. Soc. Lond. B 269, 437–445 (2002).
Pal, R. in The Use of Genetics in Insect Control (eds Pal, R. & Whitten, M. J.) 73–95 (Elsevier, 1974).
Curtis, C. F. Testing systems for the genetic control of mosquitoes. In Proceedings of XV International Congress of Entomology (eds White, D. & Packer, J. S.) 106–116 (1976)
Arunachalam, N. & Curtis, C. F. Integration of radiation with cytoplasmic incompatibility for genetic control in the Culex pipiens complex (Diptera: Culicidae). J. Med. Entomol. 22, 648–653 (1985).
Sharma, V. P., Subbarao, S. K., Adak, T. & Razdan, R. K. Integration of gamma irradiation and cytoplasmic incompatibility in Culex pipiens fatigans (Diptera: Culicidae). J. Med. Entomol. 15, 155–156 (1979).
Curtis, C. F. & Shahid, M. A. Radiation sterilization and cytoplasmic incompatibility in a “tropicalized” strain of the Culex pipiens complex (Diptera: Culicidae). J. Med. Entomol. 24, 273–274 (1987).
Mains, J. W., Brelsfoard, C. L., Rose, R. I. & Dobson, S. L. Female adult Aedes albopictus suppression by Wolbachia-infected male mosquitoes. Sci. Rep. 6, 33846 (2016).
Atyame, C. M. et al. Cytoplasmic incompatibility as a means of controlling Culex pipiens quinquefasciatus mosquito in the islands of the south-western Indian Ocean. PLoS Negl. Trop. Dis. 5, e1440 (2011).
Brelsfoard, C. L., Séchan, Y. & Dobson, S. L. Interspecific hybridization yields strategy for South Pacific filariasis vector elimination. PLoS Negl. Trop. Dis. 2, e129 (2008).
Xi, Z., Dean, J. L., Khoo, C. & Dobson, S. L. Generation of a novel Wolbachia infection in Aedes albopictus (Asian tiger mosquito) via embryonic microinjection. Insect Biochem. Mol. Biol. 35, 903–910 (2005).
Xi, Z., Khoo, C. C. & Dobson, S. L. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310, 326–328 (2005).
Brelsfoard, C. L., St Clair, W. & Dobson, S. L. Integration of irradiation with cytoplasmic incompatibility to facilitate a lymphatic filariasis vector elimination approach. Parasit. Vectors 2, 38 (2009).
Zhang, D., Lees, R. S., Xi, Z., Gilles, J. R. & Bourtzis, K. Combining the sterile insect technique with Wolbachia-based approaches: II a safer approach to Aedes albopictus population suppression programmes, designed to minimize the consequences of inadvertent female release. PLoS ONE 10, e0135194 (2015).
Zhang, D., Lees, R. S., Xi, Z., Bourtzis, K. & Gilles, J. R. Combining the sterile insect technique with the incompatible insect technique: III robust mating competitiveness of irradiated triple Wolbachia-infected Aedes albopictus males under semi-field conditions. PLoS ONE 11, e0151864 (2016).
Bourtzis, K. et al. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop. 132 (Suppl), S150–S163 (2014).
Fonseca, D. M. et al. Area-wide management of Aedes albopictus. Part 2: gauging the efficacy of traditional integrated pest control measures against urban container mosquitoes. Pest Manag. Sci. 69, 1351–1361 (2013).
Unlu, I., Farajollahi, A., Strickman, D. & Fonseca, D. M. Crouching tiger, hidden trouble: urban sources of Aedes albopictus (Diptera: Culicidae) refractory to source-reduction. PLoS ONE 8, e77999 (2013).
Xi, Z., Khoo, C. C. & Dobson, S. L. Interspecific transfer of Wolbachia into the mosquito disease vector Aedes albopictus. Proc. R. Soc. Lond. B 273, 1317–1322 (2006).
Fu, Y., Gavotte, L., Mercer, D. R. & Dobson, S. L. Artificial triple Wolbachia infection in Aedes albopictus yields a new pattern of unidirectional cytoplasmic incompatibility. Appl. Environ. Microbiol. 76, 5887–5891 (2010).
Blagrove, M. S., Arias-Goeta, C., Failloux, A. B. & Sinkins, S. P. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc. Natl Acad. Sci. USA 109, 255–260 (2012).
Suh, E., Mercer, D. R., Fu, Y. & Dobson, S. L. Pathogenicity of life-shortening Wolbachia in Aedes albopictus after transfer from Drosophila melanogaster. Appl. Environ. Microbiol. 75, 7783–7788 (2009).
Calvitti, M., Moretti, R., Lampazzi, E., Bellini, R. & Dobson, S. L. Characterization of a new Aedes albopictus (Diptera: Culicidae)–Wolbachia pipientis (Rickettsiales: Rickettsiaceae) symbiotic association generated by artificial transfer of the wPip strain from Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 47, 179–187 (2010).
Laven, H. & Aslamkhan, M. Control of Culex pipiens pipiens and C. p. fatigans with integrated genetical systems. Pak. J. Sci. 22, 303–312 (1970).
Bian, G., Xu, Y., Lu, P., Xie, Y. & Xi, Z. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog. 6, e1000833 (2010).
Moreira, L. A. et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139, 1268–1278 (2009).
Dutra, H. L. et al. Wolbachia blocks currently circulating Zika virus isolates in Brazilian Aedes aegypti mosquitoes. Cell Host Microbe 19, 771–774 (2016).
Aliota, M. T., Peinado, S. A., Velez, I. D. & Osorio, J. E. The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci. Rep. 6, 28792 (2016).
Zhang, D. et al. Establishment of a medium-scale mosquito facility: optimization of the larval mass-rearing unit for Aedes albopictus (Diptera: Culicidae). Parasit. Vectors 10, 569 (2017).
Zhang, D. et al. Establishment of a medium-scale mosquito facility: tests on mass production cages for Aedes albopictus (Diptera: Culicidae). Parasit. Vectors 11, 189 (2018).
Li, Y. et al. Comparative evaluation of the efficiency of the BG-Sentinel trap, CDC light trap and Mosquito-oviposition trap for the surveillance of vector mosquitoes. Parasit. Vectors 9, 446 (2016).
Halasa, Y. A. et al. Quantifying the impact of mosquitoes on quality of life and enjoyment of yard and porch activities in New Jersey. PLoS ONE 9, e89221 (2014).
Schmidt, T. L. et al. Genome-wide SNPs reveal the drivers of gene flow in an urban population of the Asian tiger mosquito, Aedes albopictus. PLoS Negl. Trop. Dis. 11, e0006009 (2017).
Sanogo, Y. O. & Dobson, S. L. Molecular discrimination of Wolbachia in the Culex pipiens complex: evidence for variable bacteriophage hyperparasitism. Insect Mol. Biol. 13, 365–369 (2004).
Zhou, W., Rousset, F. & O’Neil, S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. B 265, 509–515 (1998).
Heddi, A., Grenier, A. M., Khatchadourian, C., Charles, H. & Nardon, P. Four intracellular genomes direct weevil biology: nuclear, mitochondrial, principal endosymbiont, and Wolbachia. Proc. Natl Acad. Sci. USA 96, 6814–6819 (1999).
Das, S., Garver, L., Ramirez, J. R., Xi, Z. & Dimopoulos, G. Protocol for dengue infections in mosquitoes (A. aegypti) and infection phenotype determination. J. Vis. Exp. (5), PMC2557096 (2007).
Lu, P., Bian, G., Pan, X. & Xi, Z. Wolbachia induces density-dependent inhibition to dengue virus in mosquito cells. PLoS Negl. Trop. Dis. 6, e1754 (2012).
Zhang, M. et al. Quantitative analysis of replication and tropisms of Dengue virus type 2 in Aedes albopictus. Am. J. Trop. Med. Hyg. 83, 700–707 (2010).
McGraw, E. A., Merritt, D. J., Droller, J. N. & O’Neill, S. L. Wolbachia-mediated sperm modification is dependent on the host genotype in Drosophila. Proc. R. Soc. Lond. B 268, 2565–2570 (2001).
Bian, G., Zhou, G., Lu, P. & Xi, Z. Replacing a native Wolbachia with a novel strain results in an increase in endosymbiont load and resistance to dengue virus in a mosquito vector. PLoS Negl. Trop. Dis. 7, e2250 (2013).
Bian, G. et al. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science 340, 748–751 (2013).
Carvalho, D. O. et al. Mass production of genetically modified Aedes aegypti for field releases in Brazil. J. Vis. Exp. 3579, e3579 (2014). 10.3791/3579
Focks, D. A. An improved separator for the developmental stages, sexes, and species of mosquitoes (Diptera: Culicidae). J. Med. Entomol. 17, 567–568 (1980).
Methods in Anopheles research. Malaria Research and Reference Reagent Resource Center http://www.mr4.org/Publications/MethodsinAnophelesResearch.aspx (2014).
Fried, M. Determination of sterile-insect competitiveness. J. Econ. Entomol. 64, 869–872 (1971).
Acknowledgements
This work was supported by Guangdong Innovative Research Team Program (No. 2011S009), Scientific and Technological Leading Talents of Guangzhou Development District (No. 2013L-P116), Science and Technology Planning Project of Guangdong Province (2016A020251001), a grant from the Foundation for the NIH through the Grand Challenges in Global Health Initiative of the Bill and Melinda Gates Foundation, the joint Food and Agricultural Organization (FAO) of the United Nations and International Atomic Energy Agency (IAEA) Division of Nuclear Techniques in Food and Agriculture and the IAEA Department of Technical Cooperation (RAS5066, RAS5082, D42016 and D44002), the 111 Project (grant no. B12003), Key Project of NNSF of China (11631005), China Postdoctoral Innovation Program (BX20180394), and a grant-in-aid for joint research (2017-AH-04) from the NJAU-MSU Asia-Hub Project. A.A.H. was supported by an NHMRC Fellowship. We thank X. Zhou, S. O’Neill, S. L. Dobson, G. Bian and E. Walker for their support, suggestions and technical assistance.
Author information
Authors and Affiliations
Contributions
Z.X., X.Z., D.Z., Y. Li, C.Y., Y. Wu, A.G.P., J.R.L.G., K.B., Z.W., L.A.B. and A.A.H. developed the concept and methodology; D.Z. performed radiation and male mating-competitiveness assay; Y. Liang and C.Y. performed population suppression and population replacement in laboratory cages; Y. Li and X.Z. performed human-landing assay; C.Y. performed mosquito quality control; Y. Li, Y. Wu, X.L. and X.P. performed vector competence assays; A.G.P designed the X-ray irradiator; D.Z., K.B. and J.R.L.G. performed the population-suppression experiment in semi-field cages; X.Z., Z.Y., Y. Wu and J. Zhuang performed community engagement; X.L., X.P., Q.S., J.-T.G. and M.Z. performed cell culture, virus titration and Wolbachia density quantification; Z.Y., Zhigang Hu, Z.Z., L.L. and Q.L. identified the field sites; B.Z., L.H. M.T. and J.Y. developed the mathematical model and performed spatial analyses; X.W. and J. Zhu performed mosquito mass rearing; Y. Wei and W.Q. performed release and field surveillance; J. Zhu, W.Q., X.-Y.H., Zhiyong Hu and Z.W. performed coordination for the project; W.Q. obtained regulatory approvals for mosquito releases; J.L. performed mosquito crosses and maintenance of mosquito lines; J.B. and Z.X. performed cost-effectiveness analysis; Z.X. provided oversight of the project and contributed to all experimental designs, data analysis and data interpretation; Z.X., L.A.B., X.Z., D.Z., Y.L. and A.A.H. wrote the manuscript. All authors participated in manuscript editing and final approval.
Corresponding author
Ethics declarations
Competing interests
Y. Li, X.W., Y. Wei, J. Zhu, W.Q., J.L. and Z.X. are affiliated with Guangzhou Wolbaki Biotech Co., Ltd. This does not alter our adherence to all Nature policies.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Peer review information Nature thanks William Sullivan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Fig. 1 Illustration of the procedures used to establish A. albopictus HC line by embryonic microinjection and for PCR verification of the Wolbachia strains in HC.
a, The Wolbachia strain wPip from C. pipiens molestus (donor) was transinfected into the wAlbA/wAlbB superinfected HOU line of A. albopictus (recipient) by embryonic microinjection, to generate a mosquito line infected with three Wolbachia strains (HC). A circle containing a cross indicates that the line was discarded, and a tick indicates that the line was maintained. Red indicates wPip-positive, and white indicates wPip-negative individuals. b, Wolbachia infection status was verified by PCR in both male and female HC mosquitoes. Results indicate that HC contains both the native Wolbachia strains (wAlbA and wAlbB) and the new transinfected strain wPip. The experiments were repeated at least three times independently with similar results.
Extended Data Fig. 2 Inhibition of both horizontal and vertical transmission of Zika and dengue viruses in the A. albopictus HC line.
a, b, ZIKV (a) and DENV-2 (b) were significantly decreased in the saliva of HC compared to wild type, GUA and HOU, respectively. Fourteen days post-infection, mosquito saliva samples were collected, ZIKV copies were quantified by RT-qPCR, and the titre of DENV-2 was measured by plaque assay. Horizontal lines indicate the median value (two-sided Mann–Whitney test: ZIKV, n = 16 for both HC and GUA, P = 0.0049; DENV-2, n = 39 for HC and n = 36 for HOU, P < 0.0001). c, Experimental design to measure the horizontal transmission of ZIKV. d, Viral positive rate in mosquitoes at day 7 after feeding on Zika-infected suckling mice (two-sided Fisher’s exact test, n = 19 for GUA and 20 for HC, P = 0.047). e, Experimental design to measure the vertical transmission of ZIKV in mosquitoes. f, The minimum ZIKV vertical transmission rate in HC and GUA lines (two-sided Fisher’s exact test, n = 35 biologically independent samples, P = 0.004). g, h, ZIKV replication and dissemination in HC were both significantly decreased. Mosquitoes were infected with ZIKV by oral feeding. ZIKV replication was determined by viral genome copy numbers in mosquito abdomens at 7 dpi (n = 20), and dissemination was measured by ZIKV infection status in one mosquito hind leg at 14 dpi (n = 20). The observations showed that ZIKV replication (g) and dissemination (h) were both significantly inhibited in HC. The infection prevalence is shown as a percentage. Horizontal lines indicate the median number of viral copies (two-sided Mann–Whitney test: abdomen, P = 0.018; hind legs, P = 0.002).
Extended Data Fig. 3 Sensitivity analysis of the robustness of the 5:1 HC:GUA male release ratio to induce population suppression.
a–c, The mathematical model of the semi-field cage experiments shown in Fig. 1e, f and described in the Supplementary Information provides an accurate approximation to the semi-field data when r = 5 and one of three parameters listed in (5) in the Supplementary Information are varied across a wide range of values. a, R2 ∈ [0.9259, 0.9355] for ξ0 ∈ [0.6, 0.9]. b, R2 ∈ [0.9301, 0.9329] for μ ∈ [0.75, 0.95]. c, R2 ∈ [0.9325, 0.9573] for λ ∈ [0.5, 1]. d, The effect of mosquito migration and the efficiency of population suppression as measured by egg hatch rate. We fixed λ = 0.6, μ = 0.85, ξ0 = 0.80 and b0 = 75. The 5:1 ratio is sufficient to offset 20% migration with a suppression efficiency 92.20% as compared to 98.71% suppression efficiency without migration.
Extended Data Fig. 4 The proportion of egg-positive ovitraps, the average number of eggs per ovitrap, and the average percentage of eggs hatching per ovitrap in release and control sites before release of HC males.
a–c, Site 1: the proportion of egg-positive ovitraps (a), average number of eggs per ovitrap (b) and the average percentage egg hatch per ovitrap (c) in 2014, compared to the control site. d–f, Site 2: the proportion of egg-positive ovitraps (d), average number of eggs per ovitrap (e) and the average percentage egg hatch per ovitrap (f) in 2015, compared to the control site. The proportion of egg-positive ovitraps was calculated from the number of ovitraps with eggs divided by the total number of ovitraps used. The average number of eggs per ovitrap was calculated as the total number of eggs collected divided by the number of ovitraps used. The average percentage of eggs hatching per ovitrap was calculated as the mean of the percentage of hatched eggs per individual ovitrap for all the ovitraps that collected eggs. Data were collected weekly. The proportion of egg-positive ovitraps (two-sided Mann–Whitney test: site 1, n = 26, P = 0.591; site 2, n = 32, P = 0.3239), the average number of eggs per ovitrap (two-sided Mann–Whitney test: site 1, n = 26, P = 0.4516; site 2, n = 32, P = 0.6940), and the average percentage of eggs hatching per ovitrap (two-sided Mann–Whitney test: site 1, n = 26, P = 0.3186; site 2, n = 32, P = 0.8232) did not differ significantly between the control and release sites. In addition, there were significant and strong correlations across time between the release and their respective control sites for these three parameters, demonstrating similar temporal fluctuations in them: the proportion of egg-positive ovitraps (Pearson correlation: site 1, r = 0.88, n = 26, P < 0.0001; site 2, r = 0.85, n = 32, P < 0.0001), the average number of eggs per ovitrap (Pearson correlation: site 1, r = 0.77, n = 26, P < 0.0001; site 2, r = 0.96, n = 32, P < 0.0001), and the average percentage of eggs hatching per ovitrap (Pearson correlation: site 1, r = 0.67, n = 26, P = 0.0002; site 2, r = 0.70, n = 32, P < 0.0001).
Extended Data Fig. 5 Map of the ovitraps and BG traps distributed in the two release sites.
a, b, There were 110 ovitraps (grey circles) and 44 BG traps (blue circles) in release site 1 (a), and 40 ovitraps and 16 BG traps in release site 2 (b). Release site 1 was divided into 22 zones and release site 2 contained 8 zones. On average, there were five ovitraps and two BG traps in each zone, and collections from all traps were carried out weekly.
Extended Data Fig. 6 The proportion of egg-positive ovitraps, the average number of eggs per ovitrap, and the average percentage of eggs hatching per ovitrap in release and control sites after release of HC males.
a–c, Site 1: the proportion of egg-positive ovitraps (a), average number of eggs per ovitrap (b) and the average percentage egg hatch per ovitrap (c) in 2016 and 2017, compared to the control site. d–f, Site 2: the proportion of egg-positive ovitraps (d), average number of eggs per ovitrap (e) and the average percentage egg hatch per ovitrap (f) in 2016 and 2017, compared to the control site. Significant declines were observed for all three parameters in the two release sites compared to their control sites: the proportion of egg-positive ovitraps (two-sided Mann–Whitney test: site 1 2016, n = 36, P < 0.0001; site 2017, n = 35, P < 0.0001; site 2 2016, n = 32, P < 0.0001; site 2 2017, n = 35, P < 0.0001), the average number of eggs per ovitrap (two-sided Mann–Whitney test: site 1 2016, n = 36, P < 0.0001; site 1 2017, n = 35, P < 0.0001; site 2 2016, n = 32, P < 0.0001; site 2 2017, n = 35, P < 0.0001), and the average percentage of eggs hatching per ovitrap (two-sided Mann–Whitney test: site 1 2016, n = 36, P < 0.0001; site 1 2017, n = 35, P < 0.0001; site 2 2016, n = 32, P < 0.0001; site 2 2017, n = 35, P < 0.0001).
Extended Data Fig. 7 The total number of wPip-positive adult females collected monthly in release sites 1 and 2.
Females were collected weekly using BG traps and tested for wPip infection by PCR. The wPip-positive females were recorded monthly in site 1 and site 2 during the release period. No significant difference was observed in the number of wPip-positive females between 2015 (n = 3), 2016 (n = 7) and 2017 (n = 9) in site 1 (Kruskal–Wallis test, P = 0.6536), or between 2016 (n = 7) and 2017 (n = 9) in site 2 (two-sided Mann–Whitney test, P = 0.1164). No evidence of an increase in the number of wPip-positive females with time was apparent, but would have been expected if population replacement had started in the field.
Extended Data Fig. 8 Temporal and spatial distribution of wPip-positive ovitraps in the two release sites between 2015 and 2017.
a, b, Among 110 ovitraps in site 1 (a) and 40 ovitraps in site 2 (b), those from which wPip-positive larvae were detected are shown as red circles. The specific time points at which wPip-positive larvae were detected are also indicated (year.month). Overall, a total of 15 ovitraps with wPip-positive larvae were detected on 13 separate, spatially and/or temporally isolated, occasions in release site 1, whereas only one ovitrap with wPip-positive larvae was detected on a single occasion in release site 2. The first six of the ovitraps with wPip-positive larvae were detected in 2015, before the use of irradiation, while in 2017 only two were found in site 1 and none in site 2. c, Overall, wPip-positive rates of 0.9% (15/1,678 pooled larval samples taken weekly from individual ovitraps, referred to as ‘ovitrap weeks’) and 0.6% (1/166) were found during the release period in the 3 or 2 years of HC releases in sites 1 and 2, respectively. No evidence of an increase in the number of wPip-positive ovitraps with time was apparent, but would have been expected if population replacement had started in the field.
Extended Data Fig. 9 Induction of sterility in HC females after mating with irradiated HC males.
a, b, The effect of irradiating HC males on the egg hatch rate (a) and the level of induced sterility (b) in mated females. For each cross shown in the figure (x axis), a single treatment cage (30 × 30 × 30 cm) was set up containing males and females, at a 1:1 ratio, of the mosquito line with irradiation status indicated. The two control crosses (HC:HC and wild-type:wild-type) were set up with 100 individuals of each sex. All other treatment crosses used 300 individuals of each sex. IHC45Gy are HC males irradiated at the pupal stage with an X-ray dose of 45 Gy, as described in the Methods. None of the other mosquitoes used were irradiated (HC and GUA). Induced sterility was calculated as follows56: 100 − [(egg hatch rate of treatment cages)/(egg hatch rate of control cages) × 100]. Complete sterility (100%) was induced when wild-type females mated with either non-irradiated or irradiated HC males, whereas high levels of partial sterility (86.4%) were induced when HC females mated with IHC45Gy males, showing that irradiation causes sterility between the otherwise-compatible HC males and females. During HC release in the field, there is a high probability that HC females would mate with irradiated HC males owing to their high abundance relative to wild-type males.
Supplementary information
Supplementary Information
This PDF file contains Supplementary Methods, Supplementary Tables, Supplementary Discussion, and Supplementary Equations.
Rights and permissions
About this article
Cite this article
Zheng, X., Zhang, D., Li, Y. et al. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 572, 56–61 (2019). https://doi.org/10.1038/s41586-019-1407-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-019-1407-9
- Springer Nature Limited
This article is cited by
-
Identification and functional analysis of C-type lectin from mosquito Aedes albopictus in response to dengue virus infection
Parasites & Vectors (2024)
-
Response of the mosquito immune system and symbiotic bacteria to pathogen infection
Parasites & Vectors (2024)
-
Cellular sex throughout the organism underlies somatic sexual differentiation
Nature Communications (2024)
-
Computer numerical control knitting of high-resolution mosquito bite blocking textiles
Communications Engineering (2024)
-
Mass irradiation of adult Aedes mosquitoes using a coolable 3D printed canister
Scientific Reports (2024)