Abstract
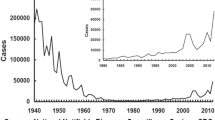
Pertussis, which is caused by Bordetella pertussis, has plagued humans for at least 800 years, is highly infectious and can be fatal in the unvaccinated, especially very young infants. Although the rollout of whole-cell pertussis (wP) vaccines in the 1940s and 1950s was associated with a drastic drop in incidence, concerns regarding the reactogenicity of wP vaccines led to the development of a new generation of safer, acellular (aP) vaccines that have been adopted mainly in high-income countries. Over the past 20 years, some countries that boast high aP coverage have experienced a resurgence in pertussis, which has led to substantial debate over the basic immunology, epidemiology and evolutionary biology of the bacterium. Controversy surrounds the duration of natural immunity and vaccine-derived immunity, the ability of vaccines to prevent transmission and severe disease, and the impact of evolution on evading vaccine immunity. Resolving these issues is made challenging by incomplete detection of pertussis cases, the absence of a serological marker of immunity, modest sequencing of the bacterial genome and heterogeneity in diagnostic methods of surveillance. In this Review, we lay out the complexities of contemporary pertussis and, where possible, propose a parsimonious explanation for apparently incongruous observations.




Similar content being viewed by others
References
Parkhill, J. et al. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 35, 32–40 (2003). This research compares whole-genome sequences for congeneric Bordetella species to demonstrate B. parapertussis and B. pertussis are independently derived from B. bronchiseptica-like ancestors and have become host-restricted species. This host adaptation is argued to be a consequence of large-scale gene loss, genome reshuffling and inactivation.
Rohani, P. & Scarpino, S. Pertussis: Epidemiology, Immunology, and Evolution (Oxford Univ. Press, 2018).
Gordon, J. E. & Hood, R. I. Whooping cough and its epidemiological anomalies. Am. J. Med. Sci. 222, 333–361 (1951).
Kendrick, P. L. Can whooping cough be eradicated? J. Infect. Dis. 132, 707–712 (1975).
Rohani, P., Earn, D. J. & Grenfell, B. T. Opposite patterns of synchrony in sympatric disease metapopulations. Science 286, 968–971 (1999).
Rohani, P. & Drake, J. M. The decline and resurgence of pertussis in the US. Epidemics 3, 183–188 (2011).
van Panhuis, W. G. et al. Contagious diseases in the United States from 1888 to the present. N. Engl. J. Med. 369, 2152–2158 (2013).
Frenkel, L. D. The global burden of vaccine-preventable infectious diseases in children less than 5 years of age: implications for COVID-19 vaccination. How can we do better? Allergy Asthma Proc. 42, 378–385 (2021).
WHO & UNICEF. Diphtheria Tetanus Toxoid and Pertussis (DTP) Vaccination Coverage. World Health Organization https://immunizationdata.who.int/global/wiise-detail-page/diphtheria-tetanus-toxoid-and-pertussis-(dtp)-vaccination-coverage (accessed 22 July 2022).
WHO & UNICEF. Vaccination Schedule for Pertussis. World Health Organization https://immunizationdata.who.int/global/wiise-detail-page/vaccination-schedule-for-pertussis?ISO_3_CODE=&TARGETPOP_GENERAL= (accessed 22 July 2022).
WHO & UNICEF. Pertussis Reported Cases and Incidence. World Health Organization https://immunizationdata.who.int/global/wiise-detail-page/pertussis-reported-cases-and-incidence (accessed 22 July 2022).
Tessier, E. et al. Impact of the COVID-19 pandemic on Bordetella pertussis infections in England. BMC Public Health 22, 405 (2022).
Matczak, S. et al. Association between the COVID-19 pandemic and pertussis derived from multiple nationwide data sources, France, 2013 to 2020. Eurosurveillance https://doi.org/10.2807/1560-7917.es.2022.27.25.2100933 (2022).
Bhatt, P., Strachan, J., Easton, M., Franklin, L. & Drewett, G. Effect of COVID-19 restrictions and border closures on vaccine preventable diseases in Victoria, Australia, 2020-2021. Commun. Dis. Intell. https://doi.org/10.33321/cdi.2022.46.29 (2022).
Shet, A. et al. Impact of the SARS-CoV-2 pandemic on routine immunisation services: evidence of disruption and recovery from 170 countries and territories. Lancet Glob. Health 10, e186–e194 (2022).
Andersen, E. K. et al. Serological studies on H. pertussis, H. para-pertussis and H. bronchisepticus. Acta Pathol. Microbiol. Scand. 33, 202–224 (1953).
Aftandelians, R. V. & Connor, J. D. Bordetella pertussis serotypes in a whooping cough outbreak. Am. J. Epidemiol. 99, 343–346 (1974).
Stanbridge, T. N. & Preston, N. W. Variation of serotype in strains of Bordetella pertussis. J. Hyg. 73, 305–310 (1974).
Bronne-Shanbury, C. J. & Dolby, J. M. The stability of the serotypes of Bordetella pertussis with particular reference to serotype 1,2,3,4. J. Hyg. 76, 277–286 (1976).
Preston, N. W. Effectiveness of pertussis vaccines. Br. Med. J. 2, 11–13 (1965).
Bronne-Shanbury, C. J., Miller, D. & Standfast, A. F. B. The serotypes of Bordetella pertussis isolated in Great Britain between 1941 and 1968 and a comparison with the serotypes observed in other countries over this period. Epidemiol. Infect. 76, 265–275 (1976).
Preston, N. W. Prevalent serotypes of Bordetella pertussis in non-vaccinated communities. J. Hyg. 77, 85–91 (1976).
Schouls, L. M., van der Heide, H. G. J., Vauterin, L., Vauterin, P. & Mooi, F. R. Multiple-locus variable-number tandem repeat analysis of Dutch Bordetella pertussis strains reveals rapid genetic changes with clonal expansion during the late 1990s. J. Bacteriol. 186, 5496–5505 (2004).
Schmidtke, A. J. et al. Population diversity among Bordetella pertussis isolates, United States, 1935-2009. Emerg. Infect. Dis. 18, 1248–1255 (2012).
Barkoff, A.-M. et al. Surveillance of circulating Bordetella pertussis strains in Europe during 1998 to 2015. J. Clin. Microbiol. 56, e01998-17 (2018).
Barkoff, A.-M. et al. Pertactin-deficient Bordetella pertussis isolates: evidence of increased circulation in Europe, 1998 to 2015. Eur. Surveill. 24, 1700832 (2019).
Weigand, M. R. et al. The history of Bordetella pertussis genome evolution includes structural rearrangement. J. Bacteriol. 199, e00806-16 (2017).
Lefrancq, N. et al. Global spatial dynamics and vaccine-induced fitness changes of Bordetella pertussis. Sci. Transl. Med. 14, eabn3253 (2022).
Safarchi, A. et al. Pertactin negative Bordetella pertussis demonstrates higher fitness under vaccine selection pressure in a mixed infection model. Vaccine 33, 6277–6281 (2015).
Lesne, E. et al. Acellular pertussis vaccines induce anti-pertactin bactericidal antibodies which drives the emergence of pertactin-negative strains. Front. Microbiol. 11, 2108 (2020).
Breakwell, L. et al. Pertussis vaccine effectiveness in the setting of pertactin-deficient pertussis. Pediatrics 137, e20153973 (2016).
Weigand, M. R. et al. Conserved patterns of symmetric inversion in the genome evolution of Bordetella respiratory pathogens. mSystems 4, e00702-19 (2019).
Hanage, W. P. Not so simple after all: bacteria, their population genetics, and recombination. Cold Spring Harb. Perspect. Biol. 8, a018069 (2016).
Weigand, M. R. et al. Genomic survey of Bordetella pertussis diversity, United States, 2000-2013. Emerg. Infect. Dis. 25, 780–783 (2019).
Ma, L., Caulfield, A., Dewan, K. K. & Harvill, E. T. Pertactin-deficient Bordetella pertussis, vaccine-driven evolution, and reemergence of pertussis. Emerg. Infect. Dis. 27, 1561–1566 (2021).
Bouchez, V., Hegerle, N., Strati, F., Njamkepo, E. & Guiso, N. New data on vaccine antigen deficient Bordetella pertussis isolates. Vaccines 3, 751–770 (2015).
Szwejser-Zawislak, E. et al. Evaluation of whole-cell and acellular pertussis vaccines in the context of long-term herd immunity. Vaccines 11, 1 (2022).
Sealey, K. L., Belcher, T. & Preston, A. Bordetella pertussis epidemiology and evolution in the light of pertussis resurgence. Infect. Genet. Evol. 40, 136–143 (2016).
Mooi, F. R. et al. Bordetella pertussis strains with increased toxin production associated with pertussis resurgence. Emerg. Infect. Dis. 15, 1206–1213 (2009).
Pittet, L. F., Emonet, S., Schrenzel, J., Siegrist, C.-A. & Posfay-Barbe, K. M. Bordetella holmesii: an under-recognised Bordetella species. Lancet Infect. Dis. 14, 510–519 (2014). This paper provides a comprehensive review of B. holmesii, exploring its history, microbiology, epidemiology, diagnosis, clinical manifestation, treatment and unknowns.
Njamkepo, E. et al. Significant finding of Bordetella holmesii DNA in nasopharyngeal samples from French patients with suspected pertussis. J. Clin. Microbiol. 49, 4347–4348 (2011).
Rodgers, L. et al. Epidemiologic and laboratory features of a large outbreak of pertussis-like illnesses associated with cocirculating Bordetella holmesii and Bordetella pertussis — Ohio, 2010–2011. Clin. Infect. Dis. 56, 322–331 (2013).
Valero-Rello, A. et al. Validation and implementation of a diagnostic algorithm for DNA detection of Bordetella pertussis, B. parapertussis, and B. holmesii in a pediatric referral hospital in Barcelona, Spain. J. Clin. Microbiol. 57, e01231–18 (2019).
David, S., van Furth, R. & Mooi, F. R. Efficacies of whole cell and acellular pertussis vaccines against Bordetella parapertussis in a mouse model. Vaccine 22, 1892–1898 (2004).
Long, G. H., Karanikas, A. T., Harvill, E. T., Read, A. F. & Hudson, P. J. Acellular pertussis vaccination facilitates Bordetella parapertussis infection in a rodent model of bordetellosis. Proc. Biol. Sci. 277, 2017–2025 (2010).
Mooi, F. R. et al. Characterization of Bordetella holmesii isolates from patients with pertussis-like illness in The Netherlands. FEMS Immunol. Med. Microbiol. 64, 289–291 (2012).
European Centre for Disease Prevention and Control. Laboratory diagnosis and molecular surveillance of Bordetella pertussis. ECDC https://www.ecdc.europa.eu/en/publications-data/bordetella-pertussis-laboratory-diagnosis-and-molecular-surveillance (2022).
van der Zee, A., Schellekens, J. F. P. & Mooi, F. R. Laboratory diagnosis of pertussis. Clin. Microbiol. Rev. 28, 1005–1026 (2015).
Barkoff, A.-M., Gröndahl-Yli-Hannuksela, K. & He, Q. Seroprevalence studies of pertussis: what have we learned from different immunized populations. Pathog. Dis. 73, ftv050 (2015).
de Melker, H. E., Versteegh, F. G. A., Schellekens, J. F. P., Teunis, P. F. M. & Kretzschmar, M. The incidence of Bordetella pertussis infections estimated in the population from a combination of serological surveys. J. Infect. 53, 106–113 (2006).
von König, C. H. W., Halperin, S., Riffelmann, M. & Guiso, N. Pertussis of adults and infants. Lancet Infect. Dis. 2, 744–750 (2002).
Domenech de Cellès, M., Magpantay, F. M. G., King, A. A. & Rohani, P. The pertussis enigma: reconciling epidemiology, immunology and evolution. Proc. Biol. Sci. 283, 20152309 (2016).
Cutts, F. T. & Hanson, M. Seroepidemiology: an underused tool for designing and monitoring vaccination programmes in low‐ and middle‐income countries. Trop. Med. Int. Health 21, 1086–1098 (2016).
Kapil, P. & Merkel, T. J. Pertussis vaccines and protective immunity. Curr. Opin. Immunol. 59, 72–78 (2019).
WHO Pertussis vaccines: WHO position paper, August 2015 — recommendations. Vaccine 34, 1423–1425 (2016).
Teunis, P. F. M., van Eijkeren, J. C. H., de Graaf, W. F., Marinović, A. B. & Kretzschmar, M. E. E. Linking the seroresponse to infection to within-host heterogeneity in antibody production. Epidemics 16, 33–39 (2016).
ECDC seroincidence R package v.2.0.0. European Centre for Disease Prevention and Control https://ecdc.europa.eu/en/publications-data/seroincidence-calculator-tool (2018).
Crowcroft, N. & Miller, E. in Pertussis: Epidemiology, Immunology, Evolution Ch. 4 (eds Rohani, P. & Scarpino, S.) 66–86 (Oxford Univ. Press, 2019).
Storsaeter, J., Hallander, H. O., Gustafsson, L. & Olin, P. Low levels of antipertussis antibodies plus lack of history of pertussis correlate with susceptibility after household exposure to Bordetella pertussis. Vaccine 21, 3542–3549 (2003).
Fine, P. E. M. Adult pertussis: a salesman’s dream — and an epidemiologist’s nightmare. Biologicals 25, 195–198 (1997).
Pertussis: adults, infants, and herds. Lancet 339, 526–527 (1992).
Guiso, N. et al. What to do and what not to do in serological diagnosis of pertussis: recommendations from EU reference laboratories. Eur. J. Clin. Microbiol. Infect. Dis. 30, 307–312 (2011).
European Centre for Disease Prevention and Control. Guidance and protocol for the serological diagnosis of human infection with Bordetella pertussis. ECDC https://www.ecdc.europa.eu/en/publications-data/guidance-and-protocol-serological-diagnosis-human-infection-bordetella-pertussis (2012).
Liu, B. C. et al. Effectiveness of acellular pertussis vaccine in older adults: nested matched case-control study. Clin. Infect. Dis. 71, 340–350 (2020). This case–control study of Tdap effectiveness in adults aged ≥45 years in New South Wales, Australia, has found a marked difference in vaccine effectiveness estimates between cases confirmed by PCR only and by serology only, suggesting the unreliability of the latter.
Graaf et al. Controlled human infection with Bordetella pertussis induces asymptomatic, immunizing colonization. Clin. Infect. Dis. 71, 403–411 (2020). This controlled human infection study for pertussis demonstrates that infection requires high inoculum dose, leading to an asymptomatic infection with no evidence of bacterial shedding.
Schellekens, J., von König, C.-H. W. & Gardner, P. Pertussis sources of infection and routes of transmission in the vaccination era. Pediatr. Infect. Dis. J. 24, S19–S24 (2005).
Tan, T. Summary: epidemiology of pertussis. Pediatr. Infect. Dis. J. 24, S35–S38 (2005).
Gill, C. J. et al. Asymptomatic Bordetella pertussis infections in a longitudinal cohort of young African infants and their mothers. eLife 10, e65663 (2021).
Mina, M. J. et al. A global immunological observatory to meet a time of pandemics. eLife 9, e58989 (2020).
Halloran, M. E., Longini, I. M., Jr & Struchiner, C. J. Design and Analysis of Vaccine Studies (Springer, 2012).
Préziosi, M.-P. & Halloran, M. E. Effects of pertussis vaccination on transmission: vaccine efficacy for infectiousness. Vaccine 21, 1853–1861 (2003).
Ionides, E. L., Nguyen, D., Atchadé, Y., Stoev, S. & King, A. A. Inference for dynamic and latent variable models via iterated, perturbed Bayes maps. Proc. Natl Acad. Sci. USA 112, 719–724 (2015).
King, A. A., Nguyen, D. & Ionides, E. L. Statistical inference for partially observed Markov processes via the R package pomp. J. Stat. Softw. 69, 1–43 (2016).
Sutter, R. W. & Cochi, S. L. Pertussis hospitalizations and mortality in the United States, 1985–1988. Evaluation of the completeness of national reporting. JAMA 267, 386–391 (1992).
Somerville, R. L. et al. Infants hospitalised with pertussis: estimating the true disease burden. J. Paediatr. Child Health 43, 617–622 (2007).
Gunning, C. E., Erhardt, E. & Wearing, H. J. Conserved patterns of incomplete reporting in pre-vaccine era childhood diseases. Proc. Biol. Sci. 281, 20140886 (2014).
Blackwood, J. C., Cummings, D. A. T., Broutin, H., Iamsirithaworn, S. & Rohani, P. Deciphering the impacts of vaccination and immunity on pertussis epidemiology in Thailand. Proc. Natl Acad. Sci. USA 110, 9595–9600 (2013).
Rohani, P., Zhong, X. & King, A. A. Contact network structure explains the changing epidemiology of pertussis. Science 330, 982–985 (2010).
Wearing, H. J. & Rohani, P. Estimating the duration of pertussis immunity using epidemiological signatures. PLoS Pathog. 5, e1000647 (2009).
Gambhir, M. et al. A change in vaccine efficacy and duration of protection explains recent rises in pertussis incidence in the United States. PLoS Comput. Biol. 11, e1004138 (2015).
Domenech de Cellès, M., Magpantay, F. M. G., King, A. A. & Rohani, P. The impact of past vaccination coverage and immunity on pertussis resurgence. Sci. Transl. Med. 10, eaau9627 (2018). Combining an age-structured model of pertussis transmission with novel statistical inference techniques to estimate the properties of pertussis vaccines from incidence data, this study shows that pertussis resurgence in Massachusetts, USA, was the predictable consequence of incomplete historical coverage with imperfect vaccines that confer slowly waning immunity — that is, an ‘end-of-honeymoon’ effect.
Kretzschmar, M., Teunis, P. F. M. & Pebody, R. G. Incidence and reproduction numbers of pertussis: estimates from serological and social contact data in five European countries. PLoS Med. 7, e1000291 (2010).
McDonald, S. A. et al. An evidence synthesis approach to estimating the incidence of symptomatic pertussis infection in the Netherlands, 2005–2011. BMC Infect. Dis. 15, 588 (2015).
Smallridge, W. E., Rolin, O. Y., Jacobs, N. T. & Harvill, E. T. Different effects of whole-cell and acellular vaccines on Bordetella transmission. J. Infect. Dis. 209, 1981–1988 (2014).
Warfel, J. M., Zimmerman, L. I. & Merkel, T. J. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc. Natl Acad. Sci. USA 111, 787–792 (2014). This paper provides a transformative baboon model of pertussis infection, teasing apart the differences in immune response elicited by whole-cell and acellular vaccines and determining their consequences for disease and transmission.
Domenech de Cellès, M., Riolo, M. A., Magpantay, F. M. G., Rohani, P. & King, A. A. Epidemiological evidence for herd immunity induced by acellular pertussis vaccines. Proc. Natl Acad. Sci. USA 111, E716–E717 (2014).
Cravitz, L. & Williams, J. W. A comparative study of the ‘immune response’ to various pertussis antigens and the disease. J. Pediatr. 28, 172–186 (1946).
Abbott, J. D., Preston, N. W. & Mackay, R. I. Agglutinin response to pertussis vaccination in the child. Br. Med. J. 1, 86–88 (1971).
Fine, P. E. & Clarkson, J. A. Distribution of immunity to pertussis in the population of England and Wales. J. Hyg. 92, 21–36 (1984).
Vaccination against whooping-cough; relation between protection in children and results of laboratory tests; a report to the Whooping-cough Immunization Committee of the Medical Research Council and to the medical officers of health for Cardiff, Leeds, Leyton, Manchester, Middlesex, Oxford, Poole, Tottenham, Walthamstow, and Wembley. Br. Med. J. 2, 454–462 (1956).
Mills, K. H. Immunity to Bordetella pertussis. Microbes Infect. 3, 655–677 (2001).
Blanchard-Rohner, G. Novel approaches to reactivate pertussis immunity. Expert Rev. Vaccines 21, 1787–1797 (2022).
Wirsing von König, C. H., Postels-Multan, S., Schmitt, H. J. & Bock, H. L. Pertussis in adults: frequency of transmission after household exposure. Lancet 346, 1326–1329 (1995).
Jenkinson, D. Natural course of 500 consecutive cases of whooping cough: a general practice population study. Br. Med. J. 310, 299–302 (1995).
Fine, P. E. & Clarkson, J. A. The recurrence of whooping cough: possible implications for assessment of vaccine efficacy. Lancet 1, 666–669 (1982).
Wendelboe, A. M., Van Rie, A., Salmaso, S. & Englund, J. A. Duration of immunity against pertussis after natural infection or vaccination. Pediatr. Infect. Dis. J. 24, S58–S61 (2005).
Fine, P. E. & Clarkson, J. A. Reflections on the efficacy of pertussis vaccines. Rev. Infect. Dis. 9, 866–883 (1987).
Rohani, P., Earn, D. J. & Grenfell, B. T. Impact of immunisation on pertussis transmission in England and Wales. Lancet 355, 285–286 (2000).
Platt, L., Thun, M. & Harriman, K. A population-based study of recurrent symptomatic Bordetella pertussis infections in children in California, 2010–2015. Clin. Infect. Dis. 65, 2099–2104 (2017).
Warfel, J. M. & Merkel, T. J. Bordetella pertussis infection induces a mucosal IL-17 response and long-lived Th17 and Th1 immune memory cells in nonhuman primates. Mucosal Immunol. 6, 787–796 (2013).
Althouse, B. M. & Scarpino, S. V. Asymptomatic transmission and the resurgence of Bordetella pertussis. BMC Med. 13, 146 (2015).
Klein, N. P., Bartlett, J., Rowhani-Rahbar, A., Fireman, B. & Baxter, R. Waning protection after fifth dose of acellular pertussis vaccine in children. N. Engl. J. Med. 367, 1012–1019 (2012).
Wilk, M. M., Allen, A. C., Misiak, A., Borkner, L. & Mills, K. H. G. The immunology of Bordetella pertussis infection and vaccination. Pertussis https://doi.org/10.1093/oso/9780198811879.003.0003 (2018).
Chit, A. et al. Acellular pertussis vaccines effectiveness over time: a systematic review, meta-analysis and modeling study. PLoS ONE 13, e0197970 (2018). This meta-analysis provides estimates of the rate of waning effectiveness of acellular pertussis vaccines and pointed out the interpretation errors in earlier US studies.
Domenech de Cellès, M., Rohani, P. & King, A. A. Duration of immunity and effectiveness of diphtheria-tetanus-acellular pertussis vaccines in children. JAMA Pediatr. 173, 588–594 (2019).
Rane, M. S., Rohani, P. & Halloran, M. E. Durability of protection after 5 doses of acellular pertussis vaccine among 5–9 year old children in King County, Washington. Vaccine 39, 6144–6150 (2021).
Crowcroft, N. S. et al. A call for caution in use of pertussis vaccine effectiveness studies to estimate waning immunity: a Canadian Immunization Research Network study. Clin. Infect. Dis. 73, 83–90 (2021). This study demonstrates that the appropriate controls for estimating pertussis waning is via a frequency-matched design, rather than a test-negative design that leads to multiple confounding factors. It further demonstrates a substantially lower estimated waning when the appropriate controls are accounted for.
Choi, Y. H., Campbell, H., Amirthalingam, G., van Hoek, A. J. & Miller, E. Investigating the pertussis resurgence in England and Wales, and options for future control. BMC Med. 14, 121 (2016).
Campbell, P. T., McCaw, J. M., McIntyre, P. & McVernon, J. Defining long-term drivers of pertussis resurgence, and optimal vaccine control strategies. Vaccine 33, 5794–5800 (2015).
Lavine, J. S. & Rohani, P. Resolving pertussis immunity and vaccine effectiveness using incidence time series. Expert Rev. Vaccines 11, 1319–1329 (2012).
Rohani, P. & Scarpino, S. V. in Pertussis: Epidemiology, Immunology, and Evolution (eds Rohani, P. & Scarpino, S.) 6–25 (Oxford Univ. Press, 2019).
King, A. A., Domenech de Cellès, M., Magpantay, F. M. G. & Rohani, P. in Pertussis: Epidemiology, Immunology, and Evolution (eds Rohani, P. & Scarpino, S. V.) Ch. 14 (Oxford Univ. Press, 2018).
Lavine, J. S., King, A. A., Andreasen, V. & Bjørnstad, O. N. Immune boosting explains regime-shifts in prevaccine-era pertussis dynamics. PLoS ONE 8, e72086 (2013).
Jackson, D. W. & Rohani, P. Perplexities of pertussis: recent global epidemiological trends and their potential causes. Epidemiol. Infect. 142, 672–684 (2014).
Broset, E. et al. BCG vaccination improves DTaP immune responses in mice and is associated with lower pertussis incidence in ecological epidemiological studies. EBioMedicine 65, 103254 (2021). This study illustrates that BCG vaccines can shift the immune response to aP vaccines towards TH1 and TH17, and it demonstrates that countries deploying both aP and BCG vaccines report an order of magnitude lower incidence of pertussis than countries with aP vaccines alone.
Gillard, J. et al. BCG-induced trained immunity enhances acellular pertussis vaccination responses in an explorative randomized clinical trial. NPJ Vaccines 7, 21 (2022).
Gillard, J. et al. Antiviral responses induced by Tdap-IPV vaccination are associated with persistent humoral immunity to Bordetella pertussis. Nat. Commun. 15, 2133 (2024).
Borkner, L., Curham, L. M., Wilk, M. M., Moran, B. & Mills, K. H. G. IL-17 mediates protective immunity against nasal infection with Bordetella pertussis by mobilizing neutrophils, especially Siglec-F+ neutrophils. Mucosal Immunol. 14, 1183–1202 (2021).
Dubois, V. et al. Suppression of mucosal Th17 memory responses by acellular pertussis vaccines enhances nasal Bordetella pertussis carriage. NPJ Vaccines 6, 6 (2021).
Wilk, M. M. et al. Immunization with whole cell but not acellular pertussis vaccines primes CD4 TRM cells that sustain protective immunity against nasal colonization with Bordetella pertussis. Emerg. Microbes Infect. 8, 169–185 (2019).
McCarthy, K. N., Hone, S., McLoughlin, R. M. & Mills, K. H. G. IL-17 and IFN-γ-producing respiratory tissue resident memory CD4 T cells persist for decades in adults immunized as children with whole cell pertussis vaccines. J. Infect. Dis. https://doi.org/10.1093/infdis/jiae034 (2024).
Winter, K., Klein, N. P., Ackley, S. & Cherry, J. D. Comment on ‘The impact of past vaccination coverage and immunity on pertussis resurgence’. Sci. Transl. Med. 10, eaau0548 (2018).
Domenech de Cellès, M., King, A. A. & Rohani, P. Response to Comment on ‘The impact of past vaccination coverage and immunity on pertussis resurgence’. Sci. Transl. Med. 10, eaau9627 (2018).
Riolo, M. A., King, A. A. & Rohani, P. Can vaccine legacy explain the British pertussis resurgence? Vaccine 31, 5903–5908 (2013).
Riolo, M. A. & Rohani, P. Combating pertussis resurgence: one booster vaccination schedule does not fit all. Proc. Natl Acad. Sci. USA 112, E472–E477 (2015). This study combines an age-structured model of pertussis transmission with a genetic optimization algorithm to identify cost-effective booster vaccination strategies, showing that not only the overall effectiveness of vaccine boosters but also the mechanism of vaccine failure predict optimal strategies.
Ward, J. I. et al. Efficacy of an acellular pertussis vaccine among adolescents and adults. N. Engl. J. Med. 353, 1555–1563 (2005).
Koepke, R. et al. Estimating the effectiveness of tetanus-diphtheria-acellular pertussis vaccine (Tdap) for preventing pertussis: evidence of rapidly waning immunity and difference in effectiveness by Tdap brand. J. Infect. Dis. 210, 942–953 (2014).
Acosta, A. M. et al. Tdap vaccine effectiveness in adolescents during the 2012 Washington State pertussis epidemic. Pediatrics 135, 981–989 (2015).
Klein, N. P., Bartlett, J., Fireman, B. & Baxter, R. Waning Tdap effectiveness in adolescents. Pediatrics 137, e20153326 (2016).
van der Lee, S., Hendrikx, L. H., Sanders, E. A. M., Berbers, G. A. M. & Buisman, A.-M. Whole-cell or acellular pertussis primary immunizations in infancy determines adolescent cellular immune profiles. Front. Immunol. 9, 51 (2018).
da Silva Antunes, R. et al. Th1/Th17 polarization persists following whole-cell pertussis vaccination despite repeated acellular boosters. J. Clin. Invest. 128, 3853–3865 (2018).
da Silva Antunes, R. et al. A system-view of Bordetella pertussis booster vaccine responses in adults primed with whole-cell versus acellular vaccine in infancy. JCI Insight 6, e141023 (2021).
Yeung, K. H. T., Duclos, P., Nelson, E. A. S. & Hutubessy, R. C. W. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect. Dis. 17, 974–980 (2017).
Kandeil, W. et al. A systematic review of the burden of pertussis disease in infants and the effectiveness of maternal immunization against pertussis. Expert Rev. Vaccines 19, 621–638 (2020).
Voysey, M. et al. The influence of maternally derived antibody and infant age at vaccination on infant vaccine responses: an individual participant meta-analysis. JAMA Pediatr. 171, 637–646 (2017).
Kretsinger, K. et al. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm. Rep. 55, 1–37 (2006).
Quinn, H. E. et al. Parental Tdap boosters and infant pertussis: a case-control study. Pediatrics 134, 713–720 (2014).
Carcione, D. et al. The impact of parental postpartum pertussis vaccination on infection in infants: a population-based study of cocooning in Western Australia. Vaccine 33, 5654–5661 (2015).
Healy, C. M., Rench, M. A., Wootton, S. H. & Castagnini, L. A. Evaluation of the impact of a pertussis cocooning program on infant pertussis infection. Pediatr. Infect. Dis. J. 34, 22–26 (2015).
Rowe, S. L. et al. Maternal vaccination and infant influenza and pertussis. Pediatrics 148, e2021051076 (2021).
Dabrera, G. et al. A case-control study to estimate the effectiveness of maternal pertussis vaccination in protecting newborn infants in England and Wales, 2012-2013. Clin. Infect. Dis. 60, 333–337 (2015).
Amirthalingam, G. et al. Sustained effectiveness of the maternal pertussis immunization program in England 3 years following introduction. Clin. Infect. Dis. 63, S236–S243 (2016).
Baxter, R., Bartlett, J., Fireman, B., Lewis, E. & Klein, N. P. Effectiveness of vaccination during pregnancy to prevent infant pertussis. Pediatrics 139, e20164091 (2017).
Skoff, T. H. et al. Impact of the US maternal tetanus, diphtheria, and acellular pertussis vaccination program on preventing pertussis in infants <2 months of age: a case-control evaluation. Clin. Infect. Dis. 65, 1977–1983 (2017).
Abu-Raya, B. et al. Global perspectives on immunization during pregnancy and priorities for future research and development: an international consensus statement. Front. Immunol. 11, 1282 (2020).
Abu-Raya, B. et al. The effect of tetanus-diphtheria-acellular-pertussis immunization during pregnancy on infant antibody responses: individual-participant data meta-analysis. Front. Immunol. https://doi.org/10.3389/fimmu.2021.689394 (2021).
Briga, M., Goult, E., Brett, T. S., Rohani, P. & Domenech de Cellès, M. Maternal pertussis immunization and the blunting of routine vaccine effectiveness: a meta-analysis and modeling study. Nat. Commun. 15, 921 (2024).
Mattoo, S. & Cherry, J. D. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin. Microbiol. Rev. 18, 326–382 (2005).
Locht, C. & Mielcarek, N. Live attenuated vaccines against pertussis. Expert Rev. Vaccines 13, 1147–1158 (2014).
Keech, C. et al. Immunogenicity and safety of BPZE1, an intranasal live attenuated pertussis vaccine, versus tetanus-diphtheria-acellular pertussis vaccine: a randomised, double-blind, phase 2b trial. Lancet 401, 843–855 (2023).
Sugai, T. et al. A CpG-containing oligodeoxynucleotide as an efficient adjuvant counterbalancing the Th1/Th2 immune response in diphtheria–tetanus–pertussis vaccine. Vaccine 23, 5450–5456 (2005).
Allen, A. C. et al. Sustained protective immunity against Bordetella pertussis nasal colonization by intranasal immunization with a vaccine-adjuvant combination that induces IL-17-secreting TRM cells. Mucosal Immunol. 11, 1763–1776 (2018).
Preston, N. in Pathogenesis and Immunity in Pertussis (eds Wardlaw, A. C. & Parton, R.) 1–19 (Wiley, 1988).
Lavine, J. S., Bjørnstad, O. N., de Blasio, B. F. & Storsaeter, J. Short-lived immunity against pertussis, age-specific routes of transmission, and the utility of a teenage booster vaccine. Vaccine 30, 544–551 (2012).
Gokhale, D. V., Brett, T. S., He, B., King, A. A. & Rohani, P. Disentangling the causes of mumps reemergence in the United States. Proc. Natl Acad. Sci. USA 120, e2207595120 (2023).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
P.R. received funding from Sanofi for a research project on pertussis vaccines. M.D.d.C. received post-doctoral funding (2017–2019) from Pfizer and consulting fees from GSK.
Peer review
Peer review information
Nature Reviews Microbiology thanks Camille Locht, Peter McIntyre, Peter Sebo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Edmond, the Open Data Repository of the Max Planck Society: https://doi.org/10.17617/3.A1KMUB
World Bank country and lending groups: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Domenech de Cellès, M., Rohani, P. Pertussis vaccines, epidemiology and evolution. Nat Rev Microbiol (2024). https://doi.org/10.1038/s41579-024-01064-8
Accepted:
Published:
DOI: https://doi.org/10.1038/s41579-024-01064-8
- Springer Nature Limited