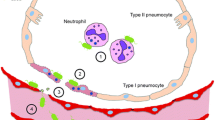
Abstract
Pseudomonas aeruginosa, a leading cause of severe hospital-acquired pneumonia, causes infections with up to 50% mortality rates in mechanically ventilated patients. Despite some knowledge of virulence factors involved, it remains unclear how P. aeruginosa disseminates on mucosal surfaces and invades the tissue barrier. Using infection of human respiratory epithelium organoids, here we observed that P. aeruginosa colonization of apical surfaces is promoted by cyclic di-GMP-dependent asymmetric division. Infection with mutant strains revealed that Type 6 Secretion System activities promote preferential invasion of goblet cells. Type 3 Secretion System activity by intracellular bacteria induced goblet cell death and expulsion, leading to epithelial rupture which increased bacterial translocation and dissemination to the basolateral epithelium. These findings show that under physiological conditions, P. aeruginosa uses coordinated activity of a specific combination of virulence factors and behaviours to invade goblet cells and breach the epithelial barrier from within, revealing mechanistic insight into lung infection dynamics.




Similar content being viewed by others
Data availability
The microscopy data that support the findings of this study are openly available in the BioImage Archive (https://doi.org/10.6019/S-BIAD1083)93, and all other data types are in Zenodo (https://zenodo.org/records/10650981)94. Unique biological materials are available from the corresponding author on reasonable request.
References
Parker, D. & Prince, A. Innate immunity in the respiratory epithelium. Am. J. Respir. Cell Mol. Biol. 45, 189–201 (2011).
Tsang, K. et al. Interaction of Pseudomonas aeruginosa with human respiratory mucosa in vitro. Eur. Respir. J. 7, 1746–1753 (1994).
Schwarzer, C., Fischer, H. & Machen, T. E. Chemotaxis and binding of Pseudomonas aeruginosa to scratch-wounded human cystic fibrosis airway epithelial cells. PLoS ONE 11, e0150109 (2016).
Happel, K. I., Nelson, S. & Summer, W. The lung in sepsis: fueling the fire. Am. J. Med. Sci. 328, 230–237 (2004).
Giantsou, E. & Manolas, K. I. Superinfections in Pseudomonas aeruginosa ventilator-associated pneumonia. Minerva Anestesiol. 77, 964–970 (2011).
Tramper‐Stranders, G. A. et al. Initial Pseudomonas aeruginosa infection in patients with cystic fibrosis: characteristics of eradicated and persistent isolates. Clin. Microbiol. Infect. 18, 567–574 (2012).
Bustamante-Marin, X. M. & Ostrowski, L. E. Cilia and mucociliary clearance. Cold Spring Harb. Perspect. Biol. 9, a028241 (2017).
Carvajal, L. A. & Pérez, C. P. Epidemiology of Respiratory Infections. in Pediatric Respiratory Diseases: A Comprehensive Textbook (eds Bertrand, P. & Sánchez, I.) 263–272 (Springer, 2020).
Hiemstra, P. S., McCray, P. B. & Bals, R. The innate immune function of airway epithelial cells in inflammatory lung disease. Eur. Respir. J. 45, 1150–1162 (2015).
Almagro, P. et al. Pseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary disease. Respiration 84, 36–43 (2012).
Gellatly, S. L. & Hancock, R. E. W. Pseudomonas aeruginosa: new insights into pathogenesis and host defenses. Pathog. Dis. 67, 159–173 (2013).
Engel, J. & Balachandran, P. Role of Pseudomonas aeruginosa type III effectors in disease. Curr. Opin. Microbiol. 12, 61–66 (2009).
Heiniger, R. W., Winther-Larsen, H. C., Pickles, R. J., Koomey, M. & Wolfgang, M. C. Infection of human mucosal tissue by Pseudomonas aeruginosa requires sequential and mutually dependent virulence factors and a novel pilus-associated adhesin. Cell. Microbiol. 12, 1158–1173 (2010).
Bucior, I., Mostov, K. & Engel, J. N. Pseudomonas aeruginosa-mediated damage requires distinct receptors at the apical and basolateral surfaces of the polarized epithelium. Infect. Immun. 78, 939–953 (2010).
Fleiszig, S. M. et al. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect. Immun. 65, 2861–2867 (1997).
Engel, J. & Eran, Y. Subversion of mucosal barrier polarity by Pseudomonas aeruginosa. Front. Microbiol. 2, 114 (2011).
Kazmierczak, B. I., Mostov, K. & Engel, J. N. Epithelial cell polarity alters rho-GTPase responses to Pseudomonas aeruginosa. Mol. Biol. Cell 15, 411–419 (2004).
Golovkine, G. et al. Pseudomonas aeruginosa transmigrates at epithelial cell-cell junctions, exploiting sites of cell division and senescent cell extrusion. PLoS Pathog. 12, e1005377 (2016).
Kierbel, A. et al. Pseudomonas aeruginosa exploits a PIP3-dependent pathway to transform apical into basolateral membrane. J. Cell Biol. 177, 21–27 (2007).
Kierbel, A., Gassama-Diagne, A., Mostov, K. & Engel, J. N. The phosphoinositol-3-kinase–protein kinase B/Akt pathway is critical for Pseudomonas aeruginosa strain PAK internalization. Mol. Biol. Cell 16, 2577–2585 (2005).
Plotkowski, M. C. et al. Pseudomonas aeruginosa internalization by human epithelial respiratory cells depends on cell differentiation, polarity, and junctional complex integrity. Am. J. Respir. Cell Mol. Biol. 20, 880–890 (1999).
Lopes, S. F. et al. Primary and immortalized human respiratory cells display different patterns of cytotoxicity and cytokine release upon exposure to deoxynivalenol, nivalenol and fusarenon-X. Toxins 9, 337 (2017).
Barron, S. L., Saez, J. & Owens, R. M. In vitro models for studying respiratory host–pathogen interactions. Adv. Biol. 5, 2000624 (2021).
Seok, J. et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl Acad. Sci. USA 110, 3507–3512 (2013).
Worp et al. Can animal models of disease reliably inform human studies? PLoS Med. 7, e1000245 (2010).
Uhl, E. W. & Warner, N. J. Mouse models as predictors of human responses: evolutionary medicine. Curr. Pathobiol. Rep. 3, 219–223 (2015).
Chia, S. P. S., Kong, S. L. Y., Pang, J. K. S. & Soh, B.-S. 3D human organoids: the next “viral” model for the molecular basis of infectious diseases. Biomedicines 10, 1541 (2022).
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772 (2011).
Rock, J. R. et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl Acad. Sci. USA 106, 12771–12775 (2009).
Youk, J. et al. Three-dimensional human alveolar stem cell culture models reveal infection response to SARS-CoV-2. Cell Stem Cell 27, 905–919.e10 (2020).
Schwank, G. et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 13, 653–658 (2013).
Sachs, N. et al. Long‐term expanding human airway organoids for disease modeling. EMBO J. 38, e100300 (2019).
Han, Y. et al. Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature 589, 270–275 (2021).
Heo, I. et al. Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat. Microbiol. 3, 814–823 (2018).
García, S. R. et al. Novel dynamics of human mucociliary differentiation revealed by single-cell RNA sequencing of nasal epithelial cultures. Development 146, dev.177428 (2019).
Ozer, E. A., Nnah, E., Didelot, X., Whitaker, R. J. & Hauser, A. R. The population structure of Pseudomonas aeruginosa is characterized by genetic isolation of exoU+ and exoS+ lineages. Genome Biol. Evol. 11, 1780–1796 (2019).
Fahy, J. V. & Dickey, B. F. Airway mucus function and dysfunction. N. Engl. J. Med. 363, 2233–2247 (2010).
Liao, C., Huang, X., Wang, Q., Yao, D. & Lu, W. Virulence factors of Pseudomonas aeruginosa and antivirulence strategies to combat its drug resistance. Front. Cell Infect. Microbiol. 12, 926758 (2022).
Williams, P. & Cámara, M. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr. Opin. Microbiol. 12, 182–191 (2009).
Jimenez, P. N. et al. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol. Mol. Biol. Rev. 76, 46–65 (2012).
Persat, A., Inclán, Y. F., Engel, J. N., Stone, H. A. & Gitai, Z. Type IV pili mechanochemically regulate virulence factors in Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA 112, 7563–7568 (2015).
Klockgether, J. & Tümmler, B. Recent advances in understanding Pseudomonas aeruginosa as a pathogen. F1000Research 6, 1261 (2017).
Bleves, S. et al. Protein secretion systems in Pseudomonas aeruginosa: a wealth of pathogenic weapons. Int. J. Med. Microbiol. 300, 534–543 (2010).
O’Toole, G. A. & Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30, 295–304 (1998).
Giltner, C. L., Nguyen, Y. & Burrows, L. L. Type IV pilin proteins: versatile molecular modules. Microbiol. Mol. Biol. Rev. 76, 740–772 (2012).
Laventie, B.-J. et al. A surface-induced asymmetric program promotes tissue colonization by Pseudomonas aeruginosa. Cell Host Microbe 25, 140–152.e6 (2019).
Luo, Y. et al. A hierarchical cascade of second messengers regulates Pseudomonas aeruginosa surface behaviors. mBio 6, e02456-14 (2015).
Kazmierczak, B. I., Schniederberend, M. & Jain, R. Cross-regulation of Pseudomonas motility systems: the intimate relationship between flagella, pili and virulence. Curr. Opin. Microbiol. 28, 78–82 (2015).
Valentini, M. & Filloux, A. Multiple roles of c-di-GMP signaling in bacterial pathogenesis. Annu. Rev. Microbiol. 73, 387–406 (2019).
Laventie, B.-J. & Jenal, U. Surface sensing and adaptation in bacteria. Annu. Rev. Microbiol. 74, 735–760 (2020).
Palmer, K. L., Aye, L. M. & Whiteley, M. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 189, 8079–8087 (2007).
Kuek, L. E. & Lee, R. J. First contact: the role of respiratory cilia in host–pathogen interactions in the airways. Am. J. Physiol. Lung Cell Mol. Physiol. 319, L603–L619 (2020).
Widdicombe, J. H. & Wine, J. J. Airway gland structure and function. Physiol. Rev. 95, 1241–1319 (2015).
Sana, T. G. et al. Internalization of Pseudomonas aeruginosa strain PAO1 into epithelial cells is promoted by interaction of a T6SS effector with the microtubule network. mBio 6, e00712-15 (2015).
Sana, T. G. et al. The second type VI secretion system of Pseudomonas aeruginosa strain PAO1 is regulated by quorum sensing and fur and modulates internalization in epithelial cells. J. Biol. Chem. 287, 27095–27105 (2012).
Hogan, B. L. M. et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15, 123–138 (2014).
Rossy, T. et al. Pseudomonas aeruginosa type IV pili actively induce mucus contraction to form biofilms in tissue-engineered human airways. PLoS Biol. 21, e3002209 (2023).
McDole, J. R. et al. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483, 345–349 (2012).
Jiang, F., Waterfield, N. R., Yang, J., Yang, G. & Jin, Q. A Pseudomonas aeruginosa type VI secretion phospholipase D effector targets both prokaryotic and eukaryotic cells. Cell Host Microbe 15, 600–610 (2014).
Rajan, S. et al. Pseudomonas aeruginosa induction of apoptosis in respiratory epithelial cells. Am. J. Respir. Cell Mol. Biol. 23, 304–312 (2000).
Yamaguchi, T. & Yamada, H. Role of mechanical injury on airway surface in the pathogenesis of Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 144, 1147–1152 (1991).
Kumar, N. G. et al. Pseudomonas aeruginosa can diversify after host cell invasion to establish multiple intracellular niches. mBio 13, e02742-22 (2022).
Chastre, J. et al. Safety, efficacy, and pharmacokinetics of gremubamab (MEDI3902), an anti-Pseudomonas aeruginosa bispecific human monoclonal antibody, in P. aeruginosa-colonised, mechanically ventilated intensive care unit patients: a randomised controlled trial. Crit. Care 26, 355 (2022).
Hotinger, J. A. & May, A. E. Antibodies inhibiting the type III secretion system of Gram-negative pathogenic bacteria. Antibodies 9, 35 (2020).
Jain, M. et al. Type III secretion phenotypes of Pseudomonas aeruginosa strains change during infection of individuals with cystic fibrosis. J. Clin. Microbiol. 42, 5229–5237 (2004).
Huus, K. E. et al. Clinical isolates of Pseudomonas aeruginosa from chronically infected cystic fibrosis patients fail to activate the inflammasome during both stable infection and pulmonary exacerbation. J. Immunol. 196, 3097–3108 (2016).
Rossi, E. et al. Pseudomonas aeruginosa adaptation and evolution in patients with cystic fibrosis. Nat. Rev. Microbiol. 19, 331–342 (2020).
Osan, J. et al. Goblet cell hyperplasia increases SARS-CoV-2 infection in chronic obstructive pulmonary disease. Microbiol. Spectr. 10, e00459-22 (2022).
Adam, D. et al. Cystic fibrosis airway epithelium remodelling: involvement of inflammation. J. Pathol. 235, 408–419 (2015).
Jeffery, P. K. Comparison of the structural and inflammatory features of COPD and asthma. Giles F. Filley Lecture. Chest 117, 251S–260S (2000).
Dovey, M. et al. Ultrastructural morphology of the lung in cystic fibrosis. J. Submicrosc. Cytol. Pathol. 21, 521–534 (1989).
Jeffery, P. K. Remodeling and inflammation of bronchi in asthma and chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 1, 176–183 (2004).
Ravindra, N. G. et al. Single-cell longitudinal analysis of SARS-CoV-2 infection in human airway epithelium identifies target cells, alterations in gene expression, and cell state changes. PLoS Biol. 19, e3001143 (2021).
Lukassen, S. et al. SARS‐CoV‐2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 39, e105114 (2020).
Ackermann, M. et al. Self-destructive cooperation mediated by phenotypic noise. Nature 454, 987–990 (2008).
Diard, M. et al. Stabilization of cooperative virulence by the expression of an avirulent phenotype. Nature 494, 353–356 (2013).
Kotte, O., Volkmer, B., Radzikowski, J. L. & Heinemann, M. Phenotypic bistability in Escherichia coli’s central carbon metabolism. Mol. Syst. Biol. 10, 736 (2014).
Basan, M. et al. A universal trade-off between growth and lag in fluctuating environments. Nature 584, 470–474 (2020).
Bakshi, S. et al. Tracking bacterial lineages in complex and dynamic environments with applications for growth control and persistence. Nat. Microbiol. 6, 783–791 (2021).
Balaban, N. Q., Merrin, J., Chait, R., Kowalik, L. & Leibler, S. Bacterial persistence as a phenotypic switch. Science 305, 1622–1625 (2004).
Arnoldini, M. et al. Bistable expression of virulence genes in Salmonella leads to the formation of an antibiotic-tolerant subpopulation. PLoS Biol. 12, e1001928 (2014).
Manina, G., Griego, A., Singh, L. K., McKinney, J. D. & Dhar, N. Preexisting variation in DNA damage response predicts the fate of single mycobacteria under stress. EMBO J. 38, e101876 (2019).
Keilberg, D., Zavros, Y., Shepherd, B., Salama, N. R. & Ottemann, K. M. Spatial and temporal shifts in bacterial biogeography and gland occupation during the development of a chronic infection. mBio 7, e01705-16 (2016).
Fung, C. et al. High-resolution mapping reveals that microniches in the gastric glands control Helicobacter pylori colonization of the stomach. PLoS Biol. 17, e3000231 (2019).
Garvis, S. et al. Caenorhabditis elegans semi-automated liquid screen reveals a specialized role for the chemotaxis gene cheB2 in Pseudomonas aeruginosa virulence. PLoS Pathog. 5, e1000540 (2009).
Laganenka, L. et al. Chemotaxis and autoinducer-2 signalling mediate colonization and contribute to co-existence of Escherichia coli strains in the murine gut. Nat. Microbiol. 8, 204–217 (2023).
Cooper, K. G. et al. Regulatory protein HilD stimulates Salmonella Typhimurium invasiveness by promoting smooth swimming via the methyl-accepting chemotaxis protein McpC. Nat. Commun. 12, 348 (2021).
Lane, M. C. et al. Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract. Infect. Immun. 73, 7644–7656 (2005).
Broder, U. N., Jaeger, T. & Jenal, U. LadS is a calcium-responsive kinase that induces acute-to-chronic virulence switch in Pseudomonas aeruginosa. Nat. Microbiol. 2, 16184 (2016).
Haubold, B., Klötzl, F. & Pfaffelhuber, P. andi: fast and accurate estimation of evolutionary distances between closely related genomes. Bioinformatics 31, 1169–1175 (2015).
Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296 (2021).
Emms, D. M. & Kelly, S. OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biol. 20, 238 (2019).
Swart, A. L. Pseudomonas aeruginosa breaches respiratory epithelia through goblet cell invasion in a microtissue model. BioImage Archive https://doi.org/10.6019/S-BIAD1083 (2024).
Swart, A. L. & Laventie, B.-J. Pseudomonas aeruginosa breaches respiratory epithelia through goblet cell invasion in a microtissue model. Zenodo https://doi.org/10.5281/zenodo.10650981 (2024).
Acknowledgements
We thank J. Sollier for critical reading and editing of the paper and L. Boeck for expert input; S. Roig, K. Schleicher, A. Loynton-Ferrand and L. Guerard (Biozentrum - Imaging core facility), J. Bögli (Biozentrum - FACS core facility), C. Alampi, C. Tiberi Schmidt and M. Chami (Biozentrum – BioEM facility) for technical support; A. Kaczmarczyk and Fabienne Hamburger for help with complementation constructs; M. Basler (Biozentrum – University of Basel) for strains; and I. Schuler for help with histology preparations. Schematics were created with BioRender.com. This study was supported by the Swiss National Science Foundation NCCR AntiResist (51NF40_180541 to U.J.) and financial support from the Roche Institute of Human Biology (IHB).
Author information
Authors and Affiliations
Contributions
A.L.S., B.-J.L., R.O. and U.J. conceptualized the project. A.L.S., B.-J.L., R.S., T.J., L.L., P.M., S.J., X.Y., E.K., R.O. and U.J. developed the methodology. A.L.S., B.-J.L., P.M., R.O. and U.J. conducted formal analysis. A.L.S., B.-J.L., R.S., T.J., L.L., S.J., X.Y. and E.K. conducted investigations. A.L.S., B.-J.L. and U.J. wrote the original paper draft. R.O. and U.J. acquired funding and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Development of in vitro lung tissues.
a. Schematics of the cell culture process to develop fully differentiated (+21 days post airlift) airway epithelium on Transwell inserts and subsequent apical infection. b, c. Development of the lung tissue post airlift (day 0 to 60) visualized by histology (b) or ICC (c). d, e. Quantification of the thickness (d) and total cell numbers (e) of tissues at day 0, 30 or 60 post airlift, inferred from ICC images. For (d) and (e): Statistical significance was calculated using Kruskal–Wallis H-test with Dunnett’s multiple comparison test. ns; not significant. For panels a-c: n = 3 independent experiments (same donor). For d and e.: n = 15 3D images (30 regions/images for d) examined over 3 independent experiments. Data are presented as mean values +/− SD. f, g. Comparison of airway epithelium on Transwell inserts from 3 donors visualized by histology (f) or ICC (g). n = 3 independent experiments (different donors). Histology: hematoxylin and eosin staining. ICC staining: nuclei: DAPI, mucus: Muc5AC, actin: phalloidin-647, cilia: acetylated-α-tubulin.
Extended Data Fig. 2 Cellular composition and functional properties of the bronchial lung epithelium model.
a, b. Visualization (a) and quantification (b) of the major cell types present in the bronchial epithelium by ICC or FACS, respectively. n = 2 independent experiments (same donor). c. Measurements of cilia beating frequencies by microscopy at day 30 or 60 post airlift. n = 36 inserts over 3 independent experiments (same donor); Box plot: center line, median; box limits, upper and lower quartiles; whiskers, min-max. d, e. Assessment of epithelial barrier integrity (TEER) of lung epithelium at day 0, 30 or 60 post airlift (d, n = 3 independent experiments, same donor) and epithelia from 3 different donors (e, n = 5 independent experiments). For (c), (d) and (e): statistical significance was calculated using Kruskal–Wallis H-test with Dunnett’s multiple comparison test. ns; not significant. f. Visualization of tight junctions by ICC of lung epithelium at day 0 or 30 post airlift. g. Assessment of epithelial barrier integrity (Lucifer yellow permeability) of lung epithelium from 3 different donors (n = 6 inserts over 3 independent experiments). h. Wound healing after physical tissue damage (indicated by the white arrow) of the lung epithelium was followed by time-lapse live imaging for 36 hours (see: Supplementary Information Movie 2). For b, c and f: data are presented as mean values +/− SD. For d, e and g: data are presented as mean values +/− SD. Staining: nuclei: DAPI, cycling basal cells: mucus: Muc5AC, cycling basal cells: α-Ki67, basal cells: α-p63, club cells: α-SCG1A1, cilia: acetylated-α-tubulin, actin: phalloidin-647, tight junctions: ZO-1 Alexa Fluor 555, membrane: CellMask.
Extended Data Fig. 3 Taxonomic distribution of virulence factors among 285 Pseudomonas aeruginosa complete genomes.
a. The phylogenetic tree depicts the isolation source of the PAO1 (blue arc) and PA14 (orange arc) clades. Purple: clinical isolates, black dots indicate clinical strains isolated from human airways, teal: environmental isolates, grey: undetermined or other sources. b. The phylogenetic trees depict the presence of an orthologue of the virulence factors (indicated in the center, using PAO1 nomenclature) among these strains. Presence: purple or pink leaflet, absence: uncolored leaflet. For pilA, two types of homologs were identified within the considered taxonomic range. Branches length relate to genetic distance with an arbitrary scale unit.
Extended Data Fig. 4 Human airway model exposes distinct steps of P. aeruginosa tissue colonization.
a. Images of the lung tissue surface before and after washing inserts with HBSS shows efficient mucus removal. Staining: mucus: Jacalin-fluorescein (green), membrane: CellMask DeepRed (purple). b. P. aeruginosa growth on the apical side of the lung tissues (continuous line) and spread to the basal compartment after tissue breaching (dotted line) with (black) and without (blue) apical mucus upon infection. c. Epithelial barrier integrity (TEER, plain lines) and tissue viability (LDH release, dashed lines) during infection in absence (blue) or presence (black) of mucus. d. Stills of live imaging (25×) of apoptotic events upon infection with P. aeruginosa. Staining: apoptotic cells: NucView (caspase-3/7 activity), membrane: CellMask DeepRed. e. Quantification of dead epithelial cells by FACS, using a viability marker (Zombie NIR), upon infection with P. aeruginosa. Unpaired t test (two-tailed) compared to uninfected; ** P = 0.0011. f. P. aeruginosa population on the airway epithelium (continuous line) and in the basal compartment (dashed line) with PneumaCult-ALI culture medium (black) or HBSS buffer (green) in the basolateral compartment. g, h, i. P. aeruginosa population on the apical (g) and basal side (h) of the lung tissues as well as tissue viability (LDH release) (i) upon infection with different inoculum sizes as indicated. For panels a-i: same donor. j, k. Comparison for 3 different donors of P. aeruginosa growth on the apical side of the lung tissues (continuous line) and spread to the basal compartment after tissue breaching (dotted line) with (black) (j) as well as tissue viability (LDH release) (k). For panels a-f, j, k: inoculum = 103. For all panels n = 3 independent experiments. For j and k: data are presented as mean values +/− SD.
Extended Data Fig. 5 Virulence factors promoting P. aeruginosa lung tissue colonization and breaching.
a. Growth of P. aeruginosa wild type and mutant strains in SCFM medium. b, e, f. Complementation of the PAO1 ΔpilA and ΔpscC mutants. Apical replication (b), basolateral invasion (e) and tissue viability (LDH release) (f) of the mutants and respective complemented strains were assessed. c. P. aeruginosa population in the basolateral compartment up to 48 h p.i. Dunnett’s One-Way ANOVA compared to wild type; P = 0.0274 (ΔfliC), <0.0001 (ΔpilA), 0.0003 (ΔpscC), 0.1585 (Δpch) or <0.0001 (ΔcyaAB); ns: not significant. d. tissue viability (LDH release) during infection with P. aeruginosa. g. Percentage actively beating cilia (>3.5Hz) of the airway epithelium during P. aeruginosa infection measured by live microscopy. h. Stills of live imaging (10x) of the tissue integrity during with P. aeruginosa wild type and Δpch mutant over time. Cell membrane staining: CellMask DeepRed. Inoculum = 101, 103 or 106 c.f.u. as indicated on the left. Representative images, quantified in Fig. 2f. i. P. aeruginosa ΔfliC colonization of the airway epithelium. 10x overview of the entire Transwell (upper panel) and 100× details (middle and lower panels) (ICC). Related to Fig. 2c, g. Staining: nuclei: DAPI, actin: phalloidin-647. j. Quantification of the airway epithelium attachment by P. aeruginosa mutants from ICC images. Related to Fig. 2h. Dunnett’s One-Way ANOVA compared to wild type; ** P < 0.01; ns: not significant. For all panels: Inoculum = 103, n = 3 independent experiments (same donor). For j: n = 51 images examined over 3 independent experiments. For b-f: data are presented as mean values +/− SD.
Extended Data Fig. 6 Selective internalization into goblet cells allows P. aeruginosa to invade lung epithelia.
a-d. Representative examples of 100× ICC images of P. aeruginosa attaching to and invading lung epithelial cells at 6 h and 12 h p.i. Attached (a), internalized (b) and replicating bacteria (c) are shown. Goblet cell invasion is illustrated in (d) using an additional stain for mucus, and was consistently observed in all three donors tested (e). For all panels: Inoculum = 103. n = 3 independent experiments. For a-d: same donor, for e: different donors. Staining: bacteria: constitutive chromosomal expression of mNeonGreen (yellow) or mCherry (pink), nuclei: DAPI, mucus: Muc5AC, membrane: CellMask, actin: phalloidin-647, tight junctions: ZO-1 Alexa Fluor 555.
Extended Data Fig. 7 Internalization of P. aeruginosa and sloughing of infected epithelial cells.
a. Quantification of the complemented T6SS mutants ΔH2 (ΔtssL2) and ΔH3 (ΔtssM3) internalized in epithelial cells 12 hours p.i. Unpaired t test (two-tailed) compared to wild type; P = <0.0001 (ΔtssL2), 0.0321 (ΔtssL2+tssL2), 0.0003 (ΔtssM3) or 0.0444 (Δ tssM3+tssM3); n = 51 images examined over 3 independent experiments. Data are presented as mean values +/− SD. b, c. Representative ICC images (100×) depicting sloughing of epithelial cells infected with P. aeruginosa (b) and colonization of sloughed cells by extracellular bacteria (c). For all panels: inoculum = 103. n = 3 independent experiments (same donor). Staining: bacteria: constitutive chromosomal expression of mNeonGreen (yellow) or mCherry (pink), nuclei: DAPI, actin: phalloidin-647.
Extended Data Fig. 8 Intracellular replication and localization of P. aeruginosa T3SS mutants.
a, b. Schematic (a) and 100× ICC images (b) of the intracellular replication of a P. aeruginosa T3SS mutant (ΔpscC) resulting in mechanical cell rupture and release of intracellular bacteria. The progression shown in (b) is a compilation of different stages of breaching sites observed in samples 24 h p.i. Staining: bacteria: constitutive chromosomal expression of mNeonGreen, nuclei: DAPI, membrane: CellMask, tight junctions: ZO-1 Alexa Fluor 555. Inoculum = 103. c. Subcellular localization in lung epithelial cells of P. aeruginosa T3SS mutant (ΔpscC) compared to wild type PAO1 using Transmission Electron Microscopy. The tissue sections were performed on the epithelium apical surface, and the cilia are visible on the left of overview images. Intracellular bacteria are marked with white asterisks. Inoculum = 106 (WT) or 103 (ΔpscC). For all panels: n = 3 independent experiments (same donor).
Extended Data Fig. 9 P. aeruginosa breaching and basolateral invasion of the lower airway epithelium.
a, b. Schematic (a) and 100× ICC images (b) of P. aeruginosa invasion of the basolateral side of the lung epithelium after successful establishment of a breaching site. c. Progression of lower airway infection by P. aeruginosa PAO1 wild type recorded by SEM. d, e. Immunocytochemistry images of P. aeruginosa breaching sites seen for all three donors tested (d) and for the PAO1 ΔpscC complemented mutants (e). For all panels: Inoculum = 103. n = 3 independent experiments (same donor [a-c and e] or different donors [d]). ICC staining: bacteria: constitutive chromosomal expression of mNeonGreen, nuclei: DAPI, tight junctions: ZO-1 Alexa Fluor 555.
Extended Data Fig. 10 Rapid colonization of breaching sites is driven by chemotaxis.
Breaching site colonization in samples infected with a 1:1 mixture of differentially labeled (pink or yellow) wild type and ΔcheA1/2 mutant strains. a. Wild type + wild type example of co-colonized breach. b. Wild type + ΔcheA1/2 example of co-colonized breach. c. Wild type + ΔcheA1/2 example of a breach solely colonize by wild type. d. ΔcheA1/2 + ΔcheA1/2 example of breaches colonized by only one of the two ΔcheA1/2 strains. For all panels: inoculum = 103, n = 3 independent experiments (same donor). Staining: bacteria: constitutive chromosomal expression of mNeonGreen or mCherry, nuclei: DAPI, actin: phalloidin-647.
Supplementary information
Supplementary Information
Suplementary Fig. 1.
Supplementary Video 1
Cilia beating and mucus flow. Real-time recording of beating cilia and mucus flow on the apical surface of the lower airway model. Staining: membrane, CellMask.
Supplementary Video 2
Tissue regeneration and P. aeruginosa infection of injured lung epithelium. Wound healing after physical tissue damage of uninfected lower airway epithelium (top panels) and selective P. aeruginosa invasion of the insured site upon infection (bottom panels). Staining: bacteria, constitutive chromosomal expression of mNeonGreen; membrane, CellMask. Inoculum = 103, n = 3 (same donor).
Supplementary Video 3
Increased frequency of apoptotic lung cell events upon infection with P. aeruginosa. Live imaging (25×) of apoptotic events upon infection with P. aeruginosa. Staining: apoptotic cells, NucView (caspase-3/7 activity); membrane, CellMask. Inoculum = 103, n = 3 (same donor).
Supplementary Video 4
Pseudomonas aeruginosa invades lung cells. Example of immunocytochemistry image (100×) where bacteria are visible both on the lung epithelium surface and internalized in a lung cell (12 h p.i.). P. aeruginosa, mNeonGreen (yellow); actin, phalloidin-647 (purple); nuclei, DAPI (blue). To facilitate the visualization, cells where segmented and rendered in 3D using IMARIS.
Supplementary Tables
Supplementary Table 1. Bacterial strains used in this study. Related to key resources table. Table 2. Key resources used in this study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Swart, A.L., Laventie, BJ., Sütterlin, R. et al. Pseudomonas aeruginosa breaches respiratory epithelia through goblet cell invasion in a microtissue model. Nat Microbiol 9, 1725–1737 (2024). https://doi.org/10.1038/s41564-024-01718-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-024-01718-6
- Springer Nature Limited