Abstract
The relationship between reduced serum uric acid (UA) levels and Parkinson’s disease (PD), particularly purine metabolic pathways, is not fully understood. Our study compared serum and cerebrospinal fluid (CSF) levels of inosine, hypoxanthine, xanthine, and UA in PD patients and healthy controls. We analyzed 132 samples (serum, 45 PD, and 29 age- and sex-matched healthy controls; CSF, 39 PD, and 19 age- and sex-matched healthy controls) using liquid chromatography-tandem mass spectrometry. Results showed significantly lower serum and CSF UA levels in PD patients than in controls (p < 0.0001; effect size r = 0.5007 in serum, p = 0.0046; r = 0.3720 in CSF). Decreased serum hypoxanthine levels were observed (p = 0.0002; r = 0.4338) in PD patients compared to controls with decreased CSF inosine and hypoxanthine levels (p < 0.0001, r = 0.5396: p = 0.0276, r = 0.2893). A general linear model analysis indicated that the reduced UA levels were mainly due to external factors such as sex and weight in serum and age and weight in CSF unrelated to the purine metabolic pathway. Our findings highlight that decreased UA levels in PD are influenced by factors beyond purine metabolism, including external factors such as sex, weight, and age, emphasizing the need for further research into the underlying mechanisms and potential therapeutic approaches.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disorder. Despite the undetermined pathogenesis of PD, serum uric acid (UA) levels are decreased in patients with PD1,2. A meta-analysis of dose–response studies established a correlation between a 6% increased risk of PD and every 1 mg/dL decrement in serum UA level3. Additionally, low serum UA levels have been associated with progression4 and non-motor dysfunction5 in PD.
Although there is evidence of decreased UA levels in patients with PD, the causal relationship between UA levels and PD onset or progression remains unclear. Additionally, the impact of blood-brain barrier (BBB) dynamics on UA transport requires further investigation. Although urate transporter gene polymorphisms determine UA levels, a Mendelian randomization study failed to determine any correlation between gene polymorphisms and PD development6,7,8. Furthermore, patients with rapid eye movement (REM) sleep behavior disorder, a prodromal feature of PD, do not exhibit a notable reduction in serum UA levels compared with healthy controls9,10. A recent double-blind study also found that increasing serum UA levels by inosine administration did not improve any clinical symptoms or inhibit PD progression11. These findings suggest that the reduction in serum UA levels in PD is not a causative factor in the onset or progression of the disease but rather a consequence of impaired mitochondrial function, altered gastrointestinal function, and impaired motor function, which may also influence the onset and progression of PD (reverse causation)12. Nevertheless, there are no reports examining the relationship between cerebrospinal fluid (CSF) UA levels and clinical symptoms in PD. Furthermore, studies on the relationship between blood and CSF UA levels have only been conducted in a limited number of cases.
Alterations in purine metabolism are also key to understanding the pathophysiology behind lower UA levels in PD patients. UA production follows this pathway: inosine monophosphate (IMP) → inosine → hypoxanthine → xanthine → UA (Fig. 1). An mRNA study revealed changes in purine metabolism gene expression in the substantia nigra of PD patients13. The metabolite hypoxanthine, recycled to the nucleotide IMP by hypoxanthine-guanine phosphoribosyltransferase, plays a significant role in producing adenosine triphosphate (ATP) alongside mitochondria in humans and is decreased in the leucine rich-repeat kinase 2 (LRRK2) -related PD sera14. Although the levels of inosine, hypoxanthine, and xanthine, which are “upstream” in the purine metabolic pathway, may affect UA levels, the “downstream” product in patients with PD, no studies have explored upstream purine metabolism in the CSF and blood of patients with PD. Addressing this issue should help us understand why UA levels are low in the blood of PD patients and explain the contradiction that, although lower UA levels are associated with worse PD symptoms, increasing these levels does not lead to symptom improvement.
UA is produced through the following process: inosine monophosphate (IMP) → inosine → hypoxanthine → xanthine → UA. We evaluated metabolites colored with yellow in this study. GTP guanosine triphosphate, GDP guanosine diphosphate, GMP guanosine monophosphate, XMP 5’-xanthylic acid, IMP inosine monophosphate, S-AMP succinyl adenosine monophosphate, AMP adenosine monophosphate, ADP adenosine diphosphate, ATP adenosine triphosphate, PPRP phosphoribosyl pyrophosphate, PNP purine nucleoside phosphorylase, HGPRT hypoxanthine-guanine phosphoribosyltransferase, XOR xanthine oxidoreductase, GDA guanine deaminase, ADA adenine deaminase.
Therefore, this study aimed to 1) determine the levels of inosine, hypoxanthine, xanthine, and UA in the serum and CSF in patients with PD in comparison with healthy controls; 2) investigate the effect of changes in the purine metabolic system on UA levels in the serum and CSF of patients with PD using a general linear model (GLM) that assumes the production of downstream substances along metabolic pathways; and 3) investigate the influence of serum UA levels on CSF UA levels, the correlation between serum and CSF UA levels, the difference in concentrations between serum and CSF UA, and the relationship between CSF UA and blood–brain barrier permeability.
Results
Demographics
This study found no significant disparities in demographic parameters, such as age, sex, height, weight, or body mass index (BMI), between the PD and control groups. The PD group exhibited significantly lower Japanese version of the Montreal Cognitive Assessment (MoCA-J) and Japanese version of Addenbrooke’s Cognitive Examination-Revised (ACE-R) scores and higher Geriatric Depression Scale-15 (GDS-15) scores than the control group (Table 1).
Serum study
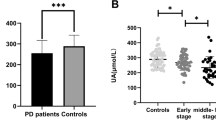
Serum UA (p < 0.0001) and hypoxanthine (p = 0.0002) levels were significantly lower in the PD group compared with the control group with a large effect size (r = 0.5007) and a moderate effect size (r = 0.4338), respectively (Fig. 2). Regarding the receiver operating characteristic (ROC) analysis, UA and hypoxanthine effectively distinguished the PD and control groups (areas under the curve [AUCs]: UA, 0.7985; hypoxanthine, 0.7586). Similar results were obtained in an additional analysis of 36 PD patients and 29 controls, excluding patients taking diuretics and/or oral aspirin or having a previous history of malignancy that affects purine metabolites (Supplementary Figure 1). As shown in Supplementary Table 1, multiple regression analyses for each metabolite, including BMI as covariable, revealed that UA concentrations were negatively affected by PD (t-value = −5.30, p < 0.0001), followed by positively affected by BMI (t-value = 2.17, p = 0.0336) but hypoxanthine concentrations were negatively affected by only PD (t-value = −2.63, p = 0.0105). No significant effects of PD and BMI on xanthine or inosine were observed.
This figure underscores the altered metabolism of serum purine compounds in PD. Serum hypoxanthine levels are significantly reduced in PD patients compared to controls (p = 0.0002). PD patients also exhibit significantly lower serum uric acid levels than controls (p < 0.0001). Analysis shows no significant variation in serum inosine and xanthine levels between the control and PD groups. Each panel presents data as mean ± standard deviation with the range in parentheses. Additionally, the effect size (r), representing the magnitude of difference between groups, along with the Area Under the Curve (AUC), specificity, and sensitivity values from Receiver Operating Characteristic (ROC) analysis, are provided to assess the diagnostic performance of these metabolites in distinguishing PD from control subjects.
The correlations between serum purine metabolites and clinical parameters in the PD group are summarized in Supplementary Table 2. The PD group demonstrated a positive correlation between UA levels and both height (rs = 0.4711, p = 0.0011) and weight (rs = 0.3128, p = 0.0364), as well as a negative correlation between hypoxanthine levels and Japanese version of the REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ-J) scores (rs = −0.3027, p = 0.0433), Odor Stick Identification Test for Japanese (OSIT-J) scores (rs = −0.3173, p = 0.0358), and the Epworth Sleepiness Scale (ESS) scores (rs = −0.3051, p = 0.0415). In the control group, UA levels were positively correlated with height (r = 0.4721, p = 0.0097), weight (r = 0.6493, p = 0.0001), and BMI (r = 0.4619, p = 0.0116). There were no gender differences in serum levels of inosine, hypoxanthine, and xanthine in either the PD group or the control. Interestingly, among patients with PD, men had significantly higher serum UA levels (P = 0.0177).
GLM analysis indicated that UA concentrations were not significantly associated with upstream xanthine concentrations. However, extrinsic factor such as weight had a significant impact on UA concentrations, and PD had a negative effect on them. Hypoxanthine and xanthine concentrations were influenced by both upstream concentrations, and sex, weight and BMI had an impact on xanthine concentrations (Table 2).
CSF study
Demographic parameters, such as age, sex, height, and weight, were similar between the PD and control groups, with no significant differences. However, a significant difference in BMI was observed, with lower values observed in the PD group than in the control group (Table 1). The PD group had significantly lower UA (p = 0.0046), hypoxanthine (p = 0.0276), and inosine (p < 0.0001) levels than the control group with moderate (r = 0.3720), moderate (r = 0.2893), and large effect sizes (r = 0.5396), respectively (Fig. 3). Regarding the ROC analysis, inosine and UA effectively distinguished the PD and control groups (AUC: inosine, 0.8354; UA, 0.7314). Similar results were obtained in an additional analysis of 32 PD patients and 16 controls, excluding patients taking diuretics and/or oral aspirin or having a previous history of malignancy that affects purine metabolites (Supplementary Fig. 2). As shown in Supplementary Table 3, multiple regression analyses for each metabolite, including BMI as covariable, revealed that inosine and UA concentrations were negatively affected by only PD (t-value = −4.65, p < 0.0001; and t-value = −3.17, p < 0.0025; respectively). Hypoxanthine concentrations were significantly positively affected by BMI (t-value = 2.62, p = 0.0114), but no significant effect by PD was confirmed. As for xanthine, no significant effects of PD and BMI were observed (Supplementary Table 3). There were no gender differences in CSF levels of inosine, hypoxanthine, and xanthine in either the PD group or controls. However, men had a significantly higher UA concentration in the CSF in the control group (p = 0.0485).
This figure underscores the altered metabolism of CSF purine compounds in PD. CSF inosine, hypoxanthine, and UA levels are significantly reduced in PD patients compared to controls. Analysis shows no significant variation in CSF xanthine levels between the control and PD groups. Each panel presents data as mean ± standard deviation with the range in parentheses. Additionally, the effect size (r), representing the magnitude of difference between groups, along with the Area Under the Curve (AUC), specificity, and sensitivity values from Receiver Operating Characteristic (ROC) analysis, are provided to assess the diagnostic performance of these metabolites in distinguishing PD from control subjects.
The correlations between CSF purine metabolites and clinical parameters in the PD group are summarized in Supplementary Table 4. The PD group demonstrated a negative correlation between UA levels and ESS scores (rs = −0.3827, p = 0.0162).
GLM analysis revealed a negative correlation between UA values and upstream xanthine concentrations. The concentration of UA is significantly affected by age. On the other hand, hypoxanthine and xanthine concentrations are positively correlated with upstream inosine and hypoxanthine concentrations, respectively. The levels of hypoxanthine are significantly influenced by sex and weight, whereas the levels of xanthine are significantly influenced by age (Fig. 3, Table 3).
Relationship between serum and CSF in PD
A significant correlation was observed between serum and CSF UA levels in the PD group (rs = 0.3794, p = 0.0188) (Supplementary Fig. 3). No significant correlation was found between the serum and CSF levels of inosine, hypoxanthine, and xanthine.
Examination of the CSF-to-plasma ratio showed that UA (0.1081 ± 0.0372) and inosine (0.396 ± 0.370) had lower CSF values than serum values. Conversely, hypoxanthine had higher CSF values than plasma values (2.721 ± 1.788), and xanthine values were relatively similar (1.089 ± 0.980).
A significant correlation was observed between CSF UA and albumin quotient (Qalb) (rs = 0.4361, p = 0.0088), but not for inosine, hypoxanthine, or xanthine. Qalb demonstrated a significant positive correlation with the CSF-to-plasma UA ratio (rs = 0.5199, p = 0.0014) but not with inosine, hypoxanthine, or xanthine.
Discussion
We assessed the levels of serum and CSF inosine, hypoxanthine, xanthine, and UA in PD patients compared to controls, exploring the impact of purine metabolism alterations on UA levels through GLM analysis. We observed significant reductions in both serum and CSF UA and hypoxanthine levels, along with decreased CSF inosine, in PD patients. These metabolites were effective in distinguishing PD patients from controls. BMI positively influenced UA levels in serum and hypoxanthine levels in CSF. According to the GLM analysis, UA levels were unaffected by upstream xanthine in both serum and CSF, and they exhibited negative associations with PD and positive associations with body weight and males. Both hypoxanthine and xanthine levels were influenced by their upstream metabolites in both serum and CSF, with hypoxanthine in CSF also positively affected by body weight and male sex. Our findings indicate that UA levels in serum and CSF are not directly related to upstream xanthine concentrations, suggesting influences beyond traditional purine metabolism pathways and highlighting the involvement of the purine recycling system in PD15.
In the current study, as reported previously1,2,3,4,5, the PD group had significantly lower serum UA levels than the control group. GLM analysis indicated that the PD and control groups were significantly influenced by factors other than purine metabolism, suggesting that there are factors contributing to the greater reduction in serum and CSF UA values in the PD group compared with the control group. This highlights the importance of considering reverse causation or involvement of other factors in understanding the relationship between UA levels and PD progression. Further studies are necessary to investigate the potential effect of changes in the gut microbiota, poor nutritional status16, weight loss17, and physical inactivity18, which affect both the pathogenesis of PD and the decline in UA levels.
This study also confirmed that CSF UA values were lower in the PD group compared with the control group19,20,21. GLM analysis suggested that factors other than the purine metabolic system might influence CSF UA values. Few xanthine oxidoreductases are present in the central nervous system, which convert xanthine to UA, and UA does not cross the blood–brain barrier22. Thus, UA in the CSF is transferred exclusively from the blood through urate transporters in the choroid plexus23. This study revealed that CSF UA values correlated with serum UA and Qalb values, suggesting that disruption of the blood–brain barrier affects CSF UA values. However, the CSF concentration of UA was low, approximately one-tenth of the serum concentration. Although a significant correlation exists between serum and CSF UA levels, the absolute concentration of UA in the CSF is approximately one-tenth of that in the serum, suggesting a constrained transfer. This may explain why inosine administration, which increases serum UA levels, does not lead to clinical improvement in PD symptoms. Additionally, while BBB disruption does affect CSF UA levels, the non-linear and regulated transport processes imply that merely increasing serum UA is insufficient to impact CSF concentrations significantly.
The current study found a significant reduction in inosine levels in the CSF of patients with PD but not in the serum. There was no significant correlation between CSF inosine and Qalb. Inosine is generated from nucleotides, such as IMP and Adenosine monophosphate (AMP)24 (Fig. 1). As inosine can cross the blood–brain barrier25, blood inosine levels may also influence CSF inosine levels. However, exogenous inosine is quickly taken up by the intestinal epithelium and hepatocytes, which metabolize most of it to hypoxanthine. This supports the view that serum inosine did not show a significant reduction in the PD group compared with the control group26. Furthermore, mouse experiments have shown that although a slight increase in brain inosine concentration was observed after administering a large dose of inosine, the concentration returned to baseline 2 h later27, suggesting a limited effect of blood inosine on CSF inosine. Several studies have reported abnormalities in mitochondrial function28, the glycolytic system29, and fatty acid metabolism30 in patients with PD. The reduced CSF inosine levels may reflect a reduction in nucleotide production in the central nervous system, and further investigation is necessary.
Compared with healthy controls, patients with PD had substantially lower serum and CSF hypoxanthine levels. Over 90% of hypoxanthine is recycled as IMP31 in the salvage pathway (Fig. 1), which plays a crucial role in preserving energy-yielding purines and ATP in humans because of the limited activity of de novo synthesis of purine nucleotide systems relative to other mammals and species32. Hypoxanthine in the blood crosses the blood–brain barrier and is used in the central nervous system for ATP production22,25. The CSF study revealed hypoxanthine levels that were twice as high as those in the serum and strongly correlated with inosine generated from IMP and AMP in the brain. GLM analysis revealed no notable effects except for the purine metabolic system in CSF. These findings suggest that the decline in CSF hypoxanthine mirrors a reduction in nucleotides in the brain, similar to inosine.
A decrease in serum hypoxanthine in PD has been previously documented in cases of LRRK2 mutations14; however, a comprehensive metabolomics study of eight patients with sporadic PD indicated elevated levels33. However, this study did not mention the duration of plasma storage. Despite reports indicating that a prolonged time for plasma separation leads to elevated hypoxanthine and xanthine values leached from erythrocytes34, our study processed blood samples within 2 h, which is not considered to affect the results35. Serum hypoxanthine is mainly produced in muscles21 and adipose tissues36, and patients with PD exhibit decreased physical activity37 and abnormalities in lipid systems38, which may contribute to the reduction in hypoxanthine levels.
Febuxostat, an inhibitor of inosine and xanthine oxidoreductase, may improve parkinsonism in PD by augmenting plasma hypoxanthine values irrespective of decreased UA levels39. As the present study found no correlation between changes in UA levels and improvements in parkinsonism, in addition to the considerably lower UA levels in the CSF than in the blood and the limited impact of serum UA on CSF UA levels, our results suggest that treatments aimed at elevating serum UA levels might have limited efficacy in improving parkinsonism. The present findings suggest that impairments in the salvage pathway related to purine recycling may serve as a therapeutic target for PD.
Evidence suggests that PD is associated with BBB disruption, endothelial cell degeneration, and neovascularization40. Our findings indicate that, while BBB disruption has a significant, albeit limited, impact on CSF UA levels, severe BBB impairment may lead to more altered central UA levels, diverging from typical patterns. Additionally, purine metabolites, such as inosine, hypoxanthine, and xanthine, are likely influenced by changes in BBB integrity. Recent advances in neuroimaging techniques have provided a more detailed visualization of BBB function, enhancing our understanding of its role in brain metabolism. Furthermore, the development of focused ultrasound (FUS) as a method for transiently opening the BBB presents new therapeutic possibilities41. While FUS can transiently open the BBB, enhancing UA levels in the CSF via inosine remains speculative. Given our findings of nonlinear UA transport across the BBB, the impact of FUS combined with inosine requires cautious interpretation. Considering the complex transport dynamics involved, future research should explore this combination’s efficacy. Multidisciplinary approaches, merging novel neuroimaging technologies with therapeutic modalities such as FUS, may further investigate the relationship between BBB permeability and UA metabolism in the CSF and unveil new therapeutic targets and strategies to alleviate PD symptoms and progression.
This study had some limitations. First, we did not assess factors such as exercise, nutritional indices, muscle volume, or adipose tissue, which may affect UA and hypoxanthine levels. Second, our cross-sectional study did not examine long-term changes in UA, inosine, hypoxanthine, and xanthine levels and their correlation with clinical indices. Third, we did not examine the influence of adenosine and guanosine on inosine levels or the purine recycling system. Fourth, this was a single-center study, and the number of participants was relatively limited. Fifth, the intervals from collection to analysis of serum and CSF samples were significantly different between the PD group and the control. Further longitudinal study will be needed to clarify whether such differences can influence the results. Sixth, we should mention a limitation of statistical analysis. While multiple molecules in the urine metabolic pathway were studied simultaneously, we did not apply multiple comparison adjustments, implying that false positive rates could be larger than the significance level. Finally, we did not explore the relationship between nucleotide status in the brain and inosine and hypoxanthine. Future longitudinal studies with a larger number of cases, along with other clinical variables, multimodal imaging, and omics data addressing these limitations, will provide novel insights into the pathogenesis of PD related to energy imbalance.
In conclusion, our findings suggest that the reduction in UA levels in PD is mainly due to sources other than the purine metabolic system. Our findings suggest that decreased UA levels in PD patients are influenced by factors beyond purine metabolism, including external factors such as sex, weight, and age. The observed reductions in serum and CSF hypoxanthine and CSF inosine highlight potential impairments in purine recycling pathways, warranting further research into alternative therapeutic strategies.
Methods
Participants
We enrolled 45 patients with PD (30 males and 15 females; age at examination, 68.4 ± 7.3 years; mean disease duration, 88.9 ± 53.7 months) admitted to Fujita Health University Hospital between July 2020 and September 2021 who met the Movement Disorder Society (MDS) diagnostic criteria42, 29 age- and sex-matched healthy participants (15 males and 14 females; age at examination, 68.7 ± 9.2 years) in the serum study, and 19 age-matched participants (13 males and six females; age at examination, 68.2 ± 9.2 years) in the CSF study. None of the participants took xanthine oxidoreductase inhibitors. In the serum study, 9 out of 45 PD patients and 1 out of 30 controls, and in the CSF study, 8 out of 39 PD patients and 4 out of 19 controls were either treated with diuretics and/or oral aspirin or had a previous history of malignancy. We are conducting an ongoing aging registry study that collects CSF from individuals with no neurodegenerative disease and undergoing epidural anesthesia for surgical treatment of urinary tract stones or prostatic hypertrophy in the urology department at Fujita Health University, Japan. We excluded patients who had structural lesions such as stroke, tumors, and moderate or severe atrophic changes on MRI. We ascertained the appropriate sample size for our study based on the mean and standard deviation from the precedent Japanese investigation14. The sample size was determined using OpenEpi (version 3), an open-source calculator, which revealed a total sample size of 74 under a confidence interval of 95%, power of 80%, and ratio of sample size 1 (http://www.openepi.com/Menu/OE_Menu.htm). Ethical approval was obtained from the ethics committees of Fujita Health University Hospital, and written informed consent was procured from all participants before their involvement.
Clinical evaluation
To elucidate the relationship between purine metabolites and various clinical indices, we assessed patients with PD using the MDS-Unified Parkinson’s Disease Rating Scale (UPDRS) Parts I–IV and various cognitive function tests, including the Frontal Assessment Battery, Japanese version of ACE-R, Mini-Mental State Examination, and MoCA-J. Additionally, we evaluated the quality of life of patients with PD using the Parkinson’s Disease Questionnaire-39, depression using the GDS-15, olfaction using the OSIT-J, sleep behavior disorder using the RBDSQ-J, autonomic dysfunction using the Scales for Outcomes in Parkinson’s Disease - Autonomic Dysfunction, excessive daytime sleepiness using the Epworth Sleepiness Scale, and impulsivity using the Japanese version of the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s disease. All neurological evaluations were performed during the ON medication state.
Blood and CSF sample preparation
We collected blood samples in the morning after >6 h of fasting. The samples were centrifuged for 10 min at 1500 g, and 500 µL aliquots of serum were immediately frozen at −80 °C until assay. We divided each aliquot to avoid repeated freezing and thawing cycles. We also obtained CSF by a standard lumbar puncture during fasting. If there were signs of bloody CSF at the time of collection, the first 1–2 mL were discarded. We collected approximately 2 mL immediately after a puncture with a sterile sample tube and used it for protein, glucose, and cell counts. We obtained the subsequent 6–7 mL directly into 15 mL of polypropylene tube, transferred it without inverted mixing, placed it on ice, centrifuged it at 1500 g for 10 min at 4 °C, aliquoted it, and froze it at −80 °C for future analysis. Blood and CSF samples were collected 12 h after L-dopa administration in patients with PD. We collected CSF and blood within 30 min of each other on the same day in patients with PD.
CSF/serum Qalb
In assessing the kinetics of each purine metabolite between CSF and serum, to estimate the blood–brain barrier permeability, Qalb was calculated as follows:
Data on CSF and serum albumin levels were retrospectively collected from medical records.
Purine metabolite measurement
Hypoxanthine, inosine, xanthine, mass spectrometry (MS)-grade acetonitrile, and ammonium acetate were purchased from Fujifilm Wako (Osaka, Japan). UA sodium salt was purchased from Merck Sigma-Aldrich (St. Louis, MO). Hypoxanthine-15N4 and UA-15N2 were purchased from Cambridge Isotope Laboratories (Tewksbury, MA).
A Nexera ultra-high-performance liquid chromatography system (Shimadzu, Kyoto, Japan) was used, consisting of a gradient pump, vacuum degassed, and an autosampler. An Intrada Organic Acid column (150 × 2 mm, Imtakt, Kyoto, Japan) was used at 60 °C. Five microliters of the analyte solution were injected into the column and eluted at a flow rate of 0.2 mL/min using a step gradient alternating between A, a mixture of acetonitrile/100 mM ammonium acetate (95:5), and B, a mixture of acetonitrile/100 mM ammonium acetate (10:90). The gradient began with 15% B and was then programmed as follows: 0–5 min, gradient to 55% B; 5–5.1 min, gradient to 70% B; 5.1–10 min, gradient to 100% B; 10–10.1 min, gradient back to 15% B; and 10.1–13 min, hold at 15% B to re-equilibrate the column.
An LCMS-8060 or LCMS-8060NX triple quadrupole mass spectrometer (Shimadzu) equipped with an electrospray ionization source was used for the MS/MS analysis. Hypoxanthine and inosine were analyzed in positive-ion multiple reaction monitoring (MRM) mode, and xanthine and UA were analyzed in negative-ion MRM mode. The mass transitions used were as follows: m/z 137 > 119 for hypoxanthine, m/z 269 > 137 for inosine, m/z 151 > 108 for xanthine, m/z 167 > 124 for UA, m/z 141 > 123 for hypoxanthine-15N4, and m/z 169 > 125 for UA-15N2.
To generate a calibration curve, a mixed standard solution of hypoxanthine, xanthine, inosine, and UA was diluted to 0.05, 0.1, 0.2, 0.4, 0.8, 1.5, 3.0, 6.0, 12, 25, 50, and 100 μM, respectively. A mixed internal standard (IS) solution of hypoxanthine-15N4 and UA-15N2 was prepared by diluting the stock solution to 10 μM. Calibration curves were prepared after mixing 25 μL of the respective standard solution, 80 μL acetonitrile, 20 μL water, and 15 μL IS solution.
In the sample preparation, 80 μL acetonitrile, 20 μL water, and 15 μL IS solution were added into 25 μL serum. After the mixture was vortexed, it was centrifuged at 12,000 g for 10 min at 4 °C. The supernatant was analyzed using liquid chromatography-MS/MS. Serum diluted to 1/10 was used for UA analysis.
The time taken for analysis after the samples were collected was longer in the PD group for both serum (PD, 368.0 ± 166.3 (mean ± SD, days, range 59–601; Control, 175.9 ± 96.7 (mean ± SD, days, range 34–363), P < 0.001) and CSF (PD, 273.7 ± 142.8 (mean ± SD, days, range 55–470), Control, 81.6 ± 39.4 (mean ± SD, days, range 28–134), P < 0.001).
Limitations and future research
This study did not assess factors such as exercise, nutritional indices, muscle volume, or adipose tissue, which may affect UA and hypoxanthine levels. Furthermore, the cross-sectional design limits our ability to infer the long-term changes in these metabolites. Future longitudinal studies should address these limitations and explore the influence of adenosine and guanosine on inosine levels, the relationship between nucleotide status in the brain, and the clinical efficacy of combining FUS with purine-modifier strategies.
Statistical analyses
We used JMP software version 16 (SAS Institute, Cary, NC) for the statistical analyses. Differences were considered statistically significant at p < 0.05. Fisher’s exact test was used to compare the sex distribution between the two groups. We tested the normality of the variables and homoscedasticity using the Shapiro–Wilk and Levene’s tests. The Wilcoxon rank-sum test (also known as the Mann-Whitney U test) was used to compare continuous variables between the two groups because assumptions of normality or homogeneity of variance were violated. After running the Wilcoxon rank-sum test, the effect size (r) is calculated as the standardized Z-score divided by the square root of the total number of samples. Correlations between continuous variables were assessed using Spearman’s rank correlation test. Continuous variables are expressed as mean ± standard deviation. ROC curve analysis was performed to evaluate the discrimination capacity of purine metabolites between PD and control.
To estimate whether low UA levels are due to internal factors, that is, upstream changes in the purine metabolic system, or other extrinsic factors, assuming that downstream metabolites were generated along metabolic pathway, we also performed the regression analysis using a GLM formulated as follows:
where x represents the upstream metabolite concentration, y the downstream metabolite concentration and z a dummy variable (1 for PD, 0 for control). For the parameter, αb is the baseline for effects due to extrinsic factors, βb is the standard effects of upstream, αp the change in effects due to different paths caused by PD, and βp the amount of change in the effect due to upstream.
We noted the outlines of the theoretical derivation of the GLM designed to analyze the dynamics of the purine metabolic pathway in PD patients compared to a control group in Supplementary information (SI). It models how inosine, hypoxanthine, xanthine, and UA concentrations change over time within this pathway, as illustrated in Figure 1SI. These changes are characterized by the influx rates from external sources to each substance, their reaction rates for conversion to downstream substances, and their efflux rates. Internal factors are defined as upstream changes in the purine metabolic pathway affecting metabolite concentrations. External factors encompass influences outside the metabolic pathway, including clinical variables such as age, sex, and weight, which are considered potential modifiers of metabolic processes.
We assumed that at a stable state, the concentrations of these substances approximate specific equilibrium values, which may vary among individuals due to factors like pathological state, age, sex, and weight. It suggests that the conversion efficiency from upstream to downstream substances within the purine metabolism pathway could be altered in PD, affecting the reaction rate coefficients. These coefficients are hypothesized to differ between the PD and control groups, reflecting the potential impact of the disease on purine metabolism.
Additionally, we considered the influence of confounding factors such as sex, age, and body weight on these coefficients, modeling their relationship linearly. The objective is to understand how PD-specific changes in the purine metabolic pathway could contribute to the disease’s pathology, offering insights that might aid in diagnosis and treatment. Figure 1SI and Table 1SI summarize the purine metabolic pathway and the GLM parameters and interpretations, respectively, providing a clear framework for this analysis.
Data availability
The data that support the findings of this study are available within the paper and its supplementary files.
References
Schwarzschild, M. A. Parkinson Study Group PRECEPT Investigators. et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch. Neurol. 65, 716–723 (2008).
Alonso, A., Rodríguez, L. A. G., Logroscino, G. & Hernán, M. A. Gout and risk of Parkinson disease: a prospective study. Neurology 69, 1696–1700 (2007).
Chang, H., Wang, B., Shi, Y. & Zhu, R. Dose-response meta-analysis on urate, gout, and the risk for Parkinson’s disease. NPJ Parkinsons Dis. 8, 160 (2022).
Ascherio, A. et al. Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch. Neurol. 66, 1460–1468 (2009).
Grażyńska, A. et al. The influence of serum uric acid level on non-motor symptoms occurrence and severity in patients with idiopathic Parkinson’s disease and atypical parkinsonisms-a systematic review. Medicina (Kaunas) 57, 972 (2021).
Kobylecki, C. J., Nordestgaard, B. G. & Afzal, S. Plasma urate and risk of Parkinson’s disease: a Mendelian randomization study. Ann. Neurol. 84, 178–190 (2018).
Kia, D. A. et al. Mendelian randomization study shows no causal relationship between circulating urate levels and Parkinson’s disease. Ann. Neurol. 84, 191–199 (2018).
Coneys, R., Storm, C. S., Kia, D. A., Almramhi, M. & Wood, N. W. Mendelian randomisation finds no causal association between urate and Parkinson’s disease progression. Mov. Disord. 36, 2182–2187 (2021).
Li, X. et al. Effect of serum uric acid on cognition in patients with idiopathic REM sleep behavior disorder. J. Neural Transm (Vienna). 125, 1805–1812 (2018).
Hasíková, L. et al. Patients with REM sleep behavior disorder have higher serum levels of allantoin. Parkinsonism Relat. Disord. 90, 38–43 (2021).
Parkinson Study Group SURE-PD3 Investigators, Schwarzschild, M. A. et al. Effect of urate-elevating inosine on early Parkinson disease progression: the SURE-PD3 randomized clinical trial. JAMA 326, 926–939 (2021).
Seifar, F., Dinasarapu, A. R. & Jinnah, H. A. Uric acid in Parkinson’s disease: what is the connection? Mov. Disord. 37, 2173–2183 (2022).
Garcia-Esparcia, P., Hernández-Ortega, K., Ansoleaga, B., Carmona, M. & Ferrer, I. Purine metabolism gene deregulation in Parkinson’s disease. Neuropathol. Appl. Neurobiol. 41, 926–940 (2015).
Johansen, K. K. et al. Metabolomic profiling in LRRK2-related Parkinson’s disease. PLoS One 4, e7551 (2009).
Ikeda, K. et al. Serological profiles of urate, paraoxonase-1, ferritin, and lipid in Parkinson’s disease: changes linked to disease progression. Neurodegener. Dis. 8, 252–258 (2011).
Yemula, N., Dietrich, C., Dostal, V. & Hornberger, M. Parkinson’s disease and the gut: symptoms, nutrition, and microbiota. J. Parkinsons Dis. 11, 1491–1505 (2021).
Ghourchian, S. et al. Weight loss and weight gain in Parkinson disease. Parkinsonism Relat. Disord. 83, 31–36 (2021).
Zhang, X., Molsberry, S. A., Schwarzschild, M. A., Ascherio, A. & Gao, X. Association of diet and physical activity with all-cause mortality among adults with Parkinson disease. JAMA Netw. Open 5, e2227738 (2022).
Maetzler, W., Stapf, A. K., Schulte, C., Hauser, A.-K., Lerche, S., Wurster, I., Schleicher, E., Melms, A. & Berg, D. Serum and cerebrospinal fluid uric acid levels in Lewy body disorders: associations with disease occurrence and amyloid-β pathway. J. Alzheimers. Dis. 27, 119–126 (2011).
Constantinescu, R., Andreasson, U., Holmberg, B. & Zetterberg, H. Serum and cerebrospinal fluid urate levels in synucleinopathies versus tauopathies. Acta Neurol. Scand. 127, e8–e12 (2013).
The Parkinson Study Group SURE-PD Investigators, Schwarzschild, M. A. et al. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol. 71, 141–150 (2014).
Johnson, T. A., Jinnah, H. A. & Kamatani, N. Shortage of cellular ATP as a cause of diseases and strategies to enhance ATP. Front. Pharmacol. 10, 98 (2019).
Chiba, Y., Murakami, R., Matsumoto, K., Wakamatsu, K., Nonaka, W., Uemura, N., Yanase, K., Kamada, M. & Ueno, M. Glucose, fructose, and urate transporters in the choroid plexus epithelium. Int. J. Mol. Sci. 21, 7230 (2020).
Srinivasan, S., Torres, A. G. & Ribas de Pouplana, L. Inosine in biology and disease. Genes 12, 600 (2021).
Cornford, E. M. & Oldendorf, W. H. Independent blood-brain barrier transport systems for nucleic acid precursors. Biochim. Biophys. Acta 394, 211–219 (1975).
Yamamoto, T. et al. Effect of inosine on the plasma concentration of uridine and purine bases. Metabolism 51, 438–442 (2002).
Muto, J. et al. Oral administration of inosine produces antidepressant-like effects in mice. Sci. Rep. 4, 4199 (2014).
Borsche, M., Pereira, S. L., Klein, C. & Grünewald, A. Mitochondria and Parkinson’s disease: clinical, molecular, and translational aspects. J. Parkinsons Dis. 11, 45–60 (2021).
Foltynie, T. Glycolysis as a therapeutic target for Parkinson’s disease. Lancet Neurol. 18, 1072–1074 (2019).
Wang, Q. et al. The role of gut dysbiosis in Parkinson’s disease: mechanistic insights and therapeutic options. Brain 144, 2571–2593 (2021).
Murray, A. W. The biological significance of purine salvage. Annu. Rev. Biochem. 40, 811–826 (1971).
Stepanova, V. et al. Reduced purine biosynthesis in humans after their divergence from Neandertals. Elife 10, e58741 (2021).
Yakhine-Diop, S. M. S. et al. Metabolic alterations in plasma from patients with familial and idiopathic Parkinson’s disease. Aging 12, 16690–16708 (2020).
Saugstad, O. D. Hypoxanthine as an indicator of hypoxia: its role in health and disease through free radical production. Pediatr. Res. 23, 143–150 (1988).
Nakamura, T. et al. Establishment of the process in blood sampling and sample handling as a biomarker of hypoxia-inducible diseases; plasma hypoxanthine and xanthine measurement. J. Mol. Biomark. Diagn. 9, https://doi.org/10.4172/2155-9929.1000404 (2018).
Furuhashi, M. New insights into purine metabolism in metabolic diseases: role of xanthine oxidoreductase activity. Am. J. Physiol. Endocrinol. Metab. 319, E827–E834 (2020).
van Nimwegen, M. et al. Physical inactivity in Parkinson’s disease. J. Neurol. 258, 2214–2221 (2011).
Galper, J. et al. Lipid pathway dysfunction is prevalent in patients with Parkinson’s disease. Brain 145, 3472–3487 (2022).
Watanabe, H. et al. Improved Parkinsons disease motor score in a single-arm open-label trial of febuxostat and inosine. Medicine (Baltimore) 99, e21576 (2020).
Sweeney, M. D. et al. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol Rev. 99, 21–78 (2019).
Gasca-Salas, C. et al. Blood-brain barrier opening with focused ultrasound in Parkinson’s disease dementia. Nat Commun. 12, 779 (2021).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Acknowledgements
We are grateful to Dr. Seiko Hirota, Dr. Ryoichi Shiroki and his colleagues in our institute for their help with the collection of CSF samples from healthy controls. This work was supported by JSPS KAKENHI Grant Number JP22K07508.
Author information
Authors and Affiliations
Contributions
S.S. managed the registry of subjects and drafted the manuscript. Y.Mi. managed and analyzed the biological data of subjects. J.Y. contributed to the analysis with a general linear model. Y.Ma. measured purine metabolites of biological samples. R.O., R.N., To.M., A.H., A.U., and M.I. performed the clinical evaluations of subjects. Ta.M. advised on biochemical aspects of the study. H.W. designed and supervised the study, advised on the statistical analyses, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Shima, S., Mizutani, Y., Yoshimoto, J. et al. Uric acid and alterations of purine recycling disorders in Parkinson’s disease: a cross-sectional study. npj Parkinsons Dis. 10, 170 (2024). https://doi.org/10.1038/s41531-024-00785-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-024-00785-0
- Springer Nature Limited