Abstract
Given the lack of regulatory approval for restorative therapies for the treatment of erectile dysfunction, we hypothesized that clinical trials would vary in methodology and endpoint measurements. Our objective was to analyze methodological approaches and outcome measures of clinical trials evaluating restorative therapies for erectile dysfunction. Data was extracted from clinicaltrials.gov on trials which contained the keywords “erectile dysfunction”. We evaluated trials initiated between 2004 and 2021 which listed a restorative therapy intervention. We identified 95 trials investigating energy-based/shockwave therapies (60/95), stem cell therapies (25/95), platelet-based therapies (6/95), and others (4/95). Only 41.1% of the trials evaluated safety. The most common efficacy endpoint was International Index of Erectile Function and Sexual Health Inventory for Men, and only 29.5% utilized penile Doppler. Thirty (31.6%) trials had been completed yet only 3 (3.2%) have published results. We found substantial heterogeneity in methodological approach in the trials. Subjective measures of erectile function were commonly reported, but definitions of inclusion criteria and objective outcome measures were inconsistent. These results provide a basis for the design of future clinical trials to improve the quality of trial data and aid in the development of standardized criteria for erectile dysfunction clinical trials.
Similar content being viewed by others
Introduction
Erectile Dysfunction (ED) is defined as the inability to attain or maintain an erection sufficient for sexual intercourse [1]. ED is one of the most prevalent sexual health conditions worldwide and is known to significantly affect the quality of life of affected men [2]. The recent increase in modifiable risk factors for ED has caused an increased prevalence of ED in recent years, with some estimates predicting that 322 million men will be affected globally by 2025 [3]. The rise in ED incidence has resulted in an increasing demand for treatment options. Current medical treatment options include oral phosphodiesterase-5 inhibitors, vacuum-assisted erection devices, intraurethral suppositories, intracavernosal vasodilator injections, and penile implants [4]. However, none of these treatments can reverse ED pathophysiology and none can restore normal penile function or spontaneous erections [5].
A variety of tools exist to determine the severity of ED [6]. These tools include both subjective, patient-reported outcome measures, which capture the patients’ view of their symptoms, and objective functional assessments and practical measures, which are generally task-based and scored on a predefined criterion.
Restorative therapies represent a new generation of treatments which aim to reverse the underlying changes that cause ED. Examples of restorative therapies for ED include shockwave therapy [7], injectable platelet-rich plasma (PRP) [8], and stem cell therapy (SCT) [9, 10]. The goal of these treatments is to cure or lessen symptomatology without causing side effects. Notably, restorative therapies have been investigated in the context of other diseases, such as plantar fasciitis [11]; however, there is currently insufficient evidence from randomized controlled clinical trials to support the safety and efficacy of these therapies for ED. Given the lack of regulatory agency approval for any restorative therapy for the treatment of ED, the Sexual Medicine Society of North America has established positions statements on their use. Restorative therapies should only be, “conducted under research protocols in compliance with Institutional Review Board approval” [12].
Nevertheless, patients continue to seek restorative therapies despite a conspicuous lack of regulation by the United States Food and Drug Administration (FDA) or credible associations [13]. With the expansion of clinics offering restorative therapy for ED, it is imperative to investigate them further for safety, efficacy, and standardization to inform eventual regulation or association guidelines.
Previous reviews of studies evaluating the efficacy of SCT [14] and shockwave therapy [15] for ED found significant limitations, such as short follow-up durations and small sample sizes. To date, no study has investigated the whole array of methodological approaches and objective outcome measures in restorative therapy clinical trials. We hypothesize that clinical trials vary in methodology and endpoint measurements. The objective of the present study is to systematically review and analyze all clinical trials that are utilizing restorative therapies for ED to assess experimental design, outcome assessments, and availability of published data.
Methods
Data was extracted from clinicaltrials.gov, the largest database of clinical trials cleared by the US FDA, on 10/27/2021 and included all trials which contained the keywords “erectile dysfunction”. We evaluated trials initiated between 2004 and 2021 which listed a restorative therapy as the intervention. This included energy-based/shockwave therapies, PRP therapy, and SCT, among others. We recorded whether each trial implemented Doppler ultrasound [16], International Index of Erectile Function (IIEF) [17], Sexual Health Inventory for Men (SHIM) [18], Erectile Hardness Scale [19], endothelial function assessment [20], and safety assessment.
Results
We identified 515 trials for ED in the clinicaltrials.gov database. Of these, 95 (18.4%) investigated a restorative therapy (Fig. 1). The trials investigated the effects of energy-based therapies (60/95), SCT (25/95), platelet-based therapies (6/95), gene therapy (1/95), Hyperbaric Oxygen Therapy (1/95), or a combination thereof (2/95). A total of 15,839 male participants were enrolled or are anticipated for enrollment in the trials.
Most trials were conducted at a single study site (77/95, 81.1%), and half were conducted in an academic setting (48/95, 50.1%). Randomization was utilized in 63.2% (60/95) of trials, yet only 30.1% were double-blinded (29/95). About half of the trials (47/95, 49.5%) were open-label, meaning the participant was aware of the treatment allocation.
Only 41.1% of the trials evaluated safety. The most common efficacy endpoints were the patient self-reported questionnaires, the IIEF and SHIM, and only 30% of trials utilized penile Doppler ultrasound (Fig. 2).
A total of 30 (31.6%) trials have been completed, yet only 3 (3.2%) have made their results available on clinicaltrials.gov. Among these, enrollment (≥80% planned sample size) was achieved in one trial (1/3), and retention (≥75% enrolled subjects) occurred in two of the three trials. A separate search was done on PubMed to determine if any of the clinical trials had published results that were not listed on their clinical trial website. Publications were identified from eight of the trials, which outlined preliminary or final results [8, 21,22,23,24,25,26,27], (Table 1).
Discussion
Meeting the increased demand for effective treatments for ED, restorative therapies are advancing the field of andrology and sexual medicine. However, despite the large number of clinical trials, there remains a need to improve the quality of trial design, data collection, and standardized criteria for ED clinical trials. Identification of clinical trial methodological approaches as well as primary and secondary outcomes are necessary for the translation of these novel therapeutics into the clinical setting, as well as to improve the rigor and reproducibility of restorative therapies overall.
In this study, we systematically evaluated multiple aspects affecting the trajectory of clinical translation among research trials conducted between 2004 and 2021 which listed a restorative therapy as the intervention for ED.
Subjective measures of ED, such as the IIEF [17] and the SHIM [18], are regularly used. These questionnaires are readily self-administered in research or clinical settings. The IIEF demonstrates the sensitivity and specificity for detecting treatment-related changes in patients with ED [28]. In addition, studies established the SHIM as a useful indicator of ED, and this measure has had positive scientific impact on understanding and improving male sexual function [29]. However, these outcome measures may be less effective in determining underlying restorative physiological changes in penile tissue than Doppler ultrasound or endothelial function assessments [30].
Penile duplex Doppler ultrasound (PDDU) is a minimally invasive tool to evaluate erectile hemodynamics and determining accurate location of deep penile arteries [31, 32]. However, PDDU protocols have marked heterogeneity and no clear consensus for normative measurements, making the interpretation of results challenging [33]. Furthermore, when arterial inflow is normal but the erectile response is poor and there is antegrade diastolic flow throughout the examination, this is called an indeterminate result or a mixed arterial and venous ED. The diagnosis of mixed arterial and venous ED cannot be made using PDDU because venous competence cannot be assessed in a patient with arterial insufficiency [34]. All in all, using PDDU as a measure of erectile function may only be used in certain circumstances.
Endothelial function is most commonly assessed via brachial artery ultrasound to determine flow-mediated vasodilation. Another method of measuring endothelial function includes culturing the circulating Endothelial Progenitor Cell—Colony Forming Units (EPC-CFUs) obtained from peripheral blood samples [35]. Improvements in these two methods are correlated with one another [36].
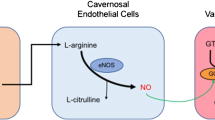
Endothelial function may be impaired in ED patients with no apparent cardiovascular disease or diabetes mellitus [37]. Erection occurs with nitric oxide (NO) release from the vascular endothelial cells leading to subsequent arterial dilation [38]. The reduction in endothelial cell production of NO results in the negative impact on the smooth muscles in the corporal bodies and results in less relaxation of the smooth muscle cells with decrease in blood supply and resulting ED. A similar phenomenon is well known to impact the coronary arterial system resulting in cardiovascular disease [39]. ED is frequently, if not usually, directly related to endothelial dysfunction, and the release of NO by the vasculature of the penile arteries is directly related to the function of intact, healthy endothelium [39]. In the face of endothelial dysfunction, the process of erection fails to occur in a normal fashion [40]. All 7 trials [41,42,43,44,45,46,47] measuring endothelial function as an endpoint utilized either SCT or Li-SWT, and 1 trial utilized a combination of Li-SWT and PRP [41]. While there are no published results, the assessment of endothelial function (whether through flow-mediated vasodilation or EPC-CFUs) may be an effective measure of erectile function recovery.
This study provides an extensive review of clinical trials utilizing restorative therapies for ED. Limitations are that clinical trials not registered under FDA did not appear in our search and therefore were not included.
Future Directions—Recommendations for Future Clinical Trials
To standardize clinical trial design for investigating novel therapies for ED, researchers should examine protocols and results from already established therapies. The nature of a diagnosis of ED warrants a consideration into the subjective nature of the disease. For this reason, currently available recommendations for clinical trial designs continue to endorse the IIEF as the most validated and reproducible tool to determine endpoints [48]. However, a significant bias in the administration and interpretation of the questionnaire exists across trials. Several studies deviate from incorporating all items of the questionnaire or focusing on specific aspects related to the trial. This has the potential to introduce significant measurement bias. In order to draw final conclusions and hopefully create more effective therapies for patients, it is imperative to adopt a systematic way of collecting data regarding outcome improvements in its entirety, whether through questionnaires, PDDU, or endothelial function assessment.
Another equally important outcome worth capturing is partner satisfaction at the completion of the trial. The IIEF currently lacks a survey of whether the therapy was beneficial in restoring normal sexual functioning, and incorporating the partner’s impression and thoughts would imbue the questionnaire with a sense of respect for both parties. This can potentially be addressed by including surveys such as the Treatment Satisfaction Scale which assesses treatment response for both the patient and the partner [49].
To minimize selection bias, a clinical trial investigating therapies for ED should also ensure that the patient population captured in the study is representative of the demographics, symptoms, and complaints of the general population suffering from ED. Because restorative therapies for ED are experimental by nature, the patient population enrolled may come from large academic centers with appropriate funding. This raises a few key issues. For example, the patients who ultimately need referral to a tertiary specialist may be different in many regards than those who receive treatment at primary community centers that lack the necessary resources and expertise to manage a condition that causes significant distress to patients [50]. The progression of their disease may be so advanced at tertiary centers that any minor benefit from a restorative therapy would be detected and recorded as a successful outcome. Although enrolling patients from primary community centers in a clinical trial is considerably challenging, striving to make these considerations before launching a clinical trial is judicious from a methodological standpoint.
Conclusion
There is substantial heterogeneity in methodological approach in clinical trials evaluating restorative therapies for ED. Subjective measures of erectile function are commonly reported, but definitions of inclusion criteria and objective outcome measures are inconsistent. These results provide a basis for the design of future clinical trials to improve the quality of trial data and aid in the development of standardized criteria for ED clinical trials.
Data availability
The data that support the findings of this study are available from www.clinicaltrials.gov.
References
Consensus development conference statement. National Institutes of Health. Impotence. December 7–9, 1992. Int J Impot Res. 1993;5:181–284.
Dunn KM, Croft PR, Hackett GI. Association of sexual problems with social, psychological, and physical problems in men and women: a cross sectional population survey. J Epidemiol Community Health. 1999;53:144–8.
Goldstein I, Goren A, Li VW, Tang WY, Hassan TA. Epidemiology update of erectile dysfunction in eight countries with high burden. Sex Med Rev. 2020;8:48–58.
Stein MJ, Lin H, Wang R. New advances in erectile technology. Ther Adv Urol. 2014;6:15–24.
McMahon CG. Current diagnosis and management of erectile dysfunction. Med J Aust. 2019;210:469–76.
Grover S, Shouan A. Assessment scales for sexual disorders—a review. J Psychosexual Health. 2020;2:121–38.
Yee CH, Chan ES, Hou SS, Ng CF. Extracorporeal shockwave therapy in the treatment of erectile dysfunction: a prospective, randomized, double-blinded, placebo controlled study. Int J Urol. 2014;21:1041–5.
Poulios E, Mykoniatis I, Pyrgidis N, Zilotis F, Kapoteli P, Kotsiris D, et al. Platelet-rich plasma (PRP) improves erectile function: a double-blind, randomized, placebo-controlled clinical trial. J Sex Med. 2021;18:926–35.
Al Demour S, Jafar H, Adwan S, AlSharif A, Alhawari H, Alrabadi A, et al. Safety and potential therapeutic effect of two intracavernous autologous bone marrow derived mesenchymal stem cells injections in diabetic patients with erectile dysfunction: an open label phase I clinical trial. Urol Int. 2018;101:358–65.
Haahr MK, Harken Jensen C, Toyserkani N, Andersen DC, Damkier P, Sorensen JA, et al. A 12-month follow-up after a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: an open-label phase I clinical trial. Urology. 2018;121:203 e6–203 e13.
Gollwitzer H, Saxena A, DiDomenico LA, Galli L, Bouche RT, Caminear DS, et al. Clinically relevant effectiveness of focused extracorporeal shock wave therapy in the treatment of chronic plantar fasciitis: a randomized, controlled multicenter study. J Bone Jt Surg Am. 2015;97:701–8.
Liu JL, Chu KY, Gabrielson AT, Wang R, Trost L, Broderick G, et al. Restorative therapies for erectile dysfunction: position statement from the Sexual Medicine Society of North America (SMSNA). Sex Med. 2021;9:100343.
Muncey W, Sellke N, Kim T, Mishra K, Thirumavalavan N, Loeb A, et al. Alternative treatment for erectile dysfunction: a growing arsenal in men’s health. Curr Urol Rep. 2021;22:11.
Lokeshwar SD, Patel P, Shah SM, Ramasamy R. A systematic review of human trials using stem cell therapy for erectile dysfunction. Sex Med Rev. 2020;8:122–30.
Pai R, Ory J, Delgado C, Ramasamy R. Energy-based therapies for erectile dysfunction: current and future directions. Urol Clin North Am. 2021;48:603–10.
Nashed A, Lokeshwar SD, Frech F, Mann U, Patel P. The efficacy of penile duplex ultrasound in erectile dysfunction management decision-making: a systematic review. Sex Med Rev. 2021;9:472–7.
Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res. 2002;14:226–44.
Vroege JA. The sexual health inventory for men (IIEF-5). Int J Impot Res. 1999;11:177.
Parisot J, Yiou R, Salomon L, de la Taille A, Lingombet O, Audureau E. Erection hardness score for the evaluation of erectile dysfunction: further psychometric assessment in patients treated by intracavernous prostaglandins injections after radical prostatectomy. J Sex Med. 2014;11:2109–18.
Tamler R, Bar-Chama N. Assessment of endothelial function in the patient with erectile dysfunction: an opportunity for the urologist. Int J Impot Res. 2008;20:370–7.
Bieri M, Said E, Antonini G, Dickerson D, Tuma J, Bartlett CE, et al. Phase I and registry study of autologous bone marrow concentrate evaluated in PDE5 inhibitor refractory erectile dysfunction. J Transl Med. 2020;18:24.
Hadanny A, Lang E, Copel L, Meir O, Bechor Y, Fishlev G, et al. Hyperbaric oxygen can induce angiogenesis and recover erectile function. Int J Impot Res. 2018;30:292–9.
Yiou R, Hamidou L, Birebent B, Bitari D, Le Corvoisier P, Contremoulins I, et al. Intracavernous injections of bone marrow mononucleated cells for postradical prostatectomy erectile dysfunction: final results of the INSTIN clinical trial. Eur Urol Focus. 2017;3:643–5.
Levy JA, Marchand M, Iorio L, Cassini W, Zahalsky MP. Determining the feasibility of managing erectile dysfunction in humans with placental-derived stem cells. J Am Osteopath Assoc. 2016;116:e1–5.
Fode M, Borre M, Ohl D, Lichtbach J, Sonksen J. Penile vibratory stimulation in the recovery of urinary continence and erectile function after nerve-sparing radical prostatectomy: a randomized, controlled trial. BJU Int. 2014;114:111–7.
Katz JE, Molina ML, Clavijo R, Prakash NS, Ramasamy R. A Phase 2 Randomized Trial To Evaluate Different Dose Regimens of Low-intensity Extracorporeal Shockwave Therapy for Erectile Dysfunction: Clinical Trial Update. Eur Urol. Focus. 2018;4:336–7.
You D, Jang MJ, Song G, Shin HC, Suh N, Kim YM, et al. Safety of autologous bone marrow-derived mesenchymal stem cells in erectile dysfunction: an open-label phase 1 clinical trial. Cytotherapy. 2021;23:931–8.
Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30.
Cappelleri JC, Rosen RC. The Sexual Health Inventory for Men (SHIM): a 5-year review of research and clinical experience. Int J Impot Res. 2005;17:307–19.
Kassouf W, Carrier S. A comparison of the International Index of Erectile Function and erectile dysfunction studies. BJU Int. 2003;91:667–9.
Golijanin D, Singer E, Davis R, Bhatt S, Seftel A, Dogra V. Doppler evaluation of erectile dysfunction - part 2. Int J Impot Res. 2007;19:43–8.
Golubinski AJ, Sikorski A. Usefulness of power Doppler ultrasonography in evaluating erectile dysfunction. BJU Int. 2002;89:779–82.
Nascimento B, Miranda EP, Terrier JE, Carneiro F, Mulhall JP. A critical analysis of methodology pitfalls in duplex doppler ultrasound in the evaluation of patients with erectile dysfunction: technical and interpretation deficiencies. J Sex Med. 2020;17:1416–22.
Golijanin D, Singer E, Davis R, Bhatt S, Seftel A, Dogra V. Doppler evaluation of erectile dysfunction—part 1. Int J Impot Res. 2007;19:37–42.
Foresta C, Ferlin A, De Toni L, Lana A, Vinanzi C, Galan A, et al. Circulating endothelial progenitor cells in subjects with erectile dysfunction. Int J Impot Res. 2005;17:288–90.
Foresta C, Carerra N, Lana A, Cabrelle A, Palu G, Ferlin A. Circulating endothelial progenitor cells and endothelial function after chronic Tadalafil treatment in subjects with erectile dysfunction. Int J Impot Res. 2006;18:484–8.
Kaya C, Uslu Z, Karaman I. Is endothelial function impaired in erectile dysfunction patients? Int J Impot Res. 2006;18:55–60.
Andersson K, Stief C. Penile erection and cardiac risk: pathophysiologic and pharmacologic mechanisms. Am J Cardiol. 2000;86:23F–26F.
Mobley DF, Khera M, Baum N. Recent advances in the treatment of erectile dysfunction. Postgrad Med J. 2017;93:679–85.
Stein RA. Endothelial dysfunction, erectile dysfunction, and coronary heart disease: the pathophysiologic and clinical linkage. Rev Urol. 2003;5:S21–7.
Shockwave therapy and platelet rich plasma for the treatment of erectile dysfunction. https://ClinicalTrials.gov/show/NCT05048667.
The effect of combination therapy using Li-ESWT and PDE-5 inhibitor in patients with erectile dysfunction. https://ClinicalTrials.gov/show/NCT05043896.
Effectiveness and safety of adipose-derived regenerative cells for treatment of erectile dysfunction. https://ClinicalTrials.gov/show/NCT02472431.
The value of enhanced external counterpulsation on erectile dysfunction. https://ClinicalTrials.gov/show/NCT01815593.
Double blind placebo controlled study on the effect of extracorporal shock wave therapy on erectile dysfunction in PDE5i responders. https://ClinicalTrials.gov/show/NCT01274923.
Double blind placebo controlled study on the effect of extracorporal shock wave therapy on erectile dysfunction. https://ClinicalTrials.gov/show/NCT01274156.
The outcomes of intracavernosal umbilical cord mesenchymal stem cells implantation in patients with diabetic erectile dysfunction. https://ClinicalTrials.gov/show/NCT04972890.
Alhathal N, Al-Qaoud T, Carrier S. Considerations in the design of clinical trials for erectile dysfunction. Clin Investig. 2012;2:733–45.
DiBenedetti DB, Gondek K, Sagnier PP, Kubin M, Marquis P, Keininger D, et al. The treatment satisfaction scale: a multidimensional instrument for the assessment of treatment satisfaction for erectile dysfunction patients and their partners. Eur Urol. 2005;48:503–11.
Sadovsky R. The role of the primary care clinician in the management of erectile dysfunction. Rev Urol. 2002;4:S54–63.
Funding
This work was supported by National Institutes of Health Grant R01 DK130991 and Clinician Scientist Development Grant from the American Cancer Society.
Author information
Authors and Affiliations
Contributions
RS conceived of the research question that led to the submission, acquired data, and helped draft the manuscript. RG acquired data and helped draft the manuscript. TM contributed to interpretation of the results and revised the manuscript. AS helped with interpretation of results and revised the manuscript. RR helped interpret the results and revised the manuscript. All authors approved of the final version of the manuscript and agreed to be accountable for all aspects of the work to ensure that questions related to accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saltzman, R.G., Golan, R., Masterson, T.A. et al. Restorative therapy clinical trials for erectile dysfunction: a scoping review of endpoint measures. Int J Impot Res 35, 720–724 (2023). https://doi.org/10.1038/s41443-022-00610-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41443-022-00610-3
- Springer Nature Limited
This article is cited by
-
A contemporary review of the treatments and challenges associated with penile rehabilitation after radical prostatectomy including a proposed optimal approach
International Journal of Impotence Research (2024)
-
Comment on: “Risk of Erectile Dysfunction in Male Patients with Gout Treated with Febuxostat or Allopurinol: A Propensity Score-Matched Cohort Study”
Drugs (2023)