Abstract
There is limited scientific literature regarding the management outcomes for end-stage erectile dysfunction (ED) following radical cystoprostatectomy (RCP). This study aims to evaluate the surgical outcomes of penile prosthesis (PP) implantation. A retrospective analysis over 17 years (2004–2017) was performed from the clinical records of patients in four tertiary referral centres, whom previously had undergone RCP, followed by PP implantation for end-stage ED. Outcome measures include both intra and postoperative complications, operative duration, a 5-point Likert hematoma scale as well as length of hospital stay. Additionally, a matched-pair cohort analysis was performed, dividing patients in 2 groups according to the type of urinary diversion (neobladder versus ileal conduit/cutaneous ureterostomy). The median time elapsed between RCP and PP implantation was 38 months (IQR 20–56). The median follow-up was 18 months (IQR 12–156). A 3-piece inflatable PP was implanted in 43 patients (91.5%) whereas a semirigid device was implanted in the remainder. Reservoir position was extra-peritoneal (utilising a separate abdominal incision) in 24 patients (54.8%), while an ectopic high-submuscular placement was preferred in the remainder. PP infection and mechanical failure occurred in 1 (2.1%) and 3 cases (6.3%) respectively. The comparative analysis of surgical outcomes did not show any statistically significant difference between the two groups. Our evidence suggests that PP implantation in patients with refractory ED following RCP may represent a safe and effective procedure associated with a low incidence of complications. The main limitation of this study is represented by the non-randomised, retrospective nature as well as the lack of patients’ functional outcomes and the limited follow-up.
Similar content being viewed by others
Introduction
Radical cystoprostatectomy (RCP) currently represents the gold standard treatment for men with localized, muscle-invasive, or refractory high risk bladder cancer (BCa) [1, 2]. Sexual dysfunction following RCP frequently occurs and may have significant negative implications for patients’ quality of life [2,3,4]. Penile prosthesis (PP) implantation is widely recognised as the gold standard treatment for medically refractory erectile dysfunction (ED) [4]. Unfortunately, there remains limited scientific literature regarding the management of the end-stage ED secondary to RCP [5]. The aim of the present study is to evaluate the surgical outcomes of PP implantation in this group of patients. To the best of our knowledge, the current study represents the first of its kind based on a multicentric series of patients.
Materials and methods
Study setting and patients
From December 2004 to September 2017, a review was performed of a series of patients who had undergone RCP, developed medically rarefractory ED and subsequently had a PP placed. Four tertiary referral centers were involved in the study. Data was retrospectively extrapolated from patients’ clinical records and operative notes. An independent blinded review of the extrapolated data was performed by MF and MP to prevent bias. Patients with incomplete clinical records or follow-up were excluded from the study.The prostheses were either 3-piece inflatable devices (AMS 700 CX® or AMS 700 CXR®, Boston Scientific, USA) or semirigid devices (Genesis®, Coloplast, Denmark).
Surgical technique
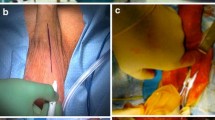
An antibiotic prophylaxis (first/second generation cephalosporin and aminoglycosides) and a preoperative scrub with chlorhexidine and iodopovidone were performed for all patients before starting the procedure. A peno-scrotal approach was utilised in all cases. Corpora cavernosa were isolated bilaterally. Two longitudinal 1.5 cm long corporotomies were carried out. Dilation of the corpora cavernosa were performed using Brooks dilators. In the event of corporal fibrosis, dilation was performed by using Rossello cavernotomes. Cylinders were inflated intraoperatively to identify the presence of a residual penile curvature. Straightening maneuvers were performed if a residual curvature >30° was detected after PP implantation [6]. If the residual curvature was still significant (i.e.,: >60 degrees), tunical incision and grafting were performed.
The reservoir was placed either in the lateral retroperitoneum, through a counter incision medial to anterior superior iliac spine, or ectopically in a high-submuscular location [7]. An indwelling urinary catheter (only for Group OIN patients) and a gently compressive penoscrotal dressing were left in place for 24 h. The same postoperative care protocol was applied in all cases. Patients were discharged with oral antibiotics for 1 week and reviewed thereafter to exclude early postoperative complications. Patients were educated and encouraged to begin cycling their device at 4 weeks postoperatively. Patients were then reviewed at 3-month intervals within the first year.
Main outcome measures
A descriptive analysis was carried out for intra and postoperative complications, operative time, a 5-point Likert hematoma scale (Grade 1: Penile oedema—Grade 2: Penile ecchymosis—Grade 3: Penile haematoma—Grade 4: Penoscrotal haematoma—Grade 5: Penoscrotal and pubic haematoma) as well as duration of hospital stay. A matched-pair analysis was also conducted, dividing patients into two groups according to the type of urinary diversion: orthotopic ileal neobladder (OIN) and incontinent urinary diversion (IUD) if they had been managed with either ileal conduit formation or a cutaneous ureterostomy.
Statistics
Categorical variables were described using frequency and percentage, and the continuous variables were described using median and interquartile range (IQR) value.
Statistical analysis was carried out using STATA statistical software package (v.14) with a p value of <0.05 being considered significant. Non-parametric, Kruskal–Wallis, Chi-square and linear trends of associations verified by Mantel-Haesenzel linear by linear associations chi-square tests were performed.
Results
According to the inclusion criteria, 65 patients were considered as eligible for the study. Eighteen patients were excluded due to incomplete post-operative follow-up data. Ultimately 47 patients were enrolled in the study. Patient features are summarised in Table 1. Most patients in this series underwent open RCP; laparoscopic or robotic assisted procedures were performed in 1 and 3 cases respectively. Adjuvant chemotherapy was utilised in 23 patients (48.9%) and the likelihood of its use was significantly higher if IUD was performed (p value = 0.002).
Orthotopic ileal neobladder was performed in 14 patients (29.8%), whilst an incontinent urinary diversion was utilised in the remainder. Patients who underwent an OIN were younger (median age 59.5 years, IQR 54–65) compared to those who received an IUD (median age 70 years, IQR 65.5–73, p value = 0.001). Regarding general pharmacological therapies, patients in IUD group were treated with antiplatelet agents and anticoagulants more often than patients in the OIN group (p value = 0.02).
In relation to the pre-PP ED management, 30 patients (63.8%) were treated with phosphodiesterase type 5 inhibitors (PDE5I) at maximal dosage as a first line treatment. OIN patients received PDE5Is more frequently (p value = 0.04). All 30 patients failed to respond satisfactorily to oral therapy. Intra-cavernosal injection (ICI) of prostaglandin E1 (PGE1) was offered in the remaining 37.2% or as a second line treatment to less than half of the patients (44.7%). Vacuum erectile device (VED) was offered as a third line treatment to 14 patients (30.4%). The median elapsed time between RCP and PP implantation was 38 months (IQR 20–56).
Surgical outcomes are summarised in Table 2. Median follow-up was 18 months (IQR 12–156). A 3-piece inflatable PP was implanted in 43 patients (91.5%), whilst semi rigid prosthesis were placed in the remainder (8.5%). The median intraoperative corporal length measurement was 19 cm (IQR 17–20.5). One patient actively complained about penile shortening (2.1%). Straightening maneuvers to address curvature after PP implantation were necessary in 5 patients (10.6%). A modelling maneuver produced adequate penile straightening in 3 of the 5 patients. Plaque incision and grafting was utilised in the remaining 2 to guarantee adequate straightening. Corporal fibrosis necessitating the use of cavernotomes was present in 5 patients. No patients required additional distal corporal incisions. A reduced-diameter cylinder was required in 5 patients (10.6%). The reservoir was inserted in the extra-peritoneal space through a second abdominal incision in 24 patients (54.8%), the remainder utilised an ectopic high-submuscular placement.
Both intraoperative and postoperative complications were rarely detected in our series. Infection and mechanical failure of the device occurred respectively in one (2.1%) and 3 cases (6.3%).
Concerning reservoir placement, a total of 4 minor intraoperative complications were detected. 2 cases of minor bleeding controlled by bipolar cauterisation and 2 cases of an extended transversalis fascia fibrosis, due to previous RCP, which lead the surgeon to perform a separate abdominal incision, were equally distributed between the groups.
Discussion
Sexual dysfunction is an inherent issue following RCP. Whilst anejacualtion is universal, ED rates approach 85% with non-nerve sparing RCP and 30–58% following nerve-sparing RCP [3]. These issues may negatively affect the quality of life of sexually active patients, particularly those of younger age [3, 8, 9].
The etiology of the postoperative ED appears multifactorial. Although damage to the accessory pudendal arteries and/or cavernosal nerves at the time of RCP may play a role in the development of postoperative ED, increasing evidence suggests that veno-occlusive dysfunction secondary to degeneration of the cavernosal smooth muscle, during the postoperative period, may also be responsible [9, 10]. The lack of oxygenation of the corpora may lead to irreversible changes of the cavernosal smooth muscle, with progressive loss of elasticity. This phenomenon leads to veno-occlusive dysfunction and penile shortening [11].
Several modifications of the standard RCP have been proposed over time aiming to minimise erectile dysfunction [12,13,14]. The introduction of the nerve-sparing approach, as well as minimally invasive techniques, have provided potential avenues for a reduction in postoperative ED rates[15,16,17,18].
Whilst it is understandable that a patients’ initial focus is towards oncological outcomes, it remains crucial to consider effects of sexual and urinary function within the context of long term quality of life [19].
Amongst the potential treatments for refractory ED, PP implantation represents the third-line approach, as suggested by international guidelines [4]. Whilst PP implantation outcomes have been widely evaluated in the literature, particularly in the context of prostate cancer treatments [19, 20], their outcomes in relation to RCP patients evokes significantly less scientific discussion.
Surgical considerations related to PP implantation in patients who have previously undergone pelvic surgery may include corporal fibrosis and associated difficulty in dilatation, as well as the absence of the space of Retzius and reservoir placement.
In the current series, despite the time lapse between RCP and the PP implant, severe corporal fibrosis was not noted to be a significant issue. Both patients and surgeons were satisfied with the length and girth of the cylinders implanted, intraoperatively from a surgeon point of view and also, for the same patient during the following outpatient visits. In the 4 patients whom underwent semi-rigid PP implantation, no particular placement difficulties were encountered. We believe that preservation of penile length and girth plays a role in influencing patient satisfaction rates.
As mentioned, reservoir positioning following RCP may represent a challenge. Previous abdominal surgery and a potential for urinary diversion are additional factors to consider when debating the preferred approach. Traditional retropubic blind insertion may be associated with an increased risk of vascular (external iliac vessels), bowel or neobladder injuries [21]. In the current series, the surgeons’ preferred choice was an extra-peritoneal placement under direct vision through an additional abdominal incision. Alternatively, an ectopic reservoir placement in a high sub-muscular position may be considered [8]. This approach affords a relatively safe placement of the reservoir avoiding the morbidity of a second abdominal incision and was utilised successfully in 19 of the patients.
Ectopic reservoir placement can be performed anteriorly (ATF) or posteriorly to the transversalis fascia (PTF). According to our experience, ATF is preferable in patients with previous pelvic surgery to avoid the risk of erosion and intraperitoneal placement of the reservoir as there are no structures interposed between the device and the peritoneum itself [22]. Independent risk factors for the development of this complication were identified in tobacco smoking patients, as well as those with a low body mass index (BMI <18.5 kg/m2) [23]. The ectopic reservoir placement is not free from complications but, when they occur, they tend to be significantly less serious compared to those reported following retropubic placement. In a large series of patients undergoing ectopic reservoir placement, caudal herniation was recorded in 0.09% of PTF and 1.34% of ATF cases respectively. These cases required surgical correction. To minimise the downward herniation, suture ligation of the neck of the ectopic tunnel, or more cranial placement of the reservoir have been suggested [21].
In non-RCT patients there remains a risk of bladder injury/erosion, whilst those who have undergone RCP risk erosion into the neo-bladder or urinary diversion, although the overall risk is as low as 0.4% [24, 25]. Risk factors for increasing reservoir erosion include previous abdominal/pelvic surgery, radiation therapy, device infection, malposition, or excess tension on the reservoir tubing [24, 25].
Erosion of the reservoir is believed to depend on the development of tissue ischaemia, compression against fixed viscera, or due to the resulting fibrosis. To minimise this complication, surgeons could consider a two-piece inflatable prosthesis or a semi-rigid PP, or utilise an ectopic or retroperitoneal reservoir placement to avoid the contact between the viscera and the PP components [25]. In the current series, no complications due to reservoir erosion were recorded at a median follow up of 18 months (IQR 12–156). Compared to the published literature to date, it appears that the strength of our study is represented by the sub-analysis focus on reservoir placement techniques, which has highlighted the role of the high-submuscular placement as a safe option despite the presence of any type of urinary diversion.
Focusing on the analysis of postoperative complications, infection and mechanical failure rates appear comparable to other published series, demonstrating that previous RCP and the urinary diversion did not represent an additional risk for PP implantation. Loh-Doyle et al. [5] reported in their retrospective series of 80 patients whom underwent inflatable PP implantation following RCP, infection and mechanical failure rates of 5% and 6% respectively after a mean follow up of 53.9 months. They also noted no statistically significant associations between infection and comorbidities, urinary diversion type, exposure to chemotherapy, radiation, or presence of an artificial urinary sphincter. Similarly in our series, a history of adjuvant chemotherapy, was not a predictive factor for peri or postoperative PP complications.
Whilst there may exist specific issues related to PP following RCP, surgical outcomes seem comparable to those following radical prostatectomy. Menard et al. in their series of post radical prostatectomy PP implantation recorded an overall incidence of surgical revision of 8.9%: 1.3% for acute infection, 2.5% for mechanical failure and 5.1% for migration / autoinflation of the device at a mean follow up of 37.6 months [19]. In our series, the overall revision rate was 8.5%.
The main limitations of this current study must be highlighted: (i) Patients were not randomised to surgical interventions. Instead, all data were retrospectively collected. (ii) No data on functional outcomes using validated questionnaires were reported. (iii) The follow-up is limited to 18 months. This may underestimate mechanical failure rates.
In conclusion PP implantation in patients with end-stage ED following RCP represents a safe and effective procedure associated with low complication rates, comparable to those in the radical prostatectomy cohort. However, further prospective, randomised multicentric studies are necessary to confirm the encouraging results that emerged from this series. Functional outcomes should be assessed by using validated questionnaires.
References
Witjes JA, Lebret T, Compérat EM, Cowan NC, De Santis M, Bruins HM, et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur Urol 2017;71:462–75.
Hernández V, Espinos EL, Dunn J, Maclennan S, Lam T, Yuan Y, et al. Oncological and functional outcomes of sexual function–preserving cystectomy compared with standard radical cystectomy in men: a systematic review. Urol Oncol Semin Orig Investig. 2017;35:539.e17–539.e29.
Zippe CD, Raina R, Massanyi EZ, Agarwal A, Jones JS, Ulchaker J, et al. Sexual function after male radical cystectomy in a sexually active population. Urology. 2004;64:682–5.
Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, Montorsi F, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. 2010;57:804–14.
Loh-Doyle J, Patil MB, Sawkar H, Wayne K, Boyd SD. 3-piece inflatable penile prosthesis placement following radical cystoprostatectomy and urinary diversion: technique and outcomes. J Sex Med. 2018:15;907–13.
Rolle L, Falcone M, Ceruti C, Timpano M, Sedigh O, Ralph DJ, et al. A prospective multicentric international study on the surgical outcomes and patients’ satisfaction rates of the “sliding” technique for end-stage Peyronie’s disease with severe shortening of the penis and erectile dysfunction. BJU Int. 2016;117:814–20.
Chung E, Ralph D, Kagioglu A, Garaffa G, Shamsodini A, Bivalacqua T, et al. Evidence-based management guidelines on Peyronie’s disease. J Sex Med. 2016;13:905–23.
Morey AF, Cefalu CA, Hudak SJ. High submuscular placement of urologic prosthetic balloons and reservoirs via transscrotal approach. J Sex Med. 2013;10:603–10.
De Luca V, Pescatori ES, Taher B, Zambolin T, Giambroni L, Frego E, et al. Damage to the erectile function following radical pelvic surgery: prevalence of veno-occlusive dysfunction. Eur Urol. 1996;29:36–40.
Hekal IA, Mosbah A, El-Bahnasawy MS, El-Assmy A, Shaaban A. Penile haemodynamic changes in post-radical cystectomy patients. Int J Androl. 2011;34:27–32.
Berookhim BM, Nelson CJ, Kunzel B, Mulhall JP, Narus JB. Prospective analysis of penile length changes after radical prostatectomy. BJU Int. 2014;113(5 B):131–6.
Davila HH, Weber T, Burday D, Thurman S, Carrion R, Salup R, et al. Total or partial prostate sparing cystectomy for invasive bladder cancer: long-term implications on erectile function. BJU Int. 2007;100:1026–9.
Kessler TM, Burkhard FC, Perimenis P, Danuser H, Thalmann GN, Hochreiter WW, et al. Attempted nerve sparing surgery and age have a significant effect on urinary continence and erectile function after radical cystoprostatectomy and ileal orthotopic bladder substitution. J Urol. 2004;172(4 Pt 1):1323–7.
Schlegel PN, Walsh PC. Neuroanatomical approach to radical cystoprostatectomy with preservation of sexual function. J Urol. 1987;138:1402–6.
Hekal IA, El-Bahnasawy MS, Mosbah A, El-Assmy A, Shaaban A. Recoverability of erectile function in post–radical cystectomy patients: subjective and objective evaluations. Eur Urol. 2009;55:275–83.
Novara G, Catto JWF, Wilson T, Annerstedt M, Chan K, Murphy DG, et al. Systematic review and cumulative analysis of perioperative outcomes and complications after robot-assisted radical cystectomy. Eur Urol. 2015;67:376–401.
Yuh B, Wilson T, Bochner B, Chan K, Palou J, Stenzl A, et al. Systematic review and cumulative analysis of oncologic and functional outcomes after robot-assisted radical cystectomy. Eur Urol. 2015;67:402–22.
Bochner BH, Dalbagni G, Sjoberg DD, Silberstein J, Keren Paz GE, Donat SM, et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: a randomized clinical trial. Eur Urol. 2015;67:1042–50.
Menard J, Tremeaux JC, Faix A, Pierrevelcin J, Staerman F. Erectile function and sexual satisfaction before and after penile prosthesis implantation in radical prostatectomy patients: a comparison with patients with vasculogenic erectile dysfunction. J Sex Med. 2011;8:3479–86.
Osterberg EC, Maganty A, Ramasamy R, Eid JF. Pharmacologically induced erect penile length and stretched penile lengh are both good predictors of post-inflatable prosthesis penile length. Int J Impot Res. 2014;26:128–31.
Karpman E, Sadeghi-Nejad H, Henry G, Khera M, Morey AF. Current opinions on alternative reservoir placement for inflatable penile prosthesis among members of the sexual medicine society of North America. J Sex Med. 2013;10:2115–20.
Stember DS, Garber BB, Perito PE. Outcomes of abdominal wall reservoir placement in inflatable penile prosthesis implantation: a safe and efficacious alternative to the space of retzius. J Sex Med. 2014;11:605–12.
Gross MS, Stember DS, Garber BB, Perito PE. A retrospective analysis of risk factors for IPP reservoir entry into the peritoneum after abdominal wall placement. Int J Impot Res. 2017;29:215–8.
Dadhich P, Hockenberry M, Kirby EW, Lipshultz L. Penile prosthesis in the management of erectile dysfunction following cancer therapy. Transl Androl Urol. 2017;6(Supplement 5):S883–889.
Tran CN, Boncher N, Montague DK, Angermeier KW. Erosion of inflatable penile prosthesis reservoir into neobladder. J Sex Med. 2013;10:2343–6.
Acknowledgements
We would like to express a great appreciation to all physicians involved in this study for the interest and efforts in data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Falcone, M., Pucci, L., Garaffa, G. et al. An outcomes analysis of penile prosthesis implantation following radical cystoprostatectomy and urinary diversion: a multicentric retrospective cohort study. Int J Impot Res 32, 126–132 (2020). https://doi.org/10.1038/s41443-019-0171-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41443-019-0171-6
- Springer Nature Limited
This article is cited by
-
Surgical tips in difficult penile prosthetic surgery: a narrative review
International Journal of Impotence Research (2023)
-
Development and content validation of a competency-based assessment tool for penile prosthesis surgery
International Journal of Impotence Research (2022)