Abstract
Hypertension in chronic kidney disease (CKD) is the most commonly observed comorbidity and is a risk factor for end-stage renal disease (ESRD) as well as cardiovascular disease (CVD) and mortality. Therefore, suitable blood pressure (BP) control in CKD patients is very important in preventing both CVD and ESRD. We herein describe the recommendations of target BP and the pharmacological drug options from the evidence-based clinical practice guidelines for CKD in 2018 by the Japanese Society of Nephrology (JSN CKD 2018) and recent advances in the management of hypertension in CKD, including sodium-glucose cotransporter (SGLT) 2 inhibitors, mineralocorticoid receptor blockers, and renal denervation. In particular, SGLT2 inhibitors are a new class of “antihypertensive drugs” that have a homeostatic mechanism that regulates body fluid volume in addition to diuretic action, which may be closely associated with their cardiorenal protective properties.
Similar content being viewed by others
Introduction
Hypertension is an important risk factor for cardiovascular disease (CVD) and mortality. Recently, the number of chronic kidney disease (CKD) patients has been globally increasing, and hypertension is the most common comorbidity in these patients [1]. In addition, hypertension in CKD patients is a risk factor for end-stage renal disease (ESRD) as well as cardiovascular events and mortality [1, 2]. Therefore, favorable blood pressure (BP) control in CKD patients is an essential treatment strategy for preventing ESRD and CVD [3].
In this review, we describe the recommendations for target BP and the pharmacological drug options from the evidence-based clinical practice guidelines for CKD established in 2018 by the Japanese Society of Nephrology (JSN CKD 2018) [4], and we discuss the recent advances in the management of hypertension in CKD, including sodium-glucose cotransporter (SGLT) 2 inhibitors, mineralocorticoid receptor (MR) blockers, and renal denervation.
Target blood pressure
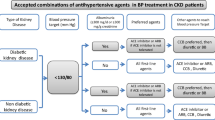
The recent guideline JSN CKD 2018 [4] recommends a target BP of <140/90 mmHg in nondiabetic CKD patients without proteinuria and of <130/80 mmHg in other CKD patients (Table 1). On the other hand, it recommends a BP target of <150/90 mmHg in elderly patients over 75 years of age, regardless of the presence or absence of diabetes mellitus. If there are no adverse events such as orthostatic hypotension, a target BP of <140/90 mmHg is recommended (Table 1). However, lowering the systolic BP to <110 mmHg is not recommended in any CKD patients because there is no beneficial effect from such a level (Table 1).
Next, we introduce the major clinical studies that were the basis for setting the BP target in the JSN CKD 2018 [4]. In the ACCORD study [5], stroke and albuminuria were significantly reduced in type 2 diabetes patients who underwent intensive therapy (target systolic BP <120 mmHg) compared with the standard therapy (target systolic BP <140 mmHg), but the reduction in renal function (estimated glomerular filtration [eGFR]) was significantly greater in the intensive therapy group. A meta-analysis of 13 randomized clinical trials (RCTs) of type 2 diabetic patients revealed that a systolic BP <130 mmHg reduced the risk for stroke but increased serious adverse events such as hypotension and hyperkalemia [6]. In the SPRINT study [7], the intensive-treatment group (target systolic BP <120 mmHg) demonstrated a significant reduction in cardiovascular events and death compared with the standard-treatment group (target systolic BP <140 mmHg) in nondiabetic patients. However, serious adverse events, including hypotension and acute kidney injury or failure, were higher in the intensive-treatment group than in the standard-treatment group [7]. In a large-scale cohort study from Japan [8], a systolic BP ≥134 mmHg in proteinuria-positive cases and a systolic BP ≥141 mmHg in proteinuria-negative cases were indicative of a significantly high risk for progressing to CKD stage 3 or higher. The post hoc analysis of the ORIENT trial [9] showed that a systolic BP ≥131 mmHg increased renal events compared with a systolic BP <130 mmHg.
Selection of antihypertensive drugs
Multiple meta-analyses and RCTs show that angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) reduce the risk of progression to ESRD and renal death, regardless of either the diabetic status or CKD stage [10, 11]. On the other hand, there is no consensus on whether ACEIs and ARBs have a superior cardiovascular protective effect compared with other antihypertensive drugs in patients with CKD [10, 12]. Based on such evidence, ACEIs and ARBs are recommended as first-line drugs for diabetic and nondiabetic patients with proteinuria, while ACEIs, ARBs, calcium channel blockers (CCBs), and thiazide diuretics are recommended for nondiabetic CKD stage 1–3 patients without proteinuria (Table 2).
There is no RCT that includes only elderly CKD patients over 75 years of age. A large cohort study of younger patients with CKD stage 5 (mean age: 64.7 years) reported that the administration of ACEIs or ARBs improved renal prognosis in comparison with other antihypertensive drugs [13]. Nevertheless, the JSN CKD 2018 recommends CCBs in CKD patients over 75 years because elderly people with severe atherosclerosis are vulnerable to dehydration and ischemia, and ACEIs and ARBs may cause a rapid onset of renal dysfunction. These inconsistencies show the difficulty of applying the results of large-scale trials in real clinical settings and the importance of balancing the risks and benefits of medications.
Controversy regarding hypertension management in CKD
To date, there remains much debate about the best target BP and antihypertensive drug selection. The main reason for this controversy depends on the goal of what is the highest priority outcome. The major outcomes of antihypertensive therapy in CKD are “cardiovascular events” and “renal prognosis”. The target BP and the selection of antihypertensive drugs are then determined based on these outcomes. Unfortunately, however, the risk of cardiovascular and renal events is not always compatible at the same target BP or for the same drug selection. For example, in the SPRINT study [7], the intensive-treatment group (target systolic BP <120 mmHg) demonstrated a significant reduction in cardiovascular events and deaths compared with the standard-treatment group (target systolic BP <140 mmHg). However, the risk of acute renal injury was conversely higher in the intensive-treatment group [14, 15]. Thus, achieving compatibility of cardiorenal protection at the same target BP is difficult.
The impact of each antihypertensive drug on cardiorenal outcomes is different in CKD patients. ACEIs and ARBs reduce the risk of progression to ESRD and renal death [10, 11], but no superior benefit of ACEIs and ARBs was observed in cardiovascular protective effects compared with other antihypertensive drugs [10, 12].
Recent advances
SGLT2 inhibitor
SGLT2 inhibitors are a new class of antidiabetic drugs that increase urinary glucose levels and sodium (Na+) excretion, thereby decreasing BP as well as blood glucose levels [16,17,18]. In particular, a higher body mass index (BMI) and baseline BP are predictors of a good BP response to SGLT2 inhibitors [18]. In the SACRA study, reductions in the daytime and 24-h systolic BP at 12 weeks with empagliflozin were significantly greater than with a placebo (–9.5 and –7.7 mmHg, respectively; p ≤ 0.002) in obese diabetes patients with uncontrolled hypertension (mean age 70 years, mean BMI 26 kg/m2, mean HbA1c 6.6%) [19].
Recent clinical trials have shown that SGLT2 inhibitors exhibit cardiorenal protective properties in patients with type 2 diabetes and heart failure [20,21,22,23,24,25]. A meta-analysis of type 2 diabetes patients showed that SGLT2 inhibitors reduced the risk of cardiovascular death or hospitalization for heart failure by 23% (hazard ratio 0.77 [95% confidence interval 0.71–0.84], p < 0.0001) and reduced the risk of progression of renal disease by 45% (0.55 [0.48–0.64], p < 0.0001), regardless of baseline atherosclerotic risk category or history of heart failure [26]. Furthermore, the CREDENCE trial, which includes type 2 diabetes and albuminuric patients with an eGFR of 30 to <90 ml/min/1.73 m2, shows that the SGLT2 inhibitor canagliflozin suppresses the composite renal endpoints (doubling of serum creatinine, ESRD, or death from renal or cardiovascular causes) regardless of baseline eGFR [24]. Therefore, the reductions in hospitalization for heart failure and progression of renal disease by SGLT2 inhibitors do not depend on baseline atherosclerotic risk category or renal function [24, 26].
The amelioration of extracellular volume expansion, including the reduction in the plasma volume, is one of the most promising BP-lowering mechanisms by SGLT2 inhibitors [17, 18, 27,28,29,30], which may lead to beneficial cardiorenal outcomes [16, 31,32,33]. Human and animal studies have shown that SGLT2 inhibitors induce natriuresis and glucose-induced osmotic diuresis by inhibiting the transporter SGLT2 in the early proximal tubule [30, 34,35,36]. We previously reported that the SGLT2 inhibitor dapagliflozin predominantly ameliorates extracellular volume expansion with a mild and transient increase in urinary Na+ levels and fluid excretion in diabetic kidney disease patients [37, 38]. On the other hand, SGLT2 inhibitors have a protective effect against hypovolemia. In euvolemic animal models, the SGLT2 inhibitor ipragliflozin did not decrease the body fluid volume (extracellular water [ECW], intracellular water, and total body water) despite causing an increase in both urinary fluid levels and Na+ excretion [39, 40]. The detailed mechanisms for this interesting finding are (1) the compensatory increase in fluid and food intake and (2) the suppression of excessive urine volume by vasopressin-induced solute-free water reabsorption in the collecting duct [39, 40]. These homeostatic mechanisms of SGLT2 inhibitors on the body fluid status were confirmed in our recent clinical study, in which dapagliflozin decreased the extracellular volume in patients with fluid retention, but it did not show any such decrease in the extracellular volume in those without fluid retention [41]. Similarly, several studies of type 2 diabetes patients without fluid retention showed that SGLT2 inhibitors transiently decreased the ECW or body fluid balance within 1 week, and then it returned to the initial value after that time [42, 43]. In contrast to SGLT2 inhibitors, the body fluid responses to the loop diuretic furosemide and vasopressin V2 receptor antagonist tolvaptan did not clearly vary depending on the pretreatment extracellular volume status (Fig. 1) [41]. The compatibility of the amelioration of extracellular fluid retention and the prevention of extracellular hypovolemia by SGLT2 inhibitors may contribute to cardiorenal protection in terms of the reduction in heart failure and BP, a lowering of acute kidney injury risk and the prevention of the persistent activation of the renin–angiotensin aldosterone (RAS) system [25, 36, 42, 44,45,46]. Thus, SGLT2 inhibitors are a new class of “antihypertensive drugs” that have a homeostatic mechanism that regulates body fluid volume in addition to diuretic action, which may play an important role in long-term cardiorenal protection.
The correlation between baseline extracellular fluid status (the ratio of extracellular water to total body water [ECW/TBW]) and the absolute change in ECW/TBW in response to loop diuretic furosemide (a), SGLT2 inhibitor dapagliflozin (b), and vasopressin V2 receptor antagonist tolvaptan (c) for 1 week. In the furosemide (a) and tolvaptan (c) groups, the ECW/TBW level was not significantly correlated, while in the dapagliflozin group (b), it was negatively and significantly correlated (modified from ref. 41)
MR blocker
The MR is a nuclear receptor that is abundantly present in the distal tubules and collecting ducts, and it serves as a regulator of the fluid volume and plasma Na+, K+, and Cl− flux mechanisms in the segment [47, 48]. MR regulates BP and therefore is involved in the pathogenesis of heart failure and CKD progression [49,50,51,52]. MR is activated mainly by aldosterone, but Ras-related C3 botulinum toxin substrate 1 (Rac1) also contributes to the activation of MRs [53]. Interestingly, in the context of salt-sensitive hypertension, high salt loading suppresses plasma aldosterone but activates MR signaling via Rac1, which causes BP elevation and kidney disease progression [47, 54].
The EVALUATE study, a randomized, placebo-controlled trial, showed that the MR blocker eplerenone significantly reduced albuminuria and BP in nondiabetic CKD patients who were already on ACEIs or ARBs [52]. In the post hoc analyses of the study, the renoprotective effects of eplerenone were prominent in those with high urinary sodium excretion, indicating the strong association between salt intake and MR signaling. Esaxerenone, a novel highly potent and selective nonsteroidal MR blocker that has been used for hypertension in Japan [55], has a stronger inhibitory effect on MR than spironolactone or eplerenone [56]. In a randomized, double-blind, placebo-controlled, phase 2 trial that enrolled 365 hypertensive or normotensive patients with type 2 diabetes mellitus and microalbuminuria, the addition of esaxerenone for 12 weeks to ongoing use of RAS inhibitors significantly reduced the urinary albumin-to-creatinine ratio [57]. Furthermore, the double-blind randomized phase 3 study comparing esaxerenone and eplerenone in patients with essential hypertension demonstrated that the proportions of patients achieving target sitting BP (<140/90 mmHg) were 31.5%, 41.2%, and 27.5% with esaxerenone 2.5 and 5 mg/day and eplerenone 50 mg/day, respectively [58]. The incidences of adverse events were similar across all treatment groups [58]. These results indicate that esaxerenone is an effective and well-tolerated MR blocker in patients with essential hypertension.
Renal denervation
Catheter-based renal denervation, which reduces sympathetic efferent and afferent sensory nerve activity, is an alternative treatment option for patients with uncontrolled hypertension [59]. The inhibition of the RAS system by sympathetic efferent nerve ablation causes a reduction in BP through an increase in renal blood flow and a reduction in salt sensitivity [59]. The SPYRAL HTN-OFF MED (SPYRAL Pivotal) trial, a recent randomized, sham-controlled trial to assess the efficacy of catheter-based renal denervation, has shown that the treatment difference between renal denervation and the sham procedure for 24-h systolic BP was –3.9 mmHg, and for office systolic BP, the difference was –6.5 mmHg from baseline to 3 months after the procedure [60]. The SYMPLICITY HTN-Japan, the first randomized controlled trial in Asia, also shows that renal denervation maintained the systolic BP reduction for up to 36 months (office systolic BP reduction −32.8 ± 20.1 mmHg) [61].
Several studies in CKD patients showed the long-term sustained BP-lowering effect of renal denervation. Ott et al. reported that renal denervation in patients with CKD stages 3 and 4 reduces office BP by 20 ± 20/8 ± 14 mmHg and average 24-h ambulatory BP by 9 ± 14/4 ± 7 mmHg after 1 year [62]. A recent report from Kiuchi et al. showed the mean change in systolic ambulatory BP monitoring compared with baseline to be −21.3 ± 14.1 mmHg at 24 months in patients with CKD stages 2–4 [63]. Furthermore, the Global SYMPLICITY Registry, a prospective, open-label registry in hypertensive patients undergoing renal denervation, showed the renal function decline in patients with CKD (eGFR <60 ml/min/1.73 m2) to be mild for 3 years (−3.7 ml/min/1.73 m2) [64]. In these reports, no apparent long-term safety concerns were observed following the renal denervation procedure [62,63,64]. From such evidence, renal denervation may therefore be suitable for individuals with resistant hypertension or noncompliant patients, including those with CKD [65].
Conclusion
The clinical guideline JSN CKD 2018 recommends new target BP and the pharmacological drug options for hypertension in CKD patients. The important change from the previous version is that CCBs are recommended in CKD stages 4 and 5 patients over 75 years of age because elderly people with severe atherosclerosis are vulnerable to dehydration and ischemia. Among the recent advances in this field, SGLT2 inhibitors are a promising “antihypertensive drug” for preventing both CVD and ESRD. In particular, they have a homeostatic mechanism to regulate the body fluid volume in addition to a diuretic action, and therefore, they may be a novel mechanism for achieving cardiorenal protection.
References
Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, et al. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2010;55:441–51.
Toto RD. Treatment of hypertension in chronic kidney disease. Semin Nephrol. 2005;25:435–9.
Townsend RR, Taler SJ. Management of hypertension in chronic kidney disease. Nat Rev Nephrol. 2015;11:555–63.
Japanese Society of Nephrology. Essential points from Evidence-based Clinical Practice Guidelines for Chronic Kidney Disease 2018. Clin Exp Nephrol. 2019;23:1–15.
Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85.
Bangalore S, Kumar S, Lobach I, Messerli FH. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and Bayesian random-effects meta-analyses of randomized trials. Circulation. 2011;123:2799–810.
Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–16.
Hirayama A, Konta T, Kamei K, Suzuki K, Ichikawa K, Fujimoto S, et al. Blood pressure, proteinuria, and renal function decline: associations in a large community-based population. Am J Hypertens. 2015;28:1150–6.
Imai E, Ito S, Haneda M, Harada A, Kobayashi F, Yamasaki T, et al. Effects of blood pressure on renal and cardiovascular outcomes in Asian patients with type 2 diabetes and overt nephropathy: a post hoc analysis (ORIENT-blood pressure). Nephrol Dial Transpl. 2016;31:447–54.
Xie X, Liu Y, Perkovic V, Li X, Ninomiya T, Hou W, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67:728–41.
Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9.
Nistor I, De Sutter J, Drechsler C, Goldsmith D, Soler MJ, Tomson C, et al. Effect of renin-angiotensin-aldosterone system blockade in adults with diabetes mellitus and advanced chronic kidney disease not on dialysis: a systematic review and meta-analysis. Nephrol Dial Transpl. 2018;33:12–22.
Hsu TW, Liu JS, Hung SC, Kuo KL, Chang YK, Chen YC, et al. Renoprotective effect of renin-angiotensin-aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern Med. 2014;174:347–54.
Rocco MV, Sink KM, Lovato LC, Wolfgram DF, Wiegmann TB, Wall BM, et al. Effects of intensive blood pressure treatment on acute kidney injury events in the systolic blood pressure intervention trial (SPRINT). Am J Kidney Dis. 2018;71:352–61.
Obi Y, Kalantar-Zadeh K, Shintani A, Kovesdy CP, Hamano T. Estimated glomerular filtration rate and the risk-benefit profile of intensive blood pressure control amongst nondiabetic patients: a post hoc analysis of a randomized clinical trial. J Intern Med. 2018;283:314–27.
Vallon V. Glucose transporters in the kidney in health and disease. Pflugers Arch. 2020. https://doi.org/10.1007/s00424-020-02361-w. Online ahead of print.
Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol. 2020;16:317–36.
Kario K, Ferdinand KC, O’Keefe JH. Control of 24-hour blood pressure with SGLT2 inhibitors to prevent cardiovascular disease. Prog Cardiovasc Dis. 2020;S0033-0620:30074-8.
Kario K, Okada K, Kato M, Nishizawa M, Yoshida T, Asano T, et al. 24-hour blood pressure-lowering effect of an SGLT-2 inhibitor in patients with diabetes and uncontrolled nocturnal hypertension: results from the randomized, placebo-controlled SACRA study. Circulation. 2019;139:2089–97.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–34.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306.
McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008.
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–9.
Cherney DZ, Kanbay M, Lovshin JA. Renal physiology of glucose handling and therapeutic implications. Nephrol Dial Transpl. 2020;35:i3–12.
Wilcox CS. Antihypertensive and renal mechanisms of SGLT2 (sodium-glucose linked transporter 2) inhibitors. Hypertension. 2020;75:894–901.
Lambers, Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–62.
Kawasoe S, Maruguchi Y, Kajiya S, Uenomachi H, Miyata M, Kawasoe M, et al. Mechanism of the blood pressure-lowering effect of sodium-glucose cotransporter 2 inhibitors in obese patients with type 2 diabetes. BMC Pharm Toxicol. 2017;18:23.
Faucon AL, Flamant M, Metzger M, Boffa JJ, Haymann JP, Houillier P, et al. Extracellular fluid volume is associated with incident end-stage kidney disease and mortality in patients with chronic kidney disease. Kidney Int. 2019;96:1020–9.
Tai R, Ohashi Y, Mizuiri S, Aikawa A, Sakai K. Association between ratio of measured extracellular volume to expected body fluid volume and renal outcomes in patients with chronic kidney disease: a retrospective single-center cohort study. BMC Nephrol. 2014;15:189.
Hung SC, Lai YS, Kuo KL, Tarng DC. Volume overload and adverse outcomes in chronic kidney disease: clinical observational and animal studies. J Am Heart Assoc. 2015;4:e001918.
Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest. 1994;93:397–404.
Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011;22:104–12.
Ansary TM, Nakano D, Nishiyama A. Diuretic effects of sodium glucose cotransporter 2 inhibitors and their influence on the renin-angiotensin system. Int J Mol Sci. 2019;20:629.
Masuda T, Ohara K, Murakami T, Imai T, Nakagawa S, Okada M, et al. Sodium-glucose cotransporter 2 inhibition with dapagliflozin ameliorates extracellular volume expansion in diabetic kidney disease patients. POJ Diabetes Obes. 2017;1:1–8.
Ohara K, Masuda T, Murakami T, Imai T, Yoshizawa H, Nakagawa S, et al. Effects of the sodium-glucose cotransporter 2 inhibitor dapagliflozin on fluid distribution: a comparison study with furosemide and tolvaptan. Nephrology (Carlton). 2019;24:904–11.
Masuda T, Muto S, Fukuda K, Watanabe M, Ohara K, Koepsell H, et al. Osmotic diuresis by SGLT2 inhibition stimulates vasopressin-induced water reabsorption to maintain body fluid volume. Physiol Rep. 2020;8:e14360.
Masuda T, Watanabe Y, Fukuda K, Watanabe M, Onishi A, Ohara K, et al. Unmasking a sustained negative effect of SGLT2 inhibition on body fluid volume in the rat. Am J Physiol Ren Physiol. 2018;315:F653–64.
Ohara K, Masuda T, Morinari M, Okada M, Miki A, Nakagawa S, et al. The extracellular volume status predicts body fluid response to SGLT2 inhibitor dapagliflozin in diabetic kidney disease. Diabetol Metab Syndr. 2020;12:37.
Schork A, Saynisch J, Vosseler A, Jaghutriz BA, Heyne N, Peter A, et al. Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy. Cardiovasc Diabetol. 2019;18:46.
Yasui A, Lee G, Hirase T, Kaneko T, Kaspers S, von Eynatten M, et al. Empagliflozin induces transient diuresis without changing long-term overall fluid balance in Japanese patients with type 2 diabetes. Diabetes Ther. 2018;9:863–71.
Gilbert RE, Thorpe KE. Acute kidney injury with sodium-glucose co-transporter-2 inhibitors: a meta-analysis of cardiovascular outcome trials. Diabetes Obes Metab. 2019;21:1996–2000.
Donnan JR, Grandy CA, Chibrikov E, Marra CA, Aubrey-Bassler K, Johnston K, et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis. BMJ Open. 2019;9:e022577.
Braam B, Lai CF, Abinader J, Bello AK. Extracellular fluid volume expansion, arterial stiffness and uncontrolled hypertension in patients with chronic kidney disease. Nephrol Dial Transplant. 2019. https://doi.org/10.1093/ndt/gfz020. Online ahead of print.
Shibata S, Ishizawa K, Uchida S. Mineralocorticoid receptor as a therapeutic target in chronic kidney disease and hypertension. Hypertens Res. 2017;40:221–5.
Azushima K, Morisawa N, Tamura K, Nishiyama A. Recent research advances in renin-angiotensin-aldosterone system receptors. Curr Hypertens Rep. 2020;22:22.
Nishiyama A. Pathophysiological mechanisms of mineralocorticoid receptor-dependent cardiovascular and chronic kidney disease. Hypertens Res. 2019;42:293–300.
Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–17.
Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21.
Ando K, Ohtsu H, Uchida S, Kaname S, Arakawa Y, Fujita T. Anti-albuminuric effect of the aldosterone blocker eplerenone in non-diabetic hypertensive patients with albuminuria: a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:944–53.
Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, et al. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nat Med. 2008;14:1370–6.
Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K, Yoshida S, et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor-dependent pathway. J Clin Invest. 2011;121:3233–43.
Yamada M, Takei M, Suzuki E, Takakusa H, Kotsuma M, Washio T, et al. Pharmacokinetics, distribution, and disposition of esaxerenone, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist, in rats and monkeys. Xenobiotica. 2017;47:1090–103.
Arai K, Homma T, Morikawa Y, Ubukata N, Tsuruoka H, Aoki K, et al. Pharmacological profile of CS-3150, a novel, highly potent and selective non-steroidal mineralocorticoid receptor antagonist. Eur J Pharm. 2015;761:226–34.
Ito S, Shikata K, Nangaku M, Okuda Y, Sawanobori T. Efficacy and safety of esaxerenone (CS-3150) for the treatment of type 2 diabetes with microalbuminuria: a randomized, double-blind, placebo-controlled, phase II trial. Clin J Am Soc Nephrol. 2019;14:1161–72.
Ito S, Itoh H, Rakugi H, Okuda Y, Yoshimura M, Yamakawa S. Double-blind randomized phase 3 study comparing esaxerenone (CS-3150) and eplerenone in patients with essential hypertension (ESAX-HTN study). Hypertension. 2020;75:51–58.
Kario K, Kim BK, Aoki J, Wong AY, Lee YH, Wongpraparut N, et al. Renal denervation in Asia: consensus statement of the Asia renal denervation consortium. Hypertension. 2020;75:590–602.
Bohm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–51.
Kario K, Yamamoto E, Tomita H, Okura T, Saito S, Ueno T, et al. Sufficient and persistent blood pressure reduction in the final long-term results from SYMPLICITY HTN-Japan- safety and efficacy of renal denervation at 3 years. Circ J. 2019;83:622–9.
Ott C, Mahfoud F, Schmid A, Toennes SW, Ewen S, Ditting T, et al. Renal denervation preserves renal function in patients with chronic kidney disease and resistant hypertension. J Hypertens. 2015;33:1261–6.
Kiuchi MG, Schlaich MP, Chen S, Villacorta H, Ho JK, Carnagarin R, et al. Relevance of targeting the distal renal artery and branches with radiofrequency renal denervation approaches—a secondary analysis from a hypertensive CKD patient cohort. J Clin Med. 2019;8:581.
Mahfoud F, Bohm M, Schmieder R, Narkiewicz K, Ewen S, Ruilope L, et al. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the Global SYMPLICITY Registry. Eur Heart J. 2019;40:3474–82.
Duni A, Dounousi E, Pavlakou P, Eleftheriadis T, Liakopoulos V. Hypertension in chronic kidney disease: novel insights. Curr Hypertens Rev. 2020;16:45–54.
Acknowledgements
We thank Keiko Fukuda and Minami Watanabe for their valuable technical support.
Funding
This study was supported in part by a Grant-in-Aid for Young Scientists 15K21321 (to TM), Jichi Medical University Young Investigator Awards (to TM), and a Grant-in-Aid for Research on Advanced Chronic Kidney Disease, Practical Research Project for Renal Diseases from the Japan Agency for Medical Research and Development (AMED to DN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Masuda, T., Nagata, D. Recent advances in the management of secondary hypertension: chronic kidney disease. Hypertens Res 43, 869–875 (2020). https://doi.org/10.1038/s41440-020-0491-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-020-0491-4
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
Fluid homeostasis induced by sodium-glucose cotransporter 2 inhibitors: novel insight for better cardio-renal outcomes in chronic kidney disease
Hypertension Research (2023)
-
Urinary free cortisol excretion is associated with lumbar bone density in patients with adrenal Cushing’s syndrome
Hypertension Research (2022)
-
Update on Hypertension Research in 2021
Hypertension Research (2022)