Abstract
Background
To investigate the prevalence of macular lesions associated with age-related macular degeneration (AMD) in eyes with pachydrusen.
Methods
Clinical records and multimodal imaging data of patients over 50 years old with drusen or drusenoid deposits were retrospectively assessed, and eyes with pachydrusen were included in this study. The presence of AMD features, including drusen or drusenoid deposits, macular pigmentary abnormalities, geographic atrophy (GA), and macular neovascularization (MNV), were evaluated.
Results
Out of 967 eyes of 494 patients with drusen or drusenoid deposits, 330 eyes of 183 patients had pachydrusen (34.1%). The mean age was 66.1 ± 9.3 years, and the subfoveal choroidal thickness (SFCT) was 292.7 ± 100.1 μm. The mean number of pachydrusen per eye was 2.22 ± 1.73. The majority of eyes with pachydrusen had no other drusen or drusenoid deposits (95.2%). Only 16 eyes (4.8%) had other deposits, including soft drusen (10 eyes, 3.0%), cuticular drusen (3 eyes, 0.9%), and reticular pseudodrusen (RPD; 3 eyes, 0.9%). Macular pigmentary abnormalities accompanied pachydrusen in 68 eyes (27.4%). None of the eyes had GA, and 82 eyes (24.8%) had MNV. The majority of MNV was polypoidal choroidal vasculopathy (PCV; 65 eyes, 19.7%), followed by type 1 (10 eyes, 3.0%), type 2 (5 eyes, 1.5%), and type 3 MNV (2 eyes, 0.6%).
Conclusions
Eyes with pachydrusen in Korean population have several characteristic AMD lesions in low frequencies. These findings indicate that pachydrusen might have diagnostic and prognostic values that are different from those of other drusen or drusenoid deposits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in elderly patients of developed countries [1]. Drusen, which is considered as the hallmark of AMD, is associated with pathogenesis and progression of AMD [1]. Several types of drusen and drusenoid deposits have been reported including soft drusen, cuticular drusen, and reticular pseudodrusen. Their clinical characteristics and natural history might differ depending on the types of drusen [2,3,4]. Drusen have been used in the diagnosis and prediction of prognosis of AMD [5, 6].
Pachydrusen is a recently recognized type of drusen, defined as large drusen (> 125 μm) with irregular borders, scattered over the posterior pole, on colour fundus photography (CFP) images [7]. Pachydrusen share many morphological and imaging aspects with other drusen. Although epidemiologic data of pachydrusen is still scarce, a previous study reported a prevalence of 7.7% among individuals over 40 years old [8]. Reportedly, pachydrusen are more prevalent than soft drusen and pseudodrusen combined and more common in Asians than in white people [8, 9]. In addition, the 5-year cumulative incidence of progression to neovascular AMD in pachydrusen eyes was reported as 7.1–17.0%, and pachydrusen was frequently accompanied by polypoidal choroidal vasculopathy (PCV) (49.3%) [2, 10]. Pachydrusen located in the macula have been used with other drusen to diagnose and predict AMD in clinical practice and studies, because the current AMD classification does not differentiate between drusen types. However, whether pachydrusen is a risk factor for advanced AMD similar to other drusen remains unclear. On the basis of the findings of less frequent development of exudative AMD and a significantly lower frequency of the ARMS2 A69S allele, a retrospective study on patients with unilateral exudative AMD in fellow eyes reported that pachydrusen have different clinical and genetic characteristics than soft drusen and pseudodrusen [11]. Thus, further studies on the nature of pachydrusen related to AMD are required to use them in the diagnosis and prognosis prediction of AMD. The accompanying rates of individual macular lesions related to AMD in pachydrusen eyes have not been thoroughly investigated before, and we believe that they may provide valuable information regarding the relation between pachydrusen and AMD.
This study aimed to analyse the prevalence of macular lesions related to AMD in pachydrusen eyes to estimate the degree of association between pachydrusen and characteristic AMD lesions, including drusen (or drusenoid deposits), macular pigmentary abnormalities, and advanced AMD lesions.
Methods
This study was approved by the institutional review board (IRB) of the Samsung Medical Center and adhered to the tenets of the Declaration of Helsinki. We performed a retrospective review of medical records of consecutive patients over 50 years old, who were referred to the retinal clinic at the Samsung Medical Center, Seoul, Korea between January 1998 and June 2021. Given the retrospective design of this study and the use of anonymized data, requirements for informed consent were waived by the IRB.
Clinical records and multimodal imaging data of patients with drusen or drusenoid deposits were retrospectively assessed. Patients were included if they were diagnosed with pachydrusen. Exclusion criteria were as follows: uveitis, severe diabetic retinopathy, severe hypertensive retinopathy, severe epiretinal membrane, retinal detachment, glaucoma, refractive error exceeding ± 6 dioptres, history of retinal laser photocoagulation, severe ocular media opacity, and insufficient ocular examinations.
Demographic information including age, sex, and comorbidities was obtained for each patient. All patients had undergone a comprehensive ophthalmologic evaluation including slit lamp examination, best corrected visual acuity (BCVA) assessment, intraocular pressure (IOP) measurements, and refractive error measurement. CFP and fundus autofluorescence (FAF) imaging were performed with a fundus camera (TRC 50 IX or DX, Topcon, Tokyo, Japan). The fundus camera had a field of view of 50° for fundus imaging. All patients underwent spectral domain optical coherence tomography (SD-OCT; Heidelberg Engineering, Heidelberg, Germany). The protocol of SD-OCT consisted of two B-scans centred on the fovea (horizontal and vertical, 12.0 mm, automatic real time (ART) 100) and raster scans (30° x 25°, 9.0 mm, centred on the fovea, 31 horizontal B-scans, ART 25) using enhanced depth imaging (EDI) protocols established by Spaide et al. [12]. In cases of suspected macular neovascularization (MNV), fluorescein angiography (FA) and indocyanine green angiography (ICGA) were performed (Spectralis HRA + OCT, Heidelberg Engineering, Heidelberg, Germany).
Drusen and drusenoid deposits
The type of drusen or drusenoid deposits was determined on the basis of multimodal imaging by the same criteria used in previous studies, which are as follows. Pachydrusen were defined as large drusen ( > 125 μm) with irregular borders, scattered over the posterior pole, on CFP images. In addition, we confirmed that the deposit was below the retinal pigment epithelium (RPE) layer using OCT [7].
Soft drusen were defined as round- or ovoid-shaped drusen with poorly defined border, which could be tightly packed and even confluent on CFP images. We confirmed that the deposit was below the RPE layer using OCT [7]. Reticular pseudodrusen (RPD) were defined as 1) multiple yellowish white lesions with a reticular network in CFP, 2) interlacing network in red-free (RF) imaging, 3) hyporeflective lesions with mild background hyperreflective in near infrared (NIR) imaging, 4) hypofluorescent lesions against a background of mild hyperfluorescence in FAF imaging, 5) ≥ 5 hyperreflective subretinal deposits above the RPE on more than one B-scan image in OCT, and 6) hypofluorescent lesions in the mid- or late-phase of ICGA. RPD were considered to be present, if they were identified in at least three imaging methods, including OCT [13]. Cuticular drusen were defined as multiple, yellow or pale, small, round lesions observed in CFP, showing a symmetric distribution pattern between bilateral eyes. There had to be at least 50 scattered, uniformly sized, small (25–75 μm) hyperfluorescent drusen with a typical “stars-in-the-sky” appearance on the FA images in each eye. The lesion had to be located beneath the RPE with RPE elevation on the OCT images [14].
Macular neovascularization (MNV) and geographic atrophy (GA)
The definition and classification of MNV followed the criteria proposed by the Consensus on Neovascular Age-related macular degeneration Nomenclature (CONAN) study group [15]. The subtypes of MNV were comprehensively diagnosed on the basis of the findings from CFP, FA, ICGA, and OCT. GA was defined as a sharply demarcated hypopigmented area with visible large choroidal vessels in CFP and hypoautofluorescent in FAF, with a diameter of at least 175 μm [16]. Only MNV and GA located in the macula were investigated.
Imaging analysis
Macula was defined by grids with a radius of 3000 µm, centred on the fovea [17]. The number of pachydrusen was counted using CFP. Clustered pachydrusen were counted as one pachydrusen. The number of pachydrusen outside and inside the macula was counted separately. Presence of macular pigmentary abnormalities (hyperpigmentation and hypopigmentation) was determined by performing CFP of each eye at baseline [5]. Macular RPE abnormalities were determined using OCT. These were classified into RPE undulation and pigment epithelial detachment (PED). Presence of MNV and GA led to the exclusion of the case in macular pigmentary abnormalities and macular RPE abnormalities.
The subfoveal choroidal thickness (SFCT) was measured manually using horizontal B-scans of SD-OCT at the foveal centre. All the photographs were assessed by two investigators (S.W.N. and H.N.). Inter and intragrader agreement on each fundus feature was regularly assessed, and consensus training was initiated when κ values were below 0.6. All uncertain diagnoses were adjudicated by a senior interpreter (D-I.H.).
Statistical analysis
Quantitative variables were presented as the means ± standard deviations. We compared the characteristics of the groups by using the independent t-test for continuous variables and the Chi-squared and Fisher’s exact test for categorical variables. We analysed linear correlations with Pearson’s correlation coefficient (r) for normally distributed continuous variables. Statistical significance was set at p < 0.05. All statistical analyses were performed using SPSS 20.0 software (SPSS, Chicago, IL).
Results
Of the 4204 eyes of 2187 patients aged over 50 years, who were referred to the retinal clinic in our hospital, 967 eyes of 494 patients were identified with drusen or drusenoid deposits via multimodal imaging. Among them, 330 eyes of 183 patients had pachydrusen (34.1% of eyes with drusen or drusenoid deposits). The mean age was 66.1 ± 9.3 years [50.0–93.8]. The male-to-female ratio was 1.17. Bilateral involvement was found in 147 patients (80.3%). The mean spherical equivalent was 0.25 ± 1.47 dioptres [−4.25– + 4.75], and 191 eyes of 330 eyes (57.9%) were hyperopic. BCVA was 0.18 ± 0.35 Log of Minimum Angle of Resolution (logMAR) [0.00–2.00]. Approximately 297 eyes (90.0%) were phakic; 74 eyes (22.4%) were fellow eyes of advanced AMD with MNV (Table 1). Representative cases are shown in Figs. 1, 2, and 3.
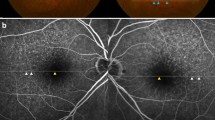
A. Two pachydrusen larger than 125 μm with irregular borders are observed in colour fundus photography (CFP). Pachydrusen at the upper position is a single form and pachydrusen at the lower position is a clustered form (arrowheads). There are no concurrent other types of drusen, macular pigmentary abnormalities, macular neovascularization, and geographic atrophy. B Two pachydrusen are located inside of the outermost ring of the Early Treatment Diabetic Retinopathy Study (ETDRS) grid with a radius of 3000 µm. C, D Spectral domain optical coherence tomography images demonstrate that two pachydrusen are sub-retinal pigment epithelium (RPE) deposits (arrows).
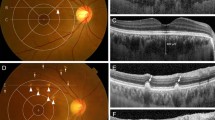
A Three pachydrusen larger than 125 μm with irregular borders are observed in colour fundus photography (CFP). (arrowheads). There are concurrent soft drusen and macular pigmentary abnormalities without macular neovascularization and geographic atrophy. B Two pachydrusen are located inside of the outermost ring of the Early Treatment Diabetic Retinopathy Study (ETDRS) grid with a radius of 3000 µm. C, D Spectral domain optical coherence tomography images demonstrate that two pachydrusen are sub-retinal pigment epithelium (RPE) deposits (arrows).
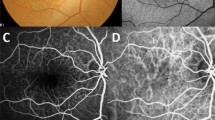
A Colour fundus photography showed three pachydrusen (arrowheads), a few retinal haemorrhages, focal elevated lesions, and macular pigmentary abnormalities. There was no concurrent other types of drusen. B Three pachydrusen are located inside of the outermost ring of the Early Treatment Diabetic Retinopathy Study (ETDRS) grid with a radius of 3000 µm. C Pachydrusen was located under the retinal pigment epithelium on the spectral domain optical coherence tomography (SD-OCT) images (arrow). D SD-OCT shows pigment epithelial detachment and double-layer sign, which are typical characteristics of polypoidal choroidal vasculopathy (PCV). The subfoveal choroidal thickness at the foveal centre was measured at 314 μm. E Fluorescein angiography shows stippled hyperfluorescences characteristic of occult macular neovascularization with localized hyperfluorescence and hypofluorescence. F Indocyanine green angiography shows an abnormal vascular branching network and multiple polypoid lesions, indicating the occurrence of PCV.
Clinical characteristics of pachydrusen eyes
The detected total number of pachydrusen was 731, and the mean number of pachydrusen per eye was 2.22 ± 1.73 [1.00–14.00]. Among the 731 pachydrusen, 445 (60.9%) were located in the macula, and 286 (39.1%) were located in the extramacula. The number of eyes with pachydrusen was 330, and 149 eyes (45.2%) had pachydrusen only in the macula, and 116 eyes (35.2%) had pachydrusen only in the extramacula. Sixty five eyes (19.6%) had pachydrusen both in the macular and extramacula. Other types of drusen or drusenoid deposits accompanied pachydrusen in 16 eyes (4.8%), including soft drusen in 10 eyes, RPD in 3 eyes, and cuticular drusen in 3 eyes. Ghost drusen was not found. MNV accompanied pachydrusen in 82 eyes (24.8%), including polypoidal choroidal vasculopathy (PCV) in 65 eyes (79.3%), type 1 MNV in 10 eyes (12.2%), type 2 MNV in 5 eyes (6.1%), and type 3 MNV in 2 eyes (2.4%). GA was not found in any of the eyes (Table 2).
In eyes with MNV, among the 167 pachydrusen, 53 (31.7%) were located in the macula, and 50.0% of eyes showed only extramacular pachydrusen. In eyes with other drusen, Among the 48 pachydrusen, 26 (54.2%) were located in the macula, and 31.3% of eyes showed only extramacular pachydrusen.
Among the eyes with PCV, 63 eyes (97.0%) showed only pachydrusen, 1 eye (1.5%) had concurrent soft drusen, and 1 eye (1.5%) had concurrent RPD. Among the type 1 and 2 MNV eyes, all eyes showed only pachydrusen. Among the type 3 MNV eyes, all eyes had concurrent RPD.
Image findings of pachydrusen eyes
Macular pigmentary abnormalities in CFP were concurrent in 68 eyes (27.4%). Macular RPE abnormalities in OCT were observed in 51 eyes (20.6%) with RPE undulation and 17 eyes (6.8%) with PED. The mean SFCT was 292.7 ± 100.1 μm [55.0–589.0].
Association between age and other features in pachydrusen eyes
The mean age was 65.77 ± 9.27 years, in eyes with pachydrusen only while that 72.56 ± 6.76 years in eyes with concurrent other types of drusen or drusenoid deposits. The mean age was 65.36 ± 9.07 years in eyes without MNV and 68.33 ± 9.60 years in eyes with MNV, respectively.
Age was significantly related to the number of pachydrusen (r = 0.215; p = 0.000), concurrent occurrence of other types of drusen or drusenoid deposits (p = 0.001), presence of MNV (p = 0.015), and SFCT (r = −0.267; p = 0.000). Age was not significantly related to macular pigmentary abnormalities (p = 0.728) or macular RPE abnormalities (p = 0.728).
Association between the number of pachydrusen and other features in pachydrusen eyes
The mean number of pachydrusen was 2.18 ± 1.70 in eyes with pachydrusen only and 3.00 ± 2.19 in eyes with other concurrent types of drusen or drusenoid deposits. The mean number of pachydrusen in eyes without and with MNV was 2.27 ± 1.81 and 2.04 ± 1.45, respectively.
The number of pachydrusen was not significantly related to sex (p = 0.151), concurrent occurrence of other types of drusen or drusenoid deposits (p = 0.157), presence of MNV (p = 0.230), macular pigmentary abnormalities (p = 0.887), macular RPE abnormalities (p = 0.887), or SFCT (r = −0.039; p = 0.484).
Association between choroidal thickness and other features in pachydrusen eyes
The mean SFCT was 296.9 ± 99.9 μm in eyes with pachydrusen only, and 209.9 ± 61.0 μm in eyes with concurrent presence of other types of drusen or drusenoid deposits. The mean SFCT in eyes without and with MNV was 294.4 ± 94.3 and 287.6 ± 116.5 μm, respectively.
SFCT was significantly related to the concurrent occurrence of other types of drusen or drusenoid deposits (p = 0.000). SFCT was not significantly related to the presence of MNV (p = 0.634), macular pigmentary abnormalities (p = 0.243), or macular RPE abnormalities (p = 0.243).
Discussion
This study investigated the basic clinical features of pachydrusen and the prevalence of characteristic macular lesions related to AMD in pachydrusen eyes. Although several previous studies have investigated pachydrusen, the association between pachydrusen and AMD lesions remains unclear, because the study purposes and methods were varied. Most previous studies used CFP with 45° or 50° for the detection of pachydrusen, and one study used a 1500 μm radius circle to assess the location of drusen [3, 8, 9, 18]. Only one study used a standard circle of 3000 μm radius centred on the fovea for the detection of drusen and pigmentary abnormalities; however, both eyes with soft drusen and pachydrusen were classified into the soft drusen group [8].
Several basic clinical features of pachydrusen observed in this study were noteworthy. Pachydrusen eyes accounted for 34.1% (330 eyes from 183 patients) of 967 drusen or drusenoid deposits in the eyes of 494 Korean patients in this study, 22.4% of which were fellow eyes of unilateral neovascular AMD. There are several previous studies on the prevalence of pachydrusen in nonexudative AMD eyes. Two studies reported a lower prevalence rate; 9.7% in 632 eyes of 418 intermediate AMD Korean patients, and 11.7% in 94 eyes of 71 nonexudative AMD patients (all white patients except one) [2, 7]. However, two other studies reported a high prevalence rate; 35.7% in the fellow eyes of treatment-naive unilateral neovascular AMD Korean patients, and 15.3% in 359 eyes with drusen (25.5% in the fellow eyes of 145 Asian patients with unilateral neovascular AMD and 8.4% in 214 white patients of whom 25.2% had neovascular AMD in the fellow eyes) [9, 10]. It appears that the prevalence rate of pachydrusen in drusen eyes vary by race and the status of fellow eyes; Asians and fellow eyes of patients with unilateral neovascular AMD are probably associated with a high prevalence rate.
More than half (60.9%) of 731 pachydrusen was located in the macula. Approximately two-thirds (64.8%) of 330 pachydrusen eyes showed macular pachydrusen, which was slightly higher than the previously reported rate (54.1%) [8]. We believe macular pachydrusen eyes should receive special attention because most of them have no other drusen or drusenoid deposits. Thus they may be diagnosed as intermediate AMD solely on the basis of pachydrusen. Contrarily, 35.2% of pachydrusen eyes showed pachydrusen only in the extramacular area, and 50.0% of pachydrusen eyes with MNV showed only extramacular pachydrusen. A previous study reported a higher rate of eyes with only extramacular pachydrusen (45.9%) [8]. This finding indicates that extramacular as well as macular pachydrusen may be associated with the risk for the occurrence of MNV.
The mean number (and standard deviation) of pachydrusen per eye (2.22 ± 1.73) appears to be relatively smaller than those of other drusen or drusenoid deposits as indicated by previous studies [1, 19,20,21]. A previous pachydrusen study reported a similar finding; 1.65 ± 1.07 per eye in the fundus area covering 12 × 9 mm2 [3]. Moreover, the mean number of pachydrusen in eyes with MNV (2.04 ± 1.45) was fewer than those in eyes without MNV (2.27 ± 1.81).
This study revealed the prevalence of characteristic macular lesions related to AMD in pachydrusen eyes. The majority of eyes with pachydrusen have no other drusen or drusenoid deposits (95.2%). Only 4.8% of pachydrusen eyes have other drusen or drusenoid deposits and only 3.0% have soft drusen. A previous study also reported a low prevalence rate (14.1%) of other drusen in pachydrusen eyes [9]. These findings showed a large difference in the results concerning RPD. It was reported that 65.2–79.2% of eyes with RPD had soft drusen [22, 23]. Only 20.8–34.8% of eyes with RPD did not have concurrent drusen or drusenoid deposits [22, 23]. Considering that the prevalence rate of AMD in the population was 8.69% (age range; 45–85, 7.4% in Asians), we suppose that other drusen might coexist with pachydrusen by mere chance, or the relation between other drusen and pachydrusen might be very weak [24].
Macular pigmentary abnormalities accompanied pachydrusen in 27.4% of the eyes. This prevalence rate was higher than that seen in previous reports of population-based AMD cohort studies; 12.6% of the urban population over 50 years old in the Blue Mountains Eye Study, and 13.1% of the population over 43 years old in the Beaver Dam Study [25, 26]. The rate of macular pigmentary abnormalities in this study was higher than the previously reported rate in the Japanese population (8.3%) [8]. The possible explanations are older age, different AMD status, and detection methods with multimodal imaging.
Advanced AMD was also investigated in this study. MNV occurred in pachydrusen eyes, and the most prevalent type was PCV (79.3%), followed by type 1 MNV (12.2%), type 2 MNV (6.1%), and type 3 MNV (2.4%). Two retrospective progressive AMD studies supported this finding, showing the association between pachydrusen and progression to PCV with a high occurrence rate [2, 11]. Furthermore, another study reported the high prevalence of pachydrusen in PCV patients [27]. Thus, it appears that pachydrusen are strongly associated with PCV. In addition, this study showed that type 1, 2, and 3 MNV also occur in pachydrusen eyes. However, all type 3 MNV occurred in eyes with both pachydrusen and RPD, suggesting the possible influence of RPD.
In this study, GA did not accompany pachydrusen, although multimodal imaging was used to detect the presence of GA. A few previous studies also reported that there were none to rare GA in pachydrusen eyes. Akushichi et al. reported that unlike soft drusen, pachydrusen eyes did not show any GA after regression of pachydrusen [8]. Lee et al. reported that focal disruption of ellipsoid zone could develop a corresponding site of pachydrusen, but GA was rarely observed [28]. The prevalence of GA in AMD eyes in Age-Related Eye Disease Study 2 (AREDS2) was 32.7%; It therefore appears that pachydrusen might have none to minimal association with the development of GA [29].
There are a few important clinical issues according to the results of this study. Pachydrusen appear to have clinical characteristics different from those of other drusen, including a low accompanying rate of other drusen and drusenoid deposits, a high rate of PCV in MNV occurrence, and no occurrence of GA. A previous retrospective, progressive study on patients with unilateral exudative AMD reported that pachydrusen have clinical and genetic characteristics different from those of soft drusen and pseudodrusen on the basis of the findings of less frequent development of exudative AMD and a significantly lower frequency of the ARMS2 A69S allele [11]. Thus, we suppose that pachydrusen probably have a prognostic value different from that of other drusen. However, eyes with only pachydrusen in the macula are diagnosed as AMD with the current AMD classification [5, 6]. In addition, the risk calculation for advanced AMD in eyes with only pachydrusen, using the current protocol, may lead to erroneous results. Moreover, some differences might occur in the results of clinical AMD studies between the Asian population and other ethnic populations, because the prevalence of pachydrusen is higher in Asians [9].
This study has some limitations. This study was a retrospective study with a cross-sectional design. We did not observe longitudinal changes in pachydrusen. Patients younger than 50 years old were not included. By contrast, the strengths of this study include a relatively larger sample size than those of previous studies, use of well-defined diagnostic criteria, evaluation of AMD lesions and pachydrusen in the macular area, and use of multimodal imaging.
In conclusion, a significant number of eyes of Korean patients with drusen or drusenoid deposits had pachydrusen, while the majority of pachydrusen eyes had no other drusen and drusenoid deposits. In addition, pachydrusen eyes had no GA but had a strong association with the occurrence of PCV. These findings indicate that pachydrusen might have clinical characteristics and prognosis different from those of other drusen and drusenoid deposits. Further studies are needed to know whether pachydrusen, similar to other drusen, can be used in AMD diagnosis and prognosis prediction. We hope that the results of this study will provide valuable insights for future studies.
Summary
What was known before
-
Pachydrusen eyes can accompany macular lesions associated with age-related macular degeneration (AMD), but epidemiological data were scarce.
What this study adds
-
Pachydrusen eyes have low frequencies of several characteristic AMD lesions.
-
Pachydrusen eyes may have different clinical values that are different from those of other drusen or drusenoid deposits.
-
Therefore, ophthalmologists need different clinical plans.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to privacy or ethical restrictions but are available from the corresponding author on reasonable request.
References
Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: The Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol. 2001;132:668–81.
Kim KL, Joo K, Park SJ, Park KH, Woo SJ. Progression from intermediate to neovascular age-related macular degeneration according to drusen subtypes: Bundang AMD cohort study report 3. Acta Ophthalmol. 2022;100:e710–e8.
Baek J, Lee JH, Chung BJ, Lee K, Lee WK. Choroidal morphology under pachydrusen. Clin Exp Ophthalmol. 2019;47:498–504.
Spaide RF, Curcio CA. Drusen characterization with multimodal imaging. Retina. 2010;30:1441–54.
Ferris FL, Davis MD, Clemons TE, Lee LY, Chew EY, Lindblad AS, et al. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol. 2005;123:1570–4.
Ferris FL 3rd, Wilkinson CP, Bird A, Chakravarthy U, Chew E, Csaky K, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–51.
Spaide RF. Disease expression in nonexudative age-related macular degeneration varies with choroidal thickness. Retina. 2018;38:708–16.
Sato-Akushichi M, Kinouchi R, Ishiko S, Hanada K, Hayashi H, Mikami D, et al. Population-based prevalence and 5-Year change of soft drusen, pseudodrusen, and pachydrusen in a Japanese population. Ophthalmol Sci. 2021;1:100081.
Cheung CMG, Gan A, Yanagi Y, Wong TY, Spaide R. Association between choroidal thickness and drusen subtypes in age-related macular degeneration. Ophthalmol Retina. 2018;2:1196–205.
Lee J, Choi S, Lee CS, Kim M, Kim SS, Koh HJ, et al. Neovascularization in fellow eye of unilateral neovascular age-related macular degeneration according to different drusen types. Am J Ophthalmol. 2019;208:103–10.
Fukuda Y, Sakurada Y, Yoneyama S, Kikushima W, Sugiyama A, Matsubara M, et al. Clinical and genetic characteristics of pachydrusen in patients with exudative age-related macular degeneration. Sci Rep. 2019;9:11906.
Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500.
Lee MY, Yoon J, Ham DI. Clinical characteristics of reticular pseudodrusen in Korean patients. Am J Ophthalmol. 2012;153:530–5.
Balaratnasingam C, Cherepanoff S, Dolz-Marco R, Killingsworth M, Chen FK, Mendis R, et al. Cuticular drusen: Clinical phenotypes and natural history defined using multimodal imaging. Ophthalmology. 2018;125:100–18.
Spaide RF, Jaffe GJ, Sarraf D, Freund KB, Sadda SR, Staurenghi G, et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: Consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology. 2020;127:616–36.
Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–34.
Wang JJ, Foran S, Smith W, Mitchell P. Risk of age-related macular degeneration in eyes with macular drusen or hyperpigmentation: The Blue Mountains Eye Study cohort. Arch Ophthalmol. 2003;121:658–63.
Teo KYC, Cheong KX, Ong R, Hamzah H, Yanagi Y, Wong TY, et al. Macular neovascularization in eyes with pachydrusen. Sci Rep. 2021;11:7495.
Diniz B, Ribeiro RM, Rodger DC, Maia M, Sadda S. Drusen detection by confocal aperture-modulated infrared scanning laser ophthalmoscopy. Br J Ophthalmol. 2013;97:285–90.
Ooto S, Ellabban AA, Ueda-Arakawa N, Oishi A, Tamura H, Yamashiro K, et al. Reduction of retinal sensitivity in eyes with reticular pseudodrusen. Am J Ophthalmol. 2013;156:1184–91.e2.
van de Ven JP, Smailhodzic D, Boon CJ, Fauser S, Groenewoud JM, Chong NV, et al. Association analysis of genetic and environmental risk factors in the cuticular drusen subtype of age-related macular degeneration. Mol Vis. 2012;18:2271–8.
Cohen SY, Dubois L, Tadayoni R, Delahaye-Mazza C, Debibie C, Quentel G. Prevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularisation. Br J Ophthalmol. 2007;91:354–9.
Kong M, Kim S, Ham DI. Incidence of late age-related macular degeneration in eyes with reticular pseudodrusen. Retina. 2019;39:1945–52.
Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob Health. 2014;2:e106–16.
Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology. 1995;102:1450–60.
Klein R, Klein BEK, Linton KLP. Prevalence of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmology. 2020;127:S122–S32.
Lee J, Byeon SH. Prevalence and clinical characteristics of pachydrusen in polypoidal choroidal vasculopathy: Multimodal image study. Retina. 2019;39:670–8.
Lee JH, Kim JY, Jung BJ, Lee WK. Focal disruptions in ellipsoid zone and interdigitation zone on spectral-domain optical coherence tomography in pachychoroid pigment epitheliopathy. Retina. 2019;39:1562–70.
Keenan TD, Agrón E, Domalpally A, Clemons TE, van Asten F, Wong WT, et al. Progression of geographic atrophy in age-related macular degeneration: AREDS2 Report Number 16. Ophthalmology. 2018;125:1913–28.
Acknowledgements
The authors would like to thank all the patients who participated in this study and Editage (www.editage.co.kr) for English language editing.
Author information
Authors and Affiliations
Contributions
SWN wrote the initial draft of the manuscript. SWN, HN, JMY, MK, and D-IH conceived the concept for this study. SWN manually extracted the original data from selected studies. HN, JMY, MK, and D-IH checked all extracted data. SWN performed the statistical analysis. SWN and D-IH had full access to all data in the study, taking responsibility for data integrity and the accuracy of the data analysis. MK and D-IH were involved in the critical revision of the manuscript. D-IH supervised the study as corresponding authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nam, S.W., Noh, H., Yoon, J.M. et al. Macular lesions associated with age-related macular degeneration in pachydrusen eyes. Eye 38, 691–697 (2024). https://doi.org/10.1038/s41433-023-02752-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02752-0
- Springer Nature Limited