Abstract
Background/Objectives
Studies based on food frequency questionnaires suggest that folate and vitamin B12 intake could protect against hearing loss. We investigated whether erythrocyte folate and serum vitamin B12 levels are independently associated with hearing loss in humans.
Subjects/Methods
Participants in the 2003–2004 US National Health and Nutrition Examination Survey who had data on hearing, folate, and vitamin B12 levels were included. Pure-tone average (PTA) at 0.5, 1.0, 2.0, and 4.0 kHz was computed for each ear. We used weighted logistic regression to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the relation between quartiles of folate and vitamin B12, and hearing loss (present if PTA > 25 dB in either ear and absent if PTA ≤ 25 dB in both ears).
Results
Participants (n = 1149) were 20–69 (mean 42) years old and 16.4% had hearing loss in at least one ear. Our data suggest a U-shaped relationship between folate and hearing loss. Compared to the 1st quartile, the ORs (95% CIs) for hearing loss were 0.87 (0.49–1.53), 0.70 (0.49–1.00), and 1.08 (0.61–1.94) for the 2nd, 3rd, and 4th quartile of erythrocyte folate in analyses adjusted for age, sex, vitamin B12, smoking, alcohol use, body mass index, race/ethnicity, exposure to noise, income, and education. Although we observed inverse associations between vitamin B12 and hearing loss, the associations were not statistically significant (P > 0.05).
Conclusions
Our data show a U-shaped relationship between erythrocyte folate levels and hearing loss, suggesting a need to evaluate whether optimizing blood folate levels could prevent hearing loss.
Similar content being viewed by others
Introduction
According to the 2012 US National Health and Nutrition Examination Survey (NHANES), about 15% of US adults reported having hearing abnormalities, with higher prevalence among men and older individuals [1]. While old age, exposure to excess noise, and infections are known risk factors, emerging data suggests that diet could also play a role. For example, healthier eating has been associated with better hearing [2].
Micronutrient deficiencies or insufficiencies could also play a role in the incidence of hearing loss. For instance, folate (natural form), and vitamin B12 are poorly absorbed in old age, and about 6–10% of elderly people experience vitamin B12 deficiency and about 10% more experience insufficiency [3,4,5]. While direct mechanistic data from hearing studies in humans are lacking, the observed associations for vitamin B12 and folate are biologically plausible. For instance, in mice overexpression of micro RNAs (miRNAs), particularly mir-34a and mir-181a, is associated with reduction in expression of SIRT1, which leads to apoptosis of cochlear hair cells and subsequent hearing loss [6, 7]. Interestingly in vitro or in vivo mouse studies on non-fatty liver disease have shown that low folate/choline diets increase expression of both mir-34a and mir-181a [8], supporting the hypothesis that folate may affect hearing loss through modulation of miRNAs. This finding is also compelling because several genetic studies [9,10,11] on age-related hearing impairment have identified genes (e.g., metabotropic glutamate receptor-7 (GRM7) gene) that contain seed sites for miRNAs that are modulated by diet e.g., GRM7 harbors a highly conserved binding site for mir-34a. Since the GRM7 gene encodes L-glutamate receptor, which binds the excitatory neurotransmitter thought to connect cochlear inner hair cells to afferent type I auditory neurons [12], it could be a target for effects of folate on hearing. Folate may also affect hearing through its effects on mir-34a which reduces expression of the hepatic nuclear factor 4-alpha [13], a transcription factor recently found to be associated with hearing loss and a target of ototoxic medications such as cyclosporine and sildenafil. Since lower levels of folate or vitamin B12 elevate homocysteine, a metabolite thought to impair blood flow to the cochlea, these nutrients may also affect hearing through their effects on homocysteine [14, 15]. While most studies have focused on folate and vitamin B12 deficiency or insufficiency as potential risk factors for hearing loss, excess folate from diet or folic acid from supplements and fortified foods could lead to high levels of circulating unmetabolized folic acid that could lead to neurological problems, altered DNA methylation and hearing loss [16,17,18,19].
While several existing human [20,21,22,23,24] and animal studies [25,26,27] have provided insights into the potential role of folate and vitamin B12 in hearing loss, they have a number of limitations. First, most studies were based on questionnaires on diet and hearing loss and did not include biomarkers of folate or vitamin B12 or more robust measures of hearing loss (e.g., otoacoustic emission tests), attributes that could lead to measurement error. Secondly, most studies that included biomarkers had small sample sizes [22, 28], did not include minorities or individuals in middle ages [22], and were mainly conducted before initiation of fortification of foods with folic acid, which began in 1998 in the US [25, 26]. Studies that include various population subgroups and use more robust measures of nutrient status are needed to further understand whether folate and vitamin B12 are associated with hearing loss in the general population where food fortification with these nutrients has already reached a steady state. As shown in different studies, tissue levels of folate and B12 are good biomarkers of intake [29, 30] and because erythrocytes live for about 120 days, folate measures in these cells are preferred to serum measures [31].
Using NHANES [32], a nationally representative sample, we investigated whether erythrocyte folate and serum vitamin B12 levels are independently associated with hearing loss in a demographically diverse population where the impact of food fortification with folic acid [33, 34] should have achieved its effects.
Methods
Study population
Participants in the current study are men and women who were between 20 and 69 years old during 2003 and 2004 NHANES examinations. In order to participate in the examination, an individual had to be free of pain in the ears and, if applicable, he/she had to remove hearing aids or devices before hearing tests [32]. The data used for this study are de-identified and already available in the public domain (https://wwwn.cdc.gov/Nchs/Nhanes/Search/Nhanes03_04.aspx). Thus, there was no need for approval by the Institutional Review Board.
Assessment of hearing
Measurement of hearing loss in NHANES was done in four steps: (a) a pre-examination questionnaire to determine factors that could affect audiometric testing, (b) an otoscopic examination for physical ear canal and eardrum problems, (c) assessment of middle ear function (tympanometry), and (d) pure-tone air conduction audiometry. Measurement protocols for middle-ear muscle reflexes and pure-tone hearing thresholds in NHANES have been described elsewhere [32, 35] and are described in detail on the NHANES website (https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/AUX_C.htm). Briefly, hearing tests were performed by a trained examiner in a dedicated, sound-isolating room. Hearing threshold testing was conducted on both ears of examinees at 6 frequencies (0.5, 1.0, 2.0, 4.0, 6.0, and 8.0 kHz for each ear) using the modified Hughson–Westlake procedure and invoking the automated testing mode of the audiometer. After excluding individuals with abnormal otoscopic examinations in either ear, we computed PTA at 0.5, 1.0, 2.0, and 4.0 kHz for each ear and participants with PTA > 25 dB in either ear were classified as having hearing loss, while those with PTA ≤ 25 dB in both ears were classified as having normal hearing in this lower frequency range. For secondary analyses, we also computed PTA at 4.0, 6.0, and 8.0 kHz for each ear and participants with PTA > 25 dB in either ear were classified as having hearing loss, while those with PTA ≤ 25 dB in both ears were classified as having normal hearing in the higher frequencies.
Measurement of erythrocyte folate and serum vitamin B12
Serum levels of vitamin B12 were determined using the Bio-Rad Laboratories “Quantaphase II Folate/Vitamin B12” radioassay kit, and erythrocyte folate was measured after 1:11 sample dilution with a solution of 1 g/dL ascorbic acid in water and either incubated for 90 min prior to assay or frozen immediately for later assay [36]. The sample was further diluted 1:2 with a protein diluent (human serum albumin), resulting in a matrix similar to that of the standards and serum samples.
Measurement of covariates
Data on age, sex, smoking, race, household income, education, and noise exposure were obtained from interviewer-administered questionnaires and data on weight, height and blood pressure were directly measured during an examination [37]. Data on diet was obtained from 24-h dietary recalls collected during the 2003/2004 NHANES cycles. From these data, we computed the total 2010 Health Eating Index (HEI 2010) and its components using the “Simple HEI Scoring Algorithm-Per Day” that has been implemented in SAS macros and is available from the National Cancer Institute [38]. The HEI was used as a covariate in the models to account for differences in the overall quality of the participants’ diets. Fasting glucose and creatinine data were obtained by direct measurement from the blood sample collected during the NHANES examination. We used the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation to compute estimated glomerular filtration rate (eGFR) [39].
Statistical analysis
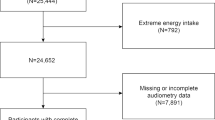
All data management and analyses were done using Statistical Analysis System software version 9.4 (SAS Institute, Cary, NC) that accounts for differences in sampling weights in NHANES. From 1889 participants with audiometry data, we excluded individuals with abnormal or missing data on otoscopic examination (n = 479) and those missing data on covariates (n = 261). In all, 1149 participants had complete analyzable data. We distributed erythrocyte folate and serum vitamin B12 into quartiles and computed descriptive statistics by quartiles of erythrocyte folate. For categorical and continuous variables, we used the SURVEYFREQ and SURVEYMEANS procedures in SAS, respectively, to estimate weighted proportions and means across quartiles of folate. We used P-values from the Wald chi square test (SURVEYFREQ procedure) and ANOVA (SURVEYREG procedure) to test for the significance of the differences in proportions and means across quartiles of folate.
Next we tested whether erythrocyte folate and serum vitamin B12 are independently associated with hearing loss based on the clinical cut-point of having a PTA > 25 dB in any ear. The SURVEYLOGISTIC Procedure in SAS was then used to estimate odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for the relation between quartiles of folate, quartiles of vitamin B12, and hearing loss. This procedure accounted for the two-year sampling weights used in NHANES. Because of the broad effects of folate and vitamin B12 on clinical variables such as blood pressure, some covariates could be potential mediators of the effects of these nutrients. Similarly, other variables such as alcohol may have effects on hearing loss partly via their effects on nutrient intake, absorption and metabolism (e.g., alcohol lowers folate absorption). Thus, we fitted models sequentially with model 1 containing erythrocyte folate (quartiles), serum vitamin B12 (quartiles), sex (men vs. women), and age as a continuous variable. Model 2 additionally adjusted for smoking (never, past, and current), alcohol use (yes or no), body mass index (continuous), race/ethnicity (non-Hispanic white, non-Hispanic black and other), exposure to loud noise or music (yes or no), annual household income (<$20,000, $20,000–54,999 and ≥$55,000) and educational attainment (not completed high school, graduated from high school/GED and college or higher). Model 3 additionally adjusted for the overall dietary pattern using the United States Department of Agriculture’s Health Eating Index (HEI) that has been shown to be inversely associated with hearing loss [2]. Additional analyses adjusted for fasting serum glucose, systolic blood pressure, diastolic blood pressure and eGFR, all as continuous variables, albeit knowing these variables could be mediators in the association between folate and hearing loss.
To test for potential interactions between folate and vitamin B12, we included the main effects and cross-products of these variables in the models. Our a priori cut-point for significance was set at P ≤ 0.05 for main effects and P ≤ 0.10 for interaction effects.
Results
The mean (min, max) age of participants in this study was 42 (20, 69) years. Most participants were women (52.0%), white (71.9%), and never smokers (50.1%). Median (25th, 75th percentile) erythrocyte folate and serum vitamin B12 levels were 584 (469, 734) nmol/L and 335 (263, 435) pmol/L, respectively. Of the 1149 participants, 189 participants (16.5%) had hearing loss in the lower frequencies and 409/1149 participants (35.6%) had hearing loss in at least one of the higher frequencies (4.0, 6.0, and 8.0 kHz).
Table 1 shows other participant characteristics by quartiles of erythrocyte folate. Most notable are the high proportions of current smokers, those exposed to loud noise/music and people with lower incomes and education in the lowest two quartiles of folate. As expected, erythrocyte folate levels were significantly higher for participants with higher scores for the overall HEI and those whose scores were high for the intake of fruits and vegetables, variables known to contribute to folate intake. Except for eGFR that decreased with increase in erythrocyte folate, other clinical variables did not vary dramatically by folate status.
Analyses of folate, vitamin B12, and hearing loss
To test for independent associations, folate and vitamin B12 were modeled simultaneously while accounting for covariates and sampling weights. There was no evidence for interaction between folate and vitamin B12. As shown in Table 2, our data suggest a U-shaped relationship between erythrocyte folate and hearing loss. In the age-adjusted, sex-adjusted, and B12-adjusted model, and using the lowest quartile as the referent, the ORs (95% CIs) for hearing loss were 0.94 (0.52–1.72), 0.66 (0.47–0.93), and 0.98 (0.62–1.56) for the 2nd, 3rd, and 4th quartile of erythrocyte folate. Additional adjustment for smoking, alcohol use, body mass index, race/ethnicity, exposure to noise, income and education strengthened the association in the lower quartiles but attenuated it in the upper quartile. The ORs (95% CIs) became 0.85 (0.49–1.49), 0.68 (0.47–0.99), and 1.05 (0.57–1.94) for the 2nd, 3rd, and 4th quartile of erythrocyte folate, respectively. Further adjustment for the HEI or serum glucose, blood pressure, and kidney function, variables that could potentially be affected by folate and/or vitamin B12, attenuated the association further but the U-shaped relationship remained evident. In this fully-adjusted model, the ORs (95% CIs) became 0.87 (0.49–1.53), 0.70 (0.49–1.00), and 1.08 (0.61–1.94) for the 2nd, 3rd, and 4th quartile of erythrocyte folate, respectively.
Although we observed inverse associations for vitamin B12 and hearing loss before and after adjustment for covariates, the associations were not statistically significant in any of the quartiles. In the fully-adjusted model and using the lowest quartile as the referent, the ORs (95% CIs) for hearing loss were 0.73 (0.48–1.11), 0.80 (0.49–1.31), and 0.70 (0.35–1.38) for the 2nd, 3rd, and 4th quartile of serum vitamin B12.
In secondary analyses with PTA computed for frequencies of 4.0, 6.0, and 8.0 kHz, neither erythrocyte folate nor serum vitamin B12 was significantly associated with hearing loss.
Discussion
Using a large dataset from the 2003–2004 NHANES we have shown that individuals with moderate (582–741 nmol/L) erythrocyte folate levels have 32% lower odds of having hearing loss in the lower frequencies (0.5–4.0 kHz) in analyses that accounted for covariates such as age, sex, and body mass index, income, education, and other factors associated with hearing loss. Serum vitamin B12 was not significantly associated with hearing loss. Neither folate nor vitamin B12 was significantly associated with hearing loss in the higher frequencies (4.0–8.0 kHz).
Our finding of a null association between serum vitamin B12 and hearing loss in the analyses adjusted for erythrocyte folate levels are consistent with those from previous studies in which vitamin B12 intake [20] or serum levels [14, 28] were not significantly associated with hearing loss but contrast a previous study [24] in which a significant inverse association between vitamin B12 intake and hearing levels was reported. While the reasons for the inconsistent results for vitamin B12 and hearing loss are not clear, it may be related to lack of adjustment for confounding by folate levels in previous studies or the relatively high levels of folate and vitamin B12 in our study sample. For instance, only 6/1149 (0.85%) participants in our sample had serum vitamin B12 levels below 103 pmol/L and only 60/1149 were below 317 nmol/L of erythrocyte folate, the lower limits for the normal range for serum vitamin B12 and erythrocyte folate, respectively. It is also possible that the effects of vitamin B12 on hearing and other auditory functions are minimal and difficult to detect in typical population studies with several confounders to adjust for. However, even in a small clinical trial among patients with tinnitus, vitamin B12 did not improve hearing [40].
Our fully-adjusted models confirm an inverse association between folate intake and hearing loss that has been observed in previous studies based on food frequency questionnaires [20, 21] and the inverse association between folic acid (supplemental form) and hearing loss that was observed in a clinical trial in the Netherlands [23]. The inverse association between erythrocyte folate and hearing loss observed in our study is also consistent with results from a study of erythrocyte folate and hearing loss in 55 older white adults in the US [22] and results from a serum folate study among 126 adult Nigerians [28] and among 2956 Australians [14]. These results are also consistent with protective effects of folate on hearing and cochlear vascularization that have been observed in animal studies [15, 25, 26].
Our finding of an inverse relationship in the general US population is important because it shows that the benefits of folate on hearing that have been observed in elderly populations are also evident in relatively young populations (20–69 years old) and racially/ethnically diverse populations such as those constituting NHANES sample. If confirmed in a clinical trial that includes both younger and older people, the association between folate and hearing loss may suggest that adequate folate intake could be beneficial for hearing health in both younger people and older populations who tend to have inadequate folate levels. We observed a non-significant increase in odds of hearing loss in the top-most quartile of folate (quartile mean = 985 nmol/L). The reason for the increase in the odds of hearing loss in the topmost quartile is unclear but could be related to neurologic damage that has been associated with excess folic acid, a likely scenario in populations with increased use of folic acid supplements and fortified foods [16, 17]. Excess folic acid from chronic use of supplements or food fortification leads to excess levels in the erythrocytes [17] and could lead to large levels of unmetabolized folic acid in the body, which has been associated with altered DNA methylation and reduced immune function [18, 19]. Although side effects of acute overconsumption of folic acid are considered reversible, effects from long-term overconsumption are unknown and may be worse in individuals with low vitamin B12 levels which has also been associated with hearing loss [41]. If replicated, this finding of increased odds of hearing loss at higher levels of folate may suggest that supplementation with folic acid should only be for population subgroups at increased risk of folate deficiency or insufficiency.
Because the 2003–2004 NHANES data were collected 5–6 years after initiation of food fortification with folic acid in 1998, our study suggests that if the effect of folate on hearing loss is confirmed, additional approaches such as targeted supplementation with folic acid, could still be used to improve folate status and potentially for preventing hearing loss, especially in the elderly and minority populations where folate intake or body folate status may be low [42].
Although previous studies have examined the role of folate and vitamin B12 independently and found inverse associations, it has been unclear as to whether both are needed to prevent hearing loss. Our study extends knowledge from previous studies by suggesting that when folate status is accounted for, vitamin B12 may not be important for hearing in populations with vitamin B12 levels as high as those observed in the majority of people in the US. Future studies in the US that evaluate the role of folate in prevention of hearing loss could focus on folic acid supplementation alone rather than both folic acid and vitamin B12.
This study has limitations. One limitation of the present study is the narrow age range (20–69 years) of the participant sample which precludes analyses that are adequately powered to determine whether folate or serum vitamin B12 have effects on presbycusis, a condition more common in older age groups. Another limitation is the use of an otoscopic examination to exclude participants with middle ear disease. We excluded all participants with abnormal otologic examination, in order to limit the study to those without middle ear disease. The sensitivity and specificity of otoscopic examination in NHANES has not been analyzed, but in other settings has been found to be variable [43]. Our study is based on secondary cross-sectional data from a nationally representative sample in the public domain and lacks some details such as information on hereditary forms of hearing loss, trauma-related hearing loss or comprehensive data on the amount and timing of noise exposure or the type, dose and timing of use of medications associated with hearing loss. The cross-sectional nature of the study precludes assessment for temporal relationships and makes it impossible to exclude the potential for reverse causality. These limitations call for caution in the interpretation of our findings.
Nonetheless, the consistency of the current findings with those from previous studies using food frequency questionnaires or biomarkers in smaller samples in multiple populations, add to the body of knowledge supporting an inverse relationship between folate and hearing loss. Our study shows that moderate erythrocyte folate levels seen in our 3rd quartile (582–741 nmol/L) are independently associated with ~30% lower odds of having hearing loss even in relatively young US adults. This is unique because the apparent protection against hearing loss observed at moderate levels of erythrocyte folate seems to affect lower hearing frequencies in relatively young adults; a finding consistent with that from a folic acid supplementation study in the Netherlands [23]. Future investigations are needed to further evaluate these associations in a larger sample of older people who are at a greater risk for folate and vitamin B12 deficiencies and at increased risk for age-related hearing loss. Moreover, these studies should determine whether supplementation with folic acid in certain population subgroups (e.g., the elderly or minority subpopulations that tend to have inadequate folate and vitamin B12 levels) could prevent or reverse hearing loss. Future prospective studies that investigate hearing loss in subgroups that use medications (e.g., methotrexate, cisplatin, carbamazepine, or phenytoin) that interfere with folate metabolism are needed.
References
Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: National Health Interview Survey, 2012. National Center for Health Statistics, Vital and Health Statistics 2014, 10: http://www.cdc.gov/nchs/data/series/sr_10/sr10_260.pdf.
Spankovich C, Le Prell CG. Healthy diets, healthy hearing: National Health and Nutrition Examination Survey, 1999-2002. Int J Audiol. 2013;52:369–76. https://doi.org/10.3109/14992027.2013.780133.
Ray JG, Cole DE, Boss SC. An Ontario-wide study of vitamin B12, serum folate, and red cell folate levels in relation to plasma homocysteine: is a preventable public health issue on the rise? Clin Biochem. 2000;33:337–43.
Allen LH. How common is vitamin B-12 deficiency? Am J Clin Nutr. 2009;89:693S–6S. https://doi.org/10.3945/ajcn.2008.26947A
de Meer K, Smulders YM, Dainty JR, Smith DE, Kok RM, Stehouwer CD, et al. [6S]5-methyltetrahydrofolate or folic acid supplementation and absorption and initial elimination of folate in young and middle-aged adults. Eur J Clin Nutr. 2005;59:1409–16. https://doi.org/10.1038/sj.ejcn.1602254.
Zhang Q, Liu H, McGee J, Walsh EJ, Soukup GA, He DZ. Identifying microRNAs involved in degeneration of the organ of corti during age-related hearing loss. PLoS One. 2013;8:e62786 https://doi.org/10.1371/journal.pone.0062786.
Xiong H, Pang J, Yang H, Dai M, Liu Y, Ou Y, et al. Activation of miR-34a/SIRT1/p53 signaling contributes to cochlear hair cell apoptosis: implications for age-related hearing loss. Neurobiol Aging. 2015;36:1692–701. https://doi.org/10.1016/j.neurobiolaging.2014.12.034.
Tryndyak VP, Latendresse JR, Montgomery B, Ross SA, Beland FA, Rusyn I, et al. Plasma microRNAs are sensitive indicators of inter-strain differences in the severity of liver injury induced in mice by a choline- and folate-deficient diet. Toxicol Appl Pharmacol. 2012;262:52–9. https://doi.org/10.1016/j.taap.2012.04.018.
Luo H, Yang T, Jin X, Pang X, Li J, Chai Y. et al. Association of GRM7 variants with different phenotype patterns of age-related hearing impairment in an elderly male Han Chinese population. PLoS One. 2013;8:e77153 https://doi.org/10.1371/journal.pone.0077153.
Van Laer L, Huyghe JR, Hannula S, Van Eyken E, Stephan DA, Maki-Torkko E, et al. A genome-wide association study for age-related hearing impairment in the Saami. Eur J Human Genet. 2010;18:685–93. https://doi.org/10.1038/ejhg.2009.234.
Newman DL, Fisher LM, Ohmen J, Parody R, Fong CT, Frisina ST, et al. GRM7 variants associated with age-related hearing loss based on auditory perception. Hear Res. 2012;294:125–32. https://doi.org/10.1016/j.heares.2012.08.016.
Oestreicher E, Wolfgang A, Felix D. Neurotransmission of the cochlear inner hair cell synapse--implications for inner ear therapy. Adv Otorhinolaryngol. 2002;59:131–9.
Xu Y, Zalzala M, Xu J, Li Y, Yin L, Zhang Y. A metabolic stress-inducible miR-34a-HNF4alpha pathway regulates lipid and lipoprotein metabolism. Nat Commun. 2015;6:7466 https://doi.org/10.1038/ncomms8466.
Gopinath B, Flood VM, Rochtchina E, McMahon CM, Mitchell P. Serum homocysteine and folate concentrations are associated with prevalent age-related hearing loss. J Nutr. 2010;140:1469–74.https://doi.org/10.3945/jn.110.122010.
Partearroyo T, Vallecillo N, Pajares MA, Varela-Moreiras G, Varela-Nieto I. Cochlear homocysteine metabolism at the crossroad of nutrition and sensorineural hearing loss. Front Mol Neurosci. 2017;10:107 https://doi.org/10.3389/fnmol.2017.00107
Devnath GP, Kumaran S, Rajiv R, Shaha KK, Nagaraj A.. Fatal folic acid toxicity in humans. J Forensic Sci. 2017;62:1668–70. https://doi.org/10.1111/1556-4029.13489.
Mudryj AN, de Groh M, Aukema HM, Yu N. Folate intakes from diet and supplements may place certain Canadians at risk for folic acid toxicity. Br J Nutr. 2016;116:1236–45. https://doi.org/10.1017/S000711451600307X
Plumptre L, Masih SP, Ly A, Aufreiter S, Sohn KJ, Croxford R, et al. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am J Clin Nutr. 2015;102:848–57. https://doi.org/10.3945/ajcn.115.110783
Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. 2006;136:189–94.
Shargorodsky J, Curhan SG, Eavey R, Curhan GC. A prospective study of vitamin intake and the risk of hearing loss in men. Otolaryngol Head Neck Surg. 2010;142:231–6. https://doi.org/10.1016/j.otohns.2009.10.049.
Curhan SG, Stankovic KM, Eavey RD, Wang M, Stampfer MJ, Curhan GC. Carotenoids, vitamin A, vitamin C, vitamin E, and folate and risk of self-reported hearing loss in women. Am J Clin Nutr. 2015;102:1167–75. https://doi.org/10.3945/ajcn.115.109314.
Houston DK, Johnson MA, Nozza RJ, Gunter EW, Shea KJ, Cutler GM, et al. Age-related hearing loss, vitamin B-12, and folate in elderly women. Am J Clin Nutr. 1999;69:564–71.
Durga J, Verhoef P, Anteunis LJ, Schouten E, Kok FJ. Effects of folic acid supplementation on hearing in older adults: a randomized, controlled trial. Ann Intern Med. 2007;146:1–9.
Peneau S, Jeandel C, Dejardin P, Andreeva VA, Hercberg S, Galan P, et al. Intake of specific nutrients and foods and hearing level measured 13 years later. Br J Nutr. 2013;109:2079–88. https://doi.org/10.1017/S0007114512004291.
Martinez-Vega R, Garrido F, Partearroyo T, Cediel R, Zeisel SH, Martinez-Alvarez C, et al. Folic acid deficiency induces premature hearing loss through mechanisms involving cochlear oxidative stress and impairment of homocysteine metabolism. FASEB J. 2015;29:418–32. https://doi.org/10.1096/fj.14-259283.
Kundu S, Munjal C, Tyagi N, Sen U, Tyagi AC, Tyagi SC. Folic acid improves inner ear vascularization in hyperhomocysteinemic mice. Hear Res. 2012;284:42–51. https://doi.org/10.1016/j.heares.2011.12.006.
Martinez-Vega R, Murillo-Cuesta S, Partearroyo T, Varela-Moreiras G, Varela-Nieto I, Pajares MA. Long-term dietary folate deficiency accelerates progressive hearing loss on CBA/Ca mice. Front Aging Neurosci. 2016;8:209 https://doi.org/10.3389/fnagi.2016.00209
Lasisi AO, Fehintola FA, Yusuf OB. Age-related hearing loss, vitamin B12, and folate in the elderly. Otolaryngol Head Neck Surg. 2010;143:826–30. https://doi.org/10.1016/j.otohns.2010.08.031.
Verkleij-Hagoort AC, de Vries JH, Stegers MP, Lindemans J, Ursem NT, Steegers-Theunissen RP. Validation of the assessment of folate and vitamin B12 intake in women of reproductive age: the method of triads. Eur J Clin Nutr. 2007;61:610–5. https://doi.org/10.1038/sj.ejcn.1602581.
Fraser GE, Jaceldo-Siegl K, Henning SM, Fan J, Knutsen SF, Haddad EH, et al. Biomarkers of dietary intake are correlated with corresponding measures from repeated dietary recalls and food-frequency questionnaires in the adventist health study-2. J Nutr. 2016;146:586–94. https://doi.org/10.3945/jn.115.225508
WHO. Serum and red blood cell folate concentrations for assessing folate status in populations. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2012. http://apps.who.int/iris/bitstream/10665/75584/1/WHO_NMH_NHD_EPG_12.1_eng.pdf.
CDC. National Health and Nutrition Examination Survey, 2003 - 2004 Data Documentation, Codebook, and Frequencies. Centers for Disease Control and Prevention 2003; http://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/AUX_C.htm.
Odewole OA, Williamson RS, Zakai NA, Berry RJ, Judd SE, Qi YP, et al. Near-elimination of folate-deficiency anemia by mandatory folic acid fortification in older US adults: Reasons for Geographic and Racial Differences in Stroke study 2003-2007. Am J Clin Nutr. 2013;98:1042–7. https://doi.org/10.3945/ajcn.113.059683.
Ganji V, Kafai MR. Hemoglobin and hematocrit values are higher and prevalence of anemia is lower in the post-folic acid fortification period than in the pre-folic acid fortification period in US adults. Am J Clin Nutr. 2009;89:363–71. https://doi.org/10.3945/ajcn.2008.26287.
Flamme GA, Deiters K, Needham T. Distributions of pure-tone hearing threshold levels among adolescents and adults in the United States by gender, ethnicity, and age: Results from the US National Health and Nutrition Examination Survey. Int J Audiol. 2011;50(S1):S11–20. https://doi.org/10.3109/14992027.2010.540582
CDC. National Health and Nutrition Examination Survey. NHANES 2003-2004 Laboratory Data. Centers for Disease Control and Prevention 2003; http://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Laboratory&CycleBeginYear=2003.
CDC. National Health and Nutrition Examination Survey. NHANES 2003-2004 Questionnaire Data. Centers for Disease Control and Prevention 2003; http://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx?Component=Questionnaire&CycleBeginYear=2003.
NCI. HEI Tools for Researchers. Division of Cancer Control and Population Sciences, The National Cancer Institute, National Institutes of Health, USA. 2010; https://epi.grants.cancer.gov/hei/tools.html#ind (Accessed 11 May 2017).
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Berkiten G, Yildirim G, Topaloglu I, Ugras H. Vitamin B12 levels in patients with tinnitus and effectiveness of vitamin B12 treatment on hearing threshold and tinnitus. B-Ent. 2013;9:111–6.
Shah T, Joshi K, Mishra S, Otiv S, Kumbar V. Molecular and cellular effects of vitamin B12 forms on human trophoblast cells in presence of excessive folate. Biomed Pharmacother. 2016;84:526–34. https://doi.org/10.1016/j.biopha.2016.09.071
McDowell MA, Lacher DA, Pfeiffer CM, Mulinare J, Picciano MF, Rader JI, et al. Blood folate levels: The latest NHANES results. NCHS data briefs, no. 6, Hyattsville, MD: National Center for Health Statistics. Centers for Disease Control and Prevention 2008; http://www.cdc.gov/nchs/data/databriefs/db06.htm.
Pelton SI. Otoscopy for the diagnosis of otitis media. Pediatr Infect Dis J. 1998;17:540–3. Discussion 580.
Acknowledgements
The NHANES data used in this study were collected with funding from the US government. We acknowledge intramural support from the Bill Wilkerson Center for Otolaryngology and Communication Disorders at Vanderbilt University School of Medicine (EKK). Salary support for DOF came from grants K23DC013559 and L30DC012687 from the National Institute for Deafness and Communication Disorders of the National Institute of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RFL reports consulting for Cochlear Corp. and Advanced Bionics Corp. in the last two years. The remaining authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kabagambe, E.K., Lipworth, L., Labadie, R.F. et al. Erythrocyte folate, serum vitamin B12, and hearing loss in the 2003-2004 National Health And Nutrition Examination Survey (NHANES). Eur J Clin Nutr 72, 720–727 (2018). https://doi.org/10.1038/s41430-018-0101-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-018-0101-6
- Springer Nature Limited
This article is cited by
-
Association of vitamins with hearing loss, vision disorder and sleep problem in the US general population
Environmental Science and Pollution Research (2023)