Abstract
Background According to the Children's Dental Health Survey 2013, around one in ten children in Wales, Northern Ireland and England will have sustained dental trauma to a permanent incisor by the of age 15. Management of an exposed pulp in an immature permanent incisor is often urgent and has an impact on the long-term outcome of the tooth; therefore, it is essential that general dental practitioners feel confident in managing such a scenario to achieve an optimal outcome. This paper discusses the indications, technique, materials and outcomes.
Aims This article aims to review the literature, which discusses various treatment modalities and materials for pulpal therapy and root canal treatment in the immature permanent tooth.
Method Electronic searches were limited to English language, human studies, published within the past five years and the medical subject heading terms used were: direct pulp capping, apexogenesis, Cvek pulpotomy, full pulpotomy/pulpectomy, partial pulpotomy, apexification, non-vital pulp therapy and mineral trioxide aggregate apexification. Older, seminal articles identified through the references sections have also been included.
Conclusion A number of options are available for the management of immature permanent teeth that have suffered an insult such as caries or trauma. This paper reviews the various methods of pulpal treatment, preservation therapy and root canal treatment options depending on the extent of the damage.
Key points
-
Highlights the management options for pulpal therapy and root canal treatment in immature permanent teeth.
-
Discusses the techniques used for carrying out pulp therapy in immature permanent teeth.
-
Discusses the literature and guidance regarding pulpal therapy in immature permanent teeth.
Similar content being viewed by others
Introduction
An immature permanent tooth can be defined as a newly erupted permanent tooth with incomplete root apex formation. Once erupted, it can take approximately three years for the root to develop and for apical closure.1 Root development involves interactions between the dental follicle, the epithelial root sheath of Hertwig and the dental papilla. Hertwig's epithelial root sheath is responsible for the future shape of the roots.2 Disturbances to the neurovascular system in the pulp can result in alterations in the pulp such as pulp canal obliteration or pulp necrosis.3 This can affect the blood supply to the root sheath and therefore disrupt or arrest root growth. Root elongation and radicular dentinogenesis no longer occur, resulting in roots with thin dentinal walls and wide, open apices. It is desirable that continued root formation, maturation and apexogenesis occurs in order to ensure longevity of the tooth.4
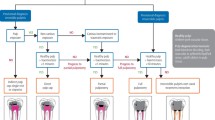
Immature teeth can become pulpally involved due to trauma, caries or iatrogenic causes. Dental trauma is a common reason for children with immature permanent teeth to require root canal treatment or pulpal therapy. It is thought to affect approximately 20% of children and adolescents in the permanent dentition.5 Furthermore, around one-third of UK 12-year-olds have been found to have 'obvious decay experience' in the 2013 Children's Dental Health Survey.6 Preservation is desirable in order to ensure long-term retention of the tooth and the type of pulp therapy indicated depends on whether the pulp is vital or non-vital. This clinical diagnosis can be obtained from a detailed history, examination and special tests.6 Pulpal diagnoses include reversible pulpitis, symptomatic irreversible pulpitis, asymptomatic irreversible pulpitis and pulp necrosis.7
Once the diagnosis is made, there are several options for vital teeth, such as pulp capping and pulpotomies, and non-vital teeth, such as apical barrier formation, regenerative endodontic procedures and extraction.
Aims
This paper aims to provide a review of the current techniques used for pulpal therapy and root canal treatment of teeth with immature apices.
Method
A literature search was carried out to identify relevant literature. Electronic searches were limited to English language, human studies, published within the past five years and the medical subject heading (MESH) terms used were: direct pulp capping (DPC), apexogenesis, Cvek pulpotomy, full pulpotomy/pulpectomy, partial pulpotomy, apexification, non-vital pulp therapy, mineral trioxide aggregate (MTA) apexification. Older, seminal articles identified through the references sections have also been included.
Vital pulp therapy
Vital pulp therapy is the appropriate treatment option for teeth which demonstrate reversible pulpitis or normal pulpal signs and symptoms, with or without exposure of the pulp due to caries or trauma, or where irreversible inflammation is limited to the most coronal portion of the pulp.8 This treatment aims to preserve pulpal vitality while encouraging apical growth completion and closure. Vital pulp therapies include indirect pulp capping, direct pulp capping, partial (superficial) pulpotomy and complete (cervical) pulpotomy.9
Indirect pulp capping
Indirect pulp capping (IPC) is a technique recommended for restoring teeth with caries in close relation to the pulp, without signs of pulpal degeneration.10 This technique involves placing a layer of cavity liner or sealer over the base of a cavity and leaving residual caries which is close to the pulp, with the aim of encouraging tertiary dentine formation and preventing pulpal exposure.11 An ideal IPC agent should be radiopaque, biocompatible, insoluble, dimensionally stable, non-toxic and non-carcinogenic.12 The material should also be able to promote dentinal healing and inhibit bacterial spread to encourage the tooth to recover favourably.13 The liner or pulp capping agent must be followed by a well-sealed restoration to minimise bacterial leakage from the dentine-restoration interface.8 Historically, calcium hydroxide [Ca(OH)2] pulp capping materials have been widely used and studied; however, some unfavourable effects of Ca(OH)2 have been identified, such as gradual disintegration of the material over a longer period of time.14 More recently, MTA has been suggested to have superior properties when compared to Ca(OH)2 when used in vital pulp therapy.12 MTA is a material composed of tricalcium aluminate, tricalcium silicate, dicalcium silicate, bismuth oxide and gypsum. Glass ionomer cements (GIC) can also be used for indirect pulp capping.
A randomised control trial examined the effectiveness of MTA, Ca(OH)2 (setting) and GIC as IPC materials when used in children aged 7-12 years.15 They concluded that all three materials were suitable for IPC; however, MTA had a 100% success rate, compared to 93.5% with Ca(OH)2 and 97% with GIC (type VII) when reviewed at 12 months clinically and radiographically.15 The success rate was measured using the American Association of Paediatric Dentistry (AAPD) criteria for success of IPC in primary teeth. The tooth should maintain vitality, have absence of pain and swelling, should not harm the permanent successor and should not have radiographic evidence of pathologic changes. The affected dentine should be completely sealed from the oral environment by the restoration.8 However, this study was only conducted over a one-year period and the results may not be representative of success rates of these materials over a longer timeframe. Furthermore, the success criteria is specific to primary teeth and may not be directly applicable to permanent teeth.
One literature review found that advantages of Ca(OH)2 include its low cost compared to other materials, low toxicity and flexible consistency.16 However, it has a high sensitivity to moisture and a lack of adhesion to dentine or bonding systems, which can lead to microleakage and sensitivity post treatment.16 It is also widely accepted that Ca(OH)2 can weaken the tooth over time and increase fracture risk. Another disadvantage of Ca(OH)2 is the formation of dentinal bridges which can be thin and irregular, causing tunnels which can increase the risk of infection; however, this can be more of an issue with direct rather than indirect pulp capping.16,17
MTA is sometimes favoured in root canal treatment for closure of perforations and to promptly create an apical seal.16 White MTA, while non-discolouring, is not as strong as grey MTA, which has ferric oxide added for greater mechanical strength.16 The setting time for MTA is approximately six hours and it must be in contact with moisture during this time. The patient will most likely need to return for their final restoration. MTA has demonstrated formation of better dentinal bridges compared to Ca(OH)2, with a more regular structure, resulting in less pulpal inflammation and it also has a better marginal seal.16,18 Biodentine (Septodont) was also found to have similar properties to MTA, including excellent marginal seal, high dimensional stability and strength. Furthermore, MTA is not suitable for indirect pulp caps in permanent teeth due to the potential to cause discolouration and this highlights the advantages of Biodentine in such applications.
Direct pulp capping
Caries removal and dental trauma can sometimes result in an inevitable pulpal exposure. Direct pulp capping (DPC) may be suitable where there are no signs of irreversible pulpitis and can help to encourage apexogenesis and pulpal recovery, without the need for conventional root canal treatment.12 A pulp may be considered irreversibly inflamed if haemostasis cannot be achieved after five minutes of attempting to control bleeding with a cotton wool pellet soaked in sodium hypochlorite (NaOCl) and using continuous pressure.13,19 However, if considering pulpal haemostasis in mature permanent teeth, the decision tree by Edwards et al. (2021) is a useful source for review.20 Different concentrations of NaOCl have been studied for this purpose, ranging from 1.25-10%.13 It has also been suggested that the use of NaOCl of up to 6% concentration may be the best for achieving haemorrhage control.13,17 As previously discussed, there are limitations to each pulp capping material used.
The European Society of Endodontology (ESE) have made the following recommendations when performing DPC:19
-
1.
Dental dam isolation and complete caries removal under magnification. Pulpal exposure before complete caries removal may lead to pulpal contamination with carious dentine. Consider partial pulpotomy if this occurs to reduce contamination risk
-
2.
Haemostasis achieved with either NaOCl (0.5-5%) or chlorhexidine (0.2-2%) for disinfection, soaked in cotton wool pellets to achieve haemostasis. If haemostasis cannot be achieved after five minutes, then consider partial pulpotomy or pulpectomy
-
3.
Hydraulic calcium silicate cement can be placed directly onto the pulp. It is recommended that the tooth is definitively restored on the day with a well-sealed restoration to prevent further microleakage
-
4.
Clinical review should occur 6 and 12 months postoperatively. Further yearly radiographs can be considered if there are any concerns regarding healing. Treatment can be considered to be successful where patients demonstrate positive responses to sensibility testing, an absence of pain and no radiological signs of apical periodontitis or internal resorption. Incomplete root apices in children should show continued root development.
Partial pulpotomy
A partial pulpotomy is a treatment option in cases where there is pulpal inflammation in the coronal section of the pulp following trauma. Only a portion of the coronal pulp is removed to a depth which is judged to be clinically appropriate by removing only the irreversibly inflamed pulpal tissue; this differs to a full pulpotomy, where the whole coronal portion of pulp is removed.21 It involves removal of 1-3 mm of inflamed coronal pulpal tissue.21,22 There is some disagreement around the accepted length of time between occurrence of dental trauma and completion of a partial pulpotomy.21,22 However, it is generally agreed that the treatment should be carried out as soon as possible.
Cvek treated patients between one hour and 90 days post-trauma and although the success rate reduced with length of time until treatment, there was still a high success rate with delayed presentation.23 In this study, 60 teeth were assessed and the overall success rate was 96.7%.23Another study found a success rate ranging from 97.5-100% for partial pulpotomies.24 This suggests that even if a patient presents late with a traumatic pulpal exposure, a partial pulpotomy should still be considered; a notion which is supported by other authors.25,26 However, if this approach were adopted, it would of course be essential to ensure that the patient and parent were fully informed of the potential outcomes and that detailed records kept, with rationale for treatment decisions. The patient would also require close monitoring and follow-up, so that if further invasive treatment is required, this can be actioned as soon as possible. A partial pulpotomy can be selected for teeth with open or closed apices, although there is some evidence to suggest that teeth with open apices have a better prognosis; however, further research in this area is needed.22,25
The 'safest' interval between occurrence of dental trauma with a complicated crown fracture and partial pulpotomy is nine days, with success being most likely within this time period, based on limited evidence.23,25 However, the authors must stress that in the general dental practice setting, any dental trauma should, of course, be treated as an emergency and the patient treated as soon as possible.
The steps of a partial pulpotomy are as follows (Fig. 1):
-
Isolation with dental dam, removal of 1-3 mm inflamed pulpal tissue with a sharp excavator, irrigation of area with sodium hypochlorite or chlorhexidine8,19
-
Haemostasis by applying continued pressure with a cotton pellet alone,22 or a cotton pellet soaked in sodium hypochlorite or chlorhexidine.19,25 If haemostasis cannot be achieved, continue to remove more pulpal tissue until haemostasis is achieved (coronal pulpotomy)22
-
Placement of dressing directly over the non-inflamed pulpal tissue. This can be Ca(OH)2, MTA or a modern calcium silicate cement (CSC), for example Biodentine.18,22,27 A GIC layer can then be placed, followed by a well-sealed restoration, or, if a modern CSC is used, there is no need for a GIC layer.
The AAPD recommend direct placement of Ca(OH)2 or MTA over the exposed pulp, followed by a resin-modified glass ionomer restoration and a well-sealed coronal restoration.8 Ca(OH)2 has good long-term outcomes but MTA has been demonstrated to create a more predictable pulp-dentine bridge and reduced pulpal inflammation.28,29 However, modern calcium silicate cements are often favoured now, owing to their faster setting time, pre-encapsulation, lower cost, increased biocompatibility and the fact that they can be used as direct restorations without the need for a GIC layer.8 The ESE recommend the use of hydraulic calcium silicate material directly over the exposed pulp in pulp capping, partial pulpotomy or full pulpotomy before placement of a definitive restoration.19 Calcium-enriched mixture, platelet-rich fibrin (PRF) (which is autologous and biocompatible) and triple antibiotic paste have also been recommended for use in the treatment of pulpotomy in immature permanent teeth.30
Full pulpotomy
A full pulpotomy can be defined as 'complete removal of the coronal pulp and application of a biomaterial directly onto the pulp tissue at the level of the root canal orifice(s), before placement of a permanent restoration'.19 Figure 2 illustrates the steps involved in carrying out a full pulpotomy. If haemostasis cannot be achieved after five minutes using the partial pulpotomy technique, then it may be appropriate to remove further irreversibly inflamed tissue, leading to a full coronal pulpotomy.19 Additionally, if clinicians are not using magnification, then it may be more feasible to complete a full pulpotomy rather than a partial pulpotomy.19 However, the partial pulpotomy technique has been shown to have a more favourable outcome as it preserves cell-rich coronal pulp tissue that is more likely to facilitate healing than radicular pulp.31,32 Therefore, clinicians should endeavour to achieve direct visualisation and haemorrhage control. If haemostasis cannot be achieved with a pulpotomy, then a full pulpectomy (complete pulpal tissue removal) and conventional root canal treatment is recommended.19
The British Endodontic Society recommend that where there is a pulpal exposure, a calcium silicate-based material is the superior and favoured material for placement over the exposed pulp in a full pulpotomy, followed by a conventional restoration.19 They recommend the monitoring of teeth following vital pulp therapy at a 6- and 12-monthly interval in the first year and then every year for four years at the clinician's discretion if needed.19 During this time, the teeth should demonstrate normal pulpal responses but this may be affected by patient age and type of original injury sustained to the tooth. There should be an absence of pain and swelling and immature teeth at the time of injury should show signs of continued root development.19 It is worth bearing in mind that teeth which have had coronal pulpotomies may not demonstrate normal pulpal responses following treatment due to the complete removal of the coronal portion of the pulp.
Non-vital pulp therapy
Non-vital pulp therapy is the suitable treatment pathway for teeth exhibiting signs and symptoms of irreversible pulpitis where it is assumed more than the coronal portion of the pulp is inflamed or there is pulpal necrosis.8 These include, but are not limited to, unprovoked throbbing, aching pain that lingers, sharp pain on thermal stimulus (usually heat) and lingering pain.33 These teeth will give negative responses to pulpal sensibility tests. The aims of non-vital pulp therapies in teeth with immature apices are to induce or form an apical barrier to allow a permanent root filling material to be placed and to allow for revitalisation and continued root formation by regenerative endodontic procedures.
If opting for obturation of the full root with a biomaterial, it is important to have a thorough understanding of the long-term effects this may have on the fracture resistance of the tooth, compared to conventional obturation with gutta-percha, for example. There is some evidence to suggest that over time, even using MTA or Biodentine may reduce fracture resistance in a tooth when used for complete obturation, but further research into this area is needed.18 There is thought to be a link between the level of calcium release of the restorative materials over time and the changes in pH this can cause, in relation to the reduction in fracture resistance.18
Apical barrier formation
Apical barrier formation can be carried out by either apexification or apical plug placement. The American Association of Endodontists (AAE) defines apexification as a method of inducing a calcified barrier in a root with an open apex.34 The purpose of apical barrier formation is to prevent bacterial invasion and allow for a root filling material and permanent restoration to be placed. This barrier also reduces the amount of root filling material that is in contact with the periapical tissues, therefore minimising the risk of sealer extrusion into the surrounding tissue fluid. In this environment, the sealer dissolves and can allow leakage of the tissue fluid into the root canal system, which can supply nutrients to any harbouring bacteria.35 Ca(OH)2, MTA and Biodentine are materials that are currently and widely used for apical barrier formation.36
The technique of using Ca(OH)2 for apexification involves (Fig. 3):
-
1.
Isolation with dental dam and extirpation of the pulp tissue, followed by chemo-mechanical debridement of the root canal system with NaOCl
-
2.
Complete filling of the canal with non-setting Ca(OH)2, which is then replaced initially after one month and then after every three months until the development of an apical barrier.37
Ca(OH)2 has excellent antibacterial properties due to the release of hydroxyl ions, which creates a high pH environment. This high pH is also thought to promote healing in the periapical tissues. Due to these properties, it is recommended that the Ca(OH)2 dressing is changed regularly.35 The effectiveness of Ca(OH)2 apexification on 26 immature permanent incisors demonstrated that 100% of the teeth were asymptomatic after treatment and apical closure was achieved in 100% of cases.38 It took an average of 3.23 dressing changes of Ca(OH)2 (range: 1-5 sessions) and 12.19 months for apical closure to occur (range: 3-20 months). Another study demonstrated that the range of apical barrier formation with Ca(OH)2 was between 11.1-13.4 weeks.39 However, in this study, the patients were seen once every 1-3 weeks and the Ca(OH)2 dressing was changed more regularly. The study also suggested that the use of ultrasonic filing and chlorhexidine irrigation solution could increase the rate of apical barrier formation. The factors reported to affect the rate of apexification are the number of dressing changes, the diameter of the apex and the type of trauma injury sustained.40 Interestingly, the rate of apexification is the same in teeth with and without the presence of periapical lesions.39
Although Ca(OH)2 is successful in inducing apical barrier formation, there are disadvantages to this technique. Multiple visits are required for apexification to occur, affecting the child, parent and dental team as time off from school and work, travelling expenses and other financial resources are required.41 Multiple visits also require compliance by patients, a factor that is difficult to control. The structure of the tooth can also become further compromised due to the repeated access and treatment of the root canal. It has also been proposed that long-term use of Ca(OH)2 as a root canal dressing can decrease the fracture strength of the root.42 Furthermore, Ca(OH)2 can reduce the flexural strength of dentine.43
An MTA plug can be used for apical barrier formation (Fig. 4). The main advantage of this is that an apical plug can be placed in a single visit following debridement, irrigation and filing of the canal. This allows immediate obturation of the root canal.44 One proposed method for this technique is isolation with dental dam, determination of working length of the canal and selection of an appropriate plugger to deposit and position a 2-3 mm plug of MTA in the canal.45 Ultrasonic vibration can improve the condensation and adaption of MTA.45 A sterile wet paper point is used to introduce moisture to the MTA in order to allow the MTA to set. An intermediate material is then placed to seal in the moisture and prevent dehydration of the MTA which allows for bonding procedures of the core without damaging the setting MTA.45 Following apical barrier formation, conventional endodontics should be completed. If an MTA plug has been placed, the use of a master cone is not advised and a thermoplastic backfill technique for obturation is preferred.
There are variable success rates in the use of an MTA plug, with one systematic review of 4 studies reporting a clinical success rate of 93-100%30 and another prospective study reporting the success rate (improvement in periapical index score and decrease in size of periapical lesion) as 81%.46 However, it was concluded that using MTA results in a predictable and reproducible procedure.46 The use of MTA can result in crown discolouration if placed close to the clinical crown, as reported in numerous studies.47,48,49 There have also been concerns regarding the cost and ease of use.49
Biodentine can be used as a suitable and effective alternative to MTA. One major advantage of Biodentine is that it requires less time to handle, use and set. A case report compared the effects of Biodentine and MTA by placing these materials in separate traumatised incisor teeth of one patient.50 Radiographic assessment of the teeth showed that initial periapical healing was superior with Biodentine; however, long-term periapical healing was better with MTA. An in vitro study investigated the microleakage between Biodentine and MTA in eight anterior teeth and concluded that there was no statistically significant difference between the microleakage values.51 The study also revealed that reducing the thickness of the apical plug increases the amount of microleakage.
Regenerative endodontic procedures
Regenerative endodontic procedures (REPs) can be defined as 'biologically based procedures designed to replace damaged structures, including dentine and root structures, as well as cells of the pulp-dentine complex'.52 The regeneration or revascularisation of a non-vital pulp can result in further root development, increasing both the length and thickness of the root and therefore increasing its resistance to fracture.53 This is one of the main advantages of the technique compared to apexification. REPs involve the principles of regenerative medicine and tissue engineering. The technique requires the following conditions: the presence of stem cells, complete disinfection of the root canals, a scaffold within the root canal to support cell differentiation and the presence of growth factors and signals to stimulate the stem cells to differentiate.54 There are four types of human dental stem cells thought to have the potential to be involved in regeneration: dental pulp stem cells, stem cells from exfoliated deciduous teeth, stem cells from apical papilla (SCAP) and periodontal ligament stem cells.55
A proposed clinical protocol for REPs is described below and illustrated in Figure 5:56
-
1.
Isolation with dental dam, prepare access cavity and extirpate pulp. Avoid mechanical instrumentation of the root canal walls to prevent further weakening on dentine walls
-
2.
Irrigate with 1.5-3% sodium hypochlorite followed by sterile saline to minimise cytotoxic effects of sodium hypochlorite on vital tissues
-
3.
Dry with paper points and irrigate with 17% EDTA
-
4.
Insert a non-discolouring and non-setting calcium hydroxide product into the canal
-
5.
Place temporary coronal seal directly onto intracanal dressing and review in 2-4 weeks for signs and symptoms of inflammation. If signs persist, refresh calcium hydroxide
-
6.
At next appointment, irrigate with 17% ethylenediaminetetraacetic acid (EDTA) followed by sterile saline. Dry canal with paper points and induce bleeding by mechanical irritation of periapical tissue and rotational movement of an apically pre-bent file (for example, size 40 Hedstrom).
-
7.
Allow canal to fill with blood until 2 mm below gingival margin to wait for blood clot formation for 15 minutes
-
8.
Cut a collagen matrix to the diameter larger than the coronal part of the root canal and a height of 2-3 mm. Place matrix on top of the blood clot and allow the matrix to soak with liquid to avoid formation of a hollow space
-
9.
Place hydraulic silicate cement (for example, MTA) on top of the matrix, 2 mm underneath the cemento-enamel junction. Place a resin-modified GIC or calcium hydroxide cement and seal with adhesive restoration
-
10.
Follow-ups should be performed after 6, 12, 18 and 24 months and after that, annually for 5 years.
A study carried out by Kontakiotis et al. (2015) analysed data from 60 publications regarding the clinical protocols used for REPs.57 With regards to irrigation, 97% of the studies used NaOCl at varying concentrations of 1-6%, with 65% using NaOCl as the only irrigant. The AAE recommend the use of 1.5% NaOCl in order to reduce the cytotoxicity to stem cells in the apical tissues.58 Chlorhexidine and EDTA were used as the final irrigant in 4% and 13% of the articles, respectively. One study investigated the effects of different root canal irrigant protocols on the survival of SCAP and concluded that irrigation with 17% EDTA had the best outcome regarding cell viability.59 Interestingly, the study also reported that cells treated with 2% chlorhexidine appeared cytotoxic and non-viable.
An in vitro study discovered that a tri-antibiotic paste containing 1% metronidazole, 1% ciprofloxacin and 1% of minocycline could be used to eliminate bacteria from carious and endodontic lesions in situ and since has been widely used as an intracanal medicament.60 A retrospective study compared the effects of triple antibiotic paste, Ca(OH)2 and formocresol on the radiographic outcome of immature teeth treated with REPs.61 The results concluded that the triple antibiotic paste produced significant greater differences in dentinal wall thickness but no significant difference in root length, compared to Ca(OH)2 and formocresol. A systematic review of the evidence of REPs protocols highlighted that minocycline can cause discolouration of teeth and the evidence showed similar antimicrobial effects of using a tri-antibiotic and bi-antibiotic paste in which minocycline had been eliminated.54 With regards to the use of antibiotic paste, the drawbacks such as discolouration, cytotoxicity, sensitisation, development of resistance and difficulty of removal from the root canal should be taken into account. The ESE advises that calcium hydroxide is used as the primary medicament; however, if signs of inflammation persist, the use of an antibiotic mix inside the root canal may be indicated.56
Kontakiotis et al. (2015) reported that 75% of the published articles used the creation of a blood clot as the scaffold.57 The blood clot acts a matrix to allow new growth of pulpal tissue.53 Platelet-rich plasma (PRP) and PRF have also been used as scaffolds. These contain a higher concentration of platelets and therefore an increased amount of growth factors to stimulate cell differentiation. A recent double-blinded randomised control trial compared the clinical and radiographic regenerative potential of PRP to PRF scaffolds in immature non-vital permanent maxillary central incisors.62 The results demonstrated 100% success and concluded no statistically significant differences between teeth treated with PRP and teeth treated with PRF with regards to clinical outcomes (resolution of pain, swelling, mobility and sinus-fistula). The results also indicated there was an increase in root length and width with PRP over PRF; however, these results were not statistically significant. Another randomised control trial investigated the use of a collagen membrane as a scaffold and found that that it promoted the development of the dentine wall in the middle third of the root.63
The main advantages of REPs include continued development of the root and fewer treatment visits compared to Ca(OH)2 apexification. However, disadvantages of this technique do exist. REPs are technically challenging as reported in a case series.64 There is also the risk of tooth discolouration with the use of minocycline. Some studies involving histological analyses after revitalisation show that in some cases repair without regeneration occurs. The ectopic tissue formed in the canal during repair has been reported as fibrous tissue, cementum or bone.56 Regenerative dentistry is a relatively new concept in dentistry and focuses on using biologically based approaches to reconstruct injured odontogenic tissues. Research using murine models is carried out world-wide; however, translation into human trials is essential in order to further our understanding and application of these techniques.
Conclusion
In a primary care setting, an understanding of the specialist treatments available can be vitally important for the management of a patient attending with a dental emergency or when an unexpected pulpal exposure occurs. It is very useful for primary care dentists to appreciate that a pulpal exposure does not necessarily mean root canal treatment is definitely indicated and thus, appropriate initial conservative management may help to prolong the life of a traumatised tooth.
References
Logan W, Kronfeld R. Development of the Human Jaws and Surrounding Structures from Birth to the Age of Fifteen. J Am Dent Assoc 1933; 20: 379-428.
Berkovitz B, Holland G, Moxham B. Oral Anatomy, Histology and Embryology. Edinburgh: Elsevier, 2009.
Andreasen F M, Kahler B. Pulpal response after acute dental injury in the permanent dentition: clinical implications - a review. J Endod 2015; 41: 299-308.
Luder H U. Malformations of the tooth root in humans. Front Physiol. 2015; DOI: 10.3389/fphys.2015.00307.
Andersson L. Epidemiology of traumatic dental injuries. J Endod 2013; DOI: 10.1016/j.joen.2012.11.021.
NHS Digital. Child Dental Health Survey 2013, England, Wales and Northern Ireland. 2015. Available at https://digital.nhs.uk/data-and-information/publications/statistical/children-s-dental-health-survey/child-dental-health-survey-2013-england-wales-and-northern-ireland (accessed March 2022).
Journal of Endodontics. AAE Consensus Conference Recommended Diagnostic Terminology. J Endod 2009; DOI: 10.1016/j.joen.2009.09.035.
American Academy of Paediatric Dentistry. Pulp therapy for primary and immature permanent teeth. The Reference Manual of Paediatric Dentistry. Am Acad Paediatr Dent 2021; 384-392.
Parirokh, M, Torabinejad, M, Dummer P. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview - part I: vital pulp therapy. Int Endod J 2018; 51: 177-205.
Thompson V, Craig R G, Curro F A, Green W S, Ship J A. Treatment of deep carious lesions by complete excavation or partial removal: a critical review. J Am Dent Assoc 2008; 139: 705-712.
Hilton T. Keys to Clinical Success with Pulp Capping: A Review of the Literature. Oper Dent 2009; 34: 615-625.
Piva E, Da Rosa W L O, Coco A R, Da Silva T M, Mesquita, L C, Galharça A D, Da Silva A F. Systematic review of dental pulp capping materials. Dent Mater 2016; DOI: 10.1016/j.dental.2016.08.185.
Bogen G, Chandler N P. Pulp preservation in immature permanent teeth. Endod Topics 2010; 23: 131-152.
Olsson H, Petersson K, Rohlin M. Formation of a hard tissue barrier after pulp cappings in humans. A systematic review. Int Endod J 2006; 39: 429-442.
Mathur V P, Dhillon J K, Logani A, Kalra G. Evaluation of indirect pulp capping using three different materials: A randomized control trial using cone-beam computed tomography. Indian J Dent Res 2016; 27: 623-629.
Piesiak-Pańczyszyn D, Wrzyszcz-Kowalczyk A, Kobierska-Brzoza J. A Comparison of Selected Regenerative Materials Used in the Management of Pulp Diseases - Review of the Literature. Dent Med Probl 2015; 52: 485-490.
Özgür B, Uysal S, Cem Güngör H. Partial Pulpotomy in Immature Permanent Molars After Carious Exposures Using Different Haemorrhage Control and Capping Materials. Paediatr Dent 2017; 39: 364-370.
Sogupinar A, Arikan V. Comparative evaluation of four endodontic biomaterials and calcium hydroxide regarding their effect on fracture resistance of simulated immature teeth. Eur J Paediatr Dent 2020; 21: 23-28.
Duncan H F, Galler K M, Tomson P L et al. European Society of Endodontology position statement: Management of deep caries and the exposed pulp. Int Endod J 2019; 52: 923-934.
Edwards D, Stone S, Bailey O, Tomson P. Preserving pulp vitality: part two - vital pulp therapies. Br Dent J 2021; 230: 148-155.
Kher M S, Rao A. Contemporary Treatment Techniques in Paediatric Dentistry. Switzerland: Springer Nature, 2019.
Barratt O, Dixon C, Barry S. Technique tips: A complicated crown fracture: The cvek pulpotomy. Dent Update 2017; 44: 1096-1097.
Cvek M. A clinical report on partial pulpotomy and capping with calcium hydroxide in permanent incisors with complicated crown fracture. J Endod 1978; 4: 232-237.
Fong C D, Davis M J. Partial pulpotomy for immature permanent teeth, its present and future. Paediatr Dent 2002; 24: 29-32.
Bimstein E, Rotstein I. Cvek pulpotomy - revisited. Dent Traumatol 2016; 32: 438-442.
Dean J A. Management of trauma to the teeth and supporting tissues. In Dean J E, Jones J E, Walker Vinson L A (eds) McDonald and Avery's Dentistry for the child and adolescent. 10th ed. pp 563-602. Missouri: Elsevier, 2016.
Andreasen J O, Andreasen F M, Andersson L. Textbook and Colour Atlas of Traumatic Injuries to the Teeth. 4th ed. New Jersey: Blackwell Publishing, 2007.
Chacko V, Kurikose S. Human pulpal response to mineral trioxide aggregate (MTA): a histological study. J Clin Paediatr Dent 2006; 30: 203-209.
Ghoddusi J, Forghani M, Parisay I. New approaches in vital pulp therapy in permanent teeth. Iran Endod J 2014; 9: 15-22.
Chen Y, Chen X, Zhang Y et al. Materials for pulpotomy in immature permanent teeth: a systematic review and meta-analysis. BMC Oral Health. 2019; DOI: 10.1186/s12903-019-0917-z.
de Blanco L P. Treatment of crown fractures with pulp exposure. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996; 82: 564-568.
Fuks A B, Gavra S, Chosac A. Long-term followup of traumatized incisors treated by partial pulpotomy. Paediatr Dent 1993; 15: 334-336.
American Association of Endodontists. Endodontics Colleagues for Excellence: Endodontic Diagnosis. 2013. Available at https://www.aae.org/specialty/wp-content/uploads/sites/2/2017/07/endodonticdiagnosisfall2013.pdf (accessed December 2020).
American Association of Endodontists. Glossary of endodontic terms. 2020. Available at https://www.aae.org/specialty/clinical-resources/glossary-endodontic-terms/ (accessed March 2022).
Abbott P V. Apexification with calcium hydroxide - when should the dressing be changed? The case for regular dressing changes. Aust Endod J 1988; 24: 27-32.
Lin J-C, Lu J-X, Zeng Q, Zhao W, Li W-Q, Ling J-Q. Comparison of mineral trioxide aggregate and calcium hydroxide for apexification of immature permanent teeth: A systematic review and meta-analysis. J Formos Med Assoc 2016; 115: 523-530.
Mackie I C, Bentley E M, Worthington H V. The closure of open apices in non-vital immature incisor teeth. Br Dent J 1988; 165: 169-173.
Dominguez Reyes A, Muñoz Muñoz L, Aznar Martin T. Study of calcium hydroxide apexification in 26 young permanent incisors. Dent Traumatol 2005; 21: 141-145.
Lee L-W, Hsiao S-H, Chang C-C, Chen L-K. Duration for apical barrier formation in necrotic immature permanent incisors treated with calcium hydroxide apexification using ultrasonic or hand filing. J Formos Med Assoc 2010; 109: 596-602.
Finucane D, Kinirons M J. Non-vital immature permanent incisors: factors that may influence treatment outcome. Endod Dent Traumatol 1999; 15: 273-277.
Phillips J M, Srinivasan V. The management of non-vital immature permanent incisors. Dent Update 2014; 41: 596-604.
Andreasen J O, Farik B, Munksgaard E C. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol 2002; 18: 134-137.
Grigoratos D, Knowles J, Ng Y L, Hulabivala K. Effect of exposing dentine to sodium hypochlorite and calcium hydroxide on its flexural strength and elastic modulus. Int Endod J 2001; 34: 113-119.
Morse D R, O'Lornic J, Yesilsoy C. Apexification: review of the literature. Quintessence Int 1990; 21: 589-598.
Steinig T H, Regan J D, Gutmann J L. The use and predictable placement of Mineral Trioxide Aggregate in one-visit apexification cases. Aust Endod J 2003; 29: 34-42.
Simon S, Rilliard F, Berdal A, Machtou P. The use of mineral trioxide aggregate in one-visit apexification treatment: a prospective study. Int Endod J 2007; 40: 186-197.
Segura-Egea J J, Castellanos-Cosano L, Martin-Gonzalez J, Alonso-Ezpeleta L O, Lopez Frias F J. Green discoloration of the crown after internal root resorption treatment with grey mineral trioxide aggregate (MTA). J Clin Exp Dent 2011; DOI: 10.4317/jced.3.e404.
Belobrov, I, Parashos P. Treatment of tooth discoloration after the use of white mineral trioxide aggregate. J Endod 2011; 37: 1017-1020.
Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review - Part III: Clinical applications, drawbacks, and mechanism of action. J Endod 2010; 36: 400-413.
Elumalai D, Kapoor B, Tewrai R K, Mishra S K. Comparison of mineral trioxide aggregate and biodentine for management of open apices. J Interdiscip Dent 2015; 5: 131-135.
Bani M, Sungurtekin-Ekçi E, Odabaş M E. Efficacy of Biodentine as an Apical Plug in Nonvital Permanent Teeth with Open Apices: An In Vitro Study. BioMed Res Int 2015; DOI: 10.1155/2015/359275.
Murray P E, Garcia-Godoy F, Hargreaves K M. Regenerative endodontics: a review of current status and a call for action. J Endod 2007; 33: 377-390.
Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod 2004; 30: 196-200.
Duggal M, Tong H J, Al-Ansary M, Twati W, Day P F, Nazzal H. Interventions for the endodontic management of non-vital traumatised immature permanent anterior teeth in children and adolescents: a systematic review of the evidence and guidelines of the European Academy of Paediatric Dentistry. Eur Arch Paediatr Dent 2017; 18: 139-151.
Huang G T-J. A paradigm shift in endodontic management of immature teeth: conservation of stem cells for regeneration. J Dent 2008; 36: 379-386.
Galler K M, Krastl G, Simon S et al. European Society of Endodontology position statement: Revitalization procedures. Int Endod J 2016; 49: 717-723.
Kontakiotis E G, Filippatos C G, Tzanetakis G N, Agrafioti A. Regenerative endodontic therapy: a data analysis of clinical protocols. J Endod 2015; 41: 146-154.
American Association of Endodontists. Endodontics Colleagues for Excellence: Regenerative Endodontics. 2013. Available at https://www.aae.org/specialty/wp-content/uploads/sites/2/2017/06/ecfespring2013.pdf (accessed December 2020).
Trevino E G, Patwardhan A N, Henry M A et al. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J Endod 2011; 37: 1109-1115.
Hoshino E, Kurihara-Ando N, Sato I et al. In-vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int Endod J 1996; 29: 125-130.
Bose R, Nummikoski P, Hargreaves K. A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod 2009; 35: 1343-1349.
Rizk H M, Al-Deen M S M S, Emam A A. Comparative evaluation of Platelet Rich Plasma (PRP) versus Platelet Rich Fibrin (PRF) scaffolds in regenerative endodontic treatment of immature necrotic permanent maxillary central incisors: A double blinded randomized controlled trial. Saudi Dent J 2019; 32: 224-231.
Jiang X, Liu H, Peng C. Clinical and Radiographic Assessment of the Efficacy of a Collagen Membrane in Regenerative Endodontics: A Randomized, Controlled Clinical Trial. J Endod 2017; 43: 1465-1471.
Petrino J A, Boda K K, Shambarger S, Bowles W R, McClanahan S B. Challenges in regenerative endodontics: a case series. J Endod 2010; 36: 536-541.
Author information
Authors and Affiliations
Contributions
Anika Shah: first author and corresponding author; involved in initial planning and establishment of MESH terms for literature search; reviewed literature and used this to write the abstract, introduction and non-vital pulp therapy techniques section; digital editing of hand-drawn diagrams; and revisions. Rachel Peacock: first author; involved in initial planning and establishment of MESH terms for literature search; reviewed literature and used this to write the abstract, introduction, vital pulp therapy techniques section and conclusion; illustration of original hand-drawn diagrams; and revisions. Shiyana Eliyas: second author; project supervisor; involved in reviewing article and editing; provided guidance on direction of the article.
Corresponding author
Ethics declarations
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Shah, A., Peacock, R. & Eliyas, S. Pulp therapy and root canal treatment techniques in immature permanent teeth: an update. Br Dent J 232, 524–530 (2022). https://doi.org/10.1038/s41415-022-4139-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41415-022-4139-4
- Springer Nature Limited