Abstract
Adjuvants for vaccines with characteristics of improving adaptive immunity particularly via leverage of antigen presenting cells (APCs) are currently lacking. In a previous work we obtained a new soluble 300 kDa homogeneous β-glucan named GFPBW1 from the fruit bodies of Granola frondosa. GFPBW1 could activate macrophages by targeting dendritic cell associated C-type lectin 1 (Dectin-1)/Syk/NF-κB signaling to achieve antitumour effects. In this study the adjuvant effects of GFPBW1 were explored with OVA-antigen and B16-OVA tumor model. We showed that GFPBW1 (5, 50, 500 μg/mL) dose-dependently promoted activation and maturation of APCs in vitro by increasing CD80, CD86 and MHC II expression. We immunized female mice with OVA in combination with GFPBW1 (50 or 300 μg) twice with an interval of two weeks. GFPBW1 markedly and dose-dependently increased OVA-specific antibody titers of different subtypes including IgG1, IgG2a, IgG2b and IgG3, suggesting that it could serve as an adjuvant for both Th1 and Th2 type immune responses. Furthermore, GFPBW1 in combination with aluminum significantly increased the titers of OVA-specific IgG2a and IgG2b, but not those of IgG1, suggesting that GFPBW1 could be used as a co-adjuvant of aluminum to compensate for Th1 deficiency. For mice immunized with OVA plus GFPBW1, no obvious pathological injury was observed in either major organs or injection sites, and no abnormalities were noted for any of the hematological parameters. When GFPBW1 served as an adjuvant in the B16-OVA cancer vaccine models, it could accomplish entire tumor suppression with preventive vaccines, and enhance antitumour efficacy with therapeutic vaccines. Differentially expressed genes were found to be enriched in antigen processing process, specifically increased tumor infiltration of DCs, B1 cells and plasma cells in the OVA plus GFPBW1 group, in accordance with its activation and maturation function of APCs. Collectively, this study systematically describes the properties of GFPBW1 as a novel potent and safe adjuvant and highlights its great potential in vaccine development.

Similar content being viewed by others
Introduction
Due to their weak immunogenicity, protein- and peptide-based cancer vaccines usually fail to elicit efficient immune responses against tumors. The reasons for this are believed to be related to the inhibition of antigen-presenting cell (APC) functionalities as well as systematic immune status. Although a few adjuvants, such as alum and CpG, and nanoparticles for antigen delivery are currently being investigated or have been approved, cancer vaccine development has been disappointing in clinical translation [1, 2]. Growing knowledge of the tumor microenvironment and immune mechanisms supports the importance of improving immunogenicity and eliminating the suppression of the innate immune system. As such, a promising adjuvant for cancer vaccines should improve adaptive immunity, which can be achieved by activating the innate immune system while processing and presenting tumor antigens, thus efficiently promoting downstream antitumour adaptive immunity.
Many traditional Chinese medicines (TCMs) have shown potential as adjuvants in recent research with the unique advantages of not only exerting non-toxic side effects, but also inducing stronger humoral and cellular immunity [3]. Polysaccharides are the main active ingredients of TCM. Recently, several polysaccharide adjuvants have been developed due to their intrinsic characteristics of immunomodulation, biocompatibility, biodegradability and safety [4,5,6,7,8].
Grifols frondosa (Fr.) S. F. Gray (G. frondose) is a culinary-medicinal mushroom that contains several physiologically active compounds, of which polysaccharides, specifically β-glucans, are known to possess a variety of potent bioactivities, such as antitumour, antioxidant and anti-inflammatory effects [9].
In our previous study, we obtained a new soluble 300 kDa homogeneous polysaccharide named GFPBW1 (Patent No.: CN 103059160A) from the fruiting bodies of G. frondosa. GFPBW1 was found to have a β-D-(1-3)-linked glucan backbone with a single β-D-(1-6)-linked glucopyranosyl residue on every third residue. GFPBW1 can activate macrophages through Dectin-1/Syk/NF-κB signaling to achieve antitumour effects [10]. Dectin-1 (gene CLEC7A), a member of the pattern recognition receptor C-type lectin family, is a crucial part of the innate immune response to recognize β-glucans of fungal pathogens [11]. Moreover, Dectin-1 is believed to be the major β-glucan recognition receptor on macrophages [12]. Its activation may induce the expression of innate cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-2, IL-8, IL-10, and IL-12 [13], further contributing to the activation of adaptive Th1/Th17 responses [14] and CD8+ T cells [15].
In the present study, the adjuvant effects of GFPBW1 were explored with OVA-antigen and B16-OVA tumor models due to its promising activation of the innate immune system by promoting the activation and maturation of APCs, including macrophages, B cells and DCs.
Materials and methods
Materials and cells
GFPBW1 was prepared as we have previously described [10], with a mean molecular mass of 300 kDa, the proposed structure of which is shown in Supplementary Fig. S1, and was not contaminated with endotoxin (<0.03 U), as confirmed by a limulus test kit (G010030, Bioendo, Xiamen, China) and G factor inhibitor (BT02, Bioendo, Xiamen, China). Briefly, the crude polysaccharide GFPB was extracted from the residue with 5% NaOH after boiled water extraction of Grifola frondosa. Subsequently, GFPB was fractioned on a DEAE-cellulose column with distilled water, which produced GFPBW1. The polysaccharide was dissolved in normal saline after passing through a 0.22 μm filter for experimental use. Ovalbumin antigen protein was obtained from Sigma (A5378, Sigma‒Aldrich, MO, USA). Aluminum hydroxide gel was purchased from InvivoGen (vac-alu-250, San Diego, USA).
The B16-OVA cell line was purchased from Crisprbio (Beijing, China) and maintained in Dulbecco’s modified Eagle’s medium (DMEM; MA0212, Meilunbio, Dalian, China) supplemented with 1% penicillin/streptomycin (P/S; 15140122, Invitrogen, MA, USA) and 10% heat-inactivated fetal bovine serum (FBS; 10100147, Life Technologies, MA, USA). The THP-1 cell line was purchased from Procell (Wuhan, China) and cultured in RPMI-1640 medium (MA0215, Meilunbio, Dalian, China) supplemented with 1% P/S and 10% FBS. All cells were cultured at 37 °C in a 5% CO2 humidified atmosphere.
Mice
All animal experiments were conducted in accordance with the ARRIVE guidelines and the U.K. Animals (Scientific Procedures) Act, 1986, and its associated guidelines. All experiments were approved by the Institutional Laboratory Animal Care and Use Committee (IACUC). The approval number of the ethics committee that endorsed this study is 2022-09-AL-49. Female C57BL/6 mice or BALB/c mice (18–22 g, 6–8 weeks) were purchased from Zhejiang Vital River Laboratory Animal Technology Co., Ltd. (Zhejiang, China) and were acclimated to the laboratory housing for 3 to 4 days. Mice were housed under specific pathogen-free (SPF) conditions with a temperature of 18–22 °C, humidity of 40%–60%, and a 12-h light/dark cycle.
Single-cell sequencing and data analysis
The single-cell RNA sequencing data used in this study were all publicly available. Data for CLEC7A (also known as Dectin-1) expression in immune cells were obtained from the GEO database (GSE127465). The SpringViewer interactive tool was used to analyze the data.
Antigen presentation cell (APC) activation/maturation assay
The spleens of the female C57BL/6 mice were removed under aseptic conditions. Single-cell suspensions of splenocytes were prepared by grinding spleen tissues in RPMI-1640 medium (MA0215, Meilunbio, Dalian, China) with a syringe rubber pad, followed by incubation with erythrocyte lysis buffer (40401ES60, Yeasen, Shanghai, China) and filtering through a 70-μm nylon cell filter. Splenocytes (1 mL, 3 × 106 cells/mL) were added to 24-well plates and stimulated with GFPBW1 at 0, 5, 50, or 500 µg/mL for 24 or 48 h. The cell samples were collected and first blocked with anti-CD16/CD32 (FcγRIII/FcγRII, 2.4G2, 553141, BD Biosciences, CA, USA) and then incubated with surface marker antibodies for 25 min at 4 °C. Antibody staining of cells was performed following the manufacturer’s recommendations. The following antibodies were used: BV786 hamster anti-mouse CD11c (563735, BD Biosciences, CA, USA), PE/Cyanine7 rat anti-mouse F4/80 (25-4801-82, eBioscience, MA, USA), PerCP-Cy™5.5 rat anti-mouse CD45R/B220 (552771, BD Biosciences, CA, USA), PE hamster anti-mouse CD80 (553769, BD Biosciences, CA, USA), APC rat anti-mouse CD86 (560747, BD Biosciences, CA, USA), and FITC rat anti-mouse I-A/I-E (MHCII) (553623, BD Biosciences, CA, USA). Flow cytometry analysis was performed using an ACEA NovoCyte flow cytometer. Data processing was carried out using NovoExpress software.
ELISA of secretion of TNF-α and IL-6 in human monocyte THP-1 cells
Human monocyte THP-1 cells (100 μL, 1 × 106 cells/mL) were added to 96-well plates and stimulated with PMA (1.6 μM) for 24 h, and the medium was discarded. GFPBW1 was added at a final concentration of 5, 50, or 500 μg/mL or saline for 24 h, and then the medium was collected for TNF-α (EK182-96, Multisciences, Hangzhou, China) and IL-6 (EK106/2-96, Multisciences, Hangzhou, China) detection with the corresponding ELISA kits.
Immunization of mice
Female C57BL/6 mice were randomly divided into 4 groups (n = 5). The mice were intramuscularly immunized with saline (control group), OVA alone (10 μg per mouse), or OVA in combination with GFPBW1 (50 or 300 μg per mouse) twice at two-week intervals (i.e., on Days 0 and 14). The serum was collected on Days 7, 14, 21, 28, and 35 after the first immunization. On Days 21, 28, and 35 after the first immunization, the corresponding collection days were 7, 14, and 21 after the second immunization, respectively.
To evaluate the coadjuvant effect of GFPBW1 and aluminum adjuvant, female C57BL/6 mice were randomly divided into 5 groups (n = 5), and the mice were immunized intramuscularly on Days 0 and 14 with saline (control group), OVA alone (10 μg per mouse), OVA in combination with alum (10 μg per mouse), or OVA in combination with both alum and GFPBW1 (50 or 300 μg per mouse), the formulations of which were physically mixed. The serum was collected as previously described.
Measurement of OVA-specific antibodies
OVA-specific antibodies against total IgG and IgG subtypes (IgG1, IgG2a, IgG2b and IgG3) in serum were detected by standard enzyme-linked immunosorbent assay (ELISA) methods. In brief, microtiter plates were coated with 100 μL of OVA solution (5 μg/mL) overnight at 4 °C. After being blocked with 3% BSA, serial dilutions of serum were added and incubated for one hour at 37 °C with shaking. After washing, 100 μL of HRP-conjugated goat anti-mouse IgG/IgG1/IgG2a/IgG2b/IgG3 (dilution ratio of 1:5,000) was added to each well and incubated for one hour. After washing again, 100 μL of tetramethyl benzidine (TMB) substrate solution was added for 20 min. The reaction was stopped with 2 M H2SO4, and the absorbance was measured at 450 nm with an Infinite F50 microplate reader (Tecan). The following antibodies were used: goat anti-mouse IgG H&L (HRP) (ab205719, Abcam, MA, USA), goat anti-mouse IgG1 (HRP) (ab97240, Abcam, MA, USA), goat anti-mouse IgG2a heavy chain (HRP) (ab97245, Abcam, MA, USA), goat anti-mouse IgG2b heavy chain (HRP) (ab97250, Abcam, MA, USA), and goat anti-mouse IgG3 heavy chain (HRP) (ab97260, Abcam, MA, USA).
Safety evaluation
C57BL/6 mice and BALB/c mice were immunized twice with saline, OVA, or OVA + GFPBW1 (10, 50 or 300 µg per mouse) on Day 0 and Day 14. Clinical observations were conducted daily, and body weight and body temperature data were collected twice a week from the first immunization to experimental completion. The animals were sacrificed on Day 28, and blood and tissues/organs were collected. Hematology was analyzed using blood counters (ADVIA 2120i). Tissues and organs were embedded and stained with haematoxylin and eosin (H&E) and then examined microscopically.
In addition, to evaluate the safety of a higher dose and a greater frequency of immunization, C57BL/6 mice were immunized four times with saline, OVA, or OVA + GFPBW1 (1000 µg per mouse) on Days 0, 3, 6, and 13 and euthanized on Day 14. The major organs (heart, liver, spleen, lung, kidney) and the injection site were collected, embedded, stained with haematoxylin and eosin (H&E) and then examined microscopically.
Efficacy of tumor prevention
C57BL/6 mice were intramuscularly injected with saline, OVA (10 µg per mouse), or OVA (10 µg per mouse) + GFPBW1 (10, 50 or 300 μg per mouse) twice, with an interval of two weeks. Six weeks after the last immunization, the mice were inoculated with 5 × 105 B16-OVA cells in the right flank. On Day 35 post tumor inoculation, tumor-free mice from the OVA + GFPBW1 groups were selected and challenged with a second tumor inoculation of 5 × 105 B16-OVA cells in the left flank. As the mice in the OVA and saline groups were euthanized prior to rechallenge due to large tumor sizes, eight additional mice incubated with B16 OVA cells were used as a control group in the rechallenge experiment.
Tumor volume was measured and recorded every two days. Tumor volume was calculated as tumor volume (mm3) = tumor length × width × width/2, and tumor growth curves were plotted. For survival studies, euthanasia endpoints were defined as tumors larger than 2000 mm3 or larger than 20 mm in length or mice with significant weight loss.
Tumor therapeutic efficacy
C57BL/6 mice were inoculated with 5 × 105 B16-OVA cells in the right flank on Day 0. Mice were immunized twice with saline, OVA (10 µg per mouse), or OVA (10 µg per mouse) + GFPBW1 (10, 50 or 300 μg per mouse) on Days 6 and 13 posttumour inoculation. Tumor growth and survival were monitored.
RNA sequencing
C57BL/6 mice (4 mice per group) bearing B16-OVA tumor cells were immunized on Days 6 and 13 post tumor inoculation with saline, OVA, OVA + GFPBW1 (300 μg per mouse), or OVA + alum and euthanized on Day 28 post tumor inoculation to collect tumor tissue for RNA sequencing.
Total RNA was isolated from tumor tissues using TRIzol Reagent (15596026, Invitrogen, California, USA). RNA purification, reverse transcription, library construction and sequencing were performed at Shanghai Majorbio Biopharm Biotechnology Co., Ltd. (Shanghai, China). The RNA sequencing transcriptome library was prepared following Illumina® Stranded mRNA Prep, Ligation from Illumina (San Diego, CA).
Based on the RNA sequencing results, we analyzed DEGs and immune cell infiltration levels. Differential expression was evaluated with DESeq. Differential gene expression was visualized by a heatmap. Genes in the heatmap were clustered according to the log2 (fold-change) value. Based on the published literature [16], immune signature scores are defined as the mean log2 (fold-change) among all genes in each gene signature during immune-related signature gene level analysis. Immune cell infiltration within tumor tissues was analyzed using ImmuCellAI.
Statistical methods and software
Statistical analysis was carried out using GraphPad Prism 9 software. Multiple comparisons were performed using one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparisons test. Student’s t test was used to compare the differences between two groups. The detailed statistical methods and sample sizes used in the experiment are shown in the figure legends. No statistical methods were used to predetermine the sample size. All the statistical tests were two-sided, and P values less than 0.05 were considered to indicate statistical significance. *P < 0.05, **P < 0.01, ***P < 0.001.
Results
GFPBW1 promotes the activation and maturation of APCs in vitro
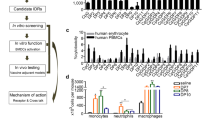
GFPBW1, a polysaccharide isolated from Grifola frondosa, was identified as a novel ligand of Dectin-1 in our previous work [10]. The extraction and structural information of GFPBW1 are described in the supplementary information (Supplementary Fig. S1). Using open-source human immune cell single-cell sequencing data, we first analyzed the expression of Dectin-1 and found that Dectin-1 was predominantly expressed in monocytes, macrophages, dendritic cells (DCs), and neutrophils (Fig. 1a). A similar expression profile was observed in the single-cell sequencing data of immune cells from mice (Fig. 1b), suggesting that Dectin-1 is highly expressed in APCs. Considering that Dectin-1 is also a pattern recognition receptor (PRR), we hypothesized that GFPBW1 might activate APCs by stimulating Dectin-1. To test this hypothesis, spleen cells extracted from C57BL/6 mice were treated with 0, 5, 50, or 500 µg/mL GFPBW1, and the maturation and activation markers of DCs, macrophages and B cells were detected by flow cytometry. After 48 h of stimulation, 50 and 500 μg/mL GFPBW1 significantly increased the expression of CD80, CD86, and major histocompatibility complex (MHC) II in DCs (Fig. 1c). At 50 μg/mL, GFPBW1 significantly activated DCs, as indicated by 70.4%, 36.2%, and 8.61% increases in the mean fluorescence intensity (MFI) of CD80, CD86, and MHC II, respectively. Similarly, increased CD80, CD86, and MHCII were noted in both macrophages and B cells after 500 μg/mL treatment. However, at 50 μg/mL, no significant increase was noted in macrophages or B cells except for increases in CD86 in macrophages and in CD80 in B cells, which may be due to differences in Dectin-1 expression among DCs, macrophages and B cells. No significant changes in the activation or maturation of APCs were observed in the 5 μg/mL group compared to the saline control group (Fig. 1d, e). In addition, the CD80-expressing macrophages that appeared to be downregulated in this group were not significantly different, as the values were within the normal range of variation (Fig. 1d). In addition, we also found that GFPBW1 acting on splenocytes for only 24 h promoted the expression of APC activation markers in all three cell types (Supplementary Fig. S2).
At the single-cell transcription level, the expression of human (a) and murine (b) CLEC7A in immune cells was analyzed. c–e Splenocytes isolated from C57BL/6 mice were treated with 0, 5, 50, or 500 µg/mL GFPBW1 for 48 h. CD80, CD86 and MHC II expression on DCs (c), macrophages (d), and B cells (e) was determined by flow cytometry (n = 5). THP-1 cells were treated with 0, 5, 50, or 500 µg/mL GFPBW1 for 24 h. TNF-α (f) and IL-6 (g) in culture supernatants were detected by ELISA (n = 4). The data are presented as the mean ± SEM. *P < 0.05; ***P < 0.001; determined by one-way ANOVA followed by Tukey’s multiple comparisons test.
Next, we analyzed the effects of 0, 5, 50, and 500 µg/mL GFPBW1 on the secretion of TNF-α and IL-6 in human monocyte THP-1 cells by ELISA. The results demonstrated that GFPBW1 may increase TNF-α and IL-6 release from THP-1 cells in a dose-dependent manner (Fig. 1f, g). For instance, the three doses of GFPBW1 increased TNF-α secretion by 4.47-fold, 40.85-fold, and 49.28-fold, respectively. These results demonstrate that GFPBW1 could act on APCs to promote their activation and maturation, resulting in an increase in antigen-presenting function and innate immune effects.
GFPBW1 promotes OVA protein-induced humoral immune responses
APCs play a crucial role in vaccine immunization, which is a prerequisite for vaccines to stimulate protective humoral and cellular immunity; therefore, agonists of APCs are frequently developed for vaccine adjuvants. As GFPBW1 can activate three types of APCs, we next asked whether GFPBW1 has a potential adjuvant effect. To address this issue, we used the model antigen OVA protein to evaluate the adjuvant effect of GFPBW1 on OVA-induced immune responses. C57BL/6 mice were immunized intramuscularly on Days 0 and 14 with saline, OVA, OVA + GFPBW1 (50 µg per mouse), or OVA + GFPBW1 (300 µg per mouse) (Fig. 2a). ELISA was used to determine the level of anti-OVA IgG in mouse serum from 7 days after the first immunization to 21 days after the second immunization. An obvious increase in the anti-OVA IgG antibody level was observed from 14 days after the first immunization to 21 days after the second immunization in the OVA + GFPBW1 (50 or 300 µg per mouse) groups in a dose-dependent manner compared with that in the OVA group (Fig. 2b–e). In addition, GFPBW1 elevated the serum titer of anti-OVA IgG to a maximum of 1:409,600, compared with a maximum of 1:6,400 in the OVA-alone immunization group, suggesting that GFPBW1 exerted a significant adjuvant effect by promoting the generation of OVA-specific humoral immune responses.
a Vaccination schedule. C57BL/6 mice were immunized twice with saline, OVA, or OVA + GFPBW1 (50 or 300 µg per mouse) at two-week intervals between the 1st and 2nd immunizations (n = 5). Serum collection was conducted on Days 7, 14, 21, 28, and 35. b–e Anti-OVA IgG levels were determined by ELISA. f–i IgG1, IgG2a, IgG2b and IgG3 levels in the serum of immunized C57BL/6 mice on Day 14 after the second immunization (n = 5). Antibody levels were measured in 400-fold diluted serum. The data are presented as the mean ± SEM. **P < 0.01; ***P< 0.001; determined by one-way ANOVA followed by Tukey’s multiple comparisons test.
GFPBW1 assists the antigen in producing both Th1- and Th2-type immunity
Different IgG antibody isotypes were used to evaluate the type of T helper (Th) response. The predominant presence of IgG2a/IgG2b/IgG3 or IgG1 antibodies suggests a Th1 or Th2 response, respectively. To further evaluate the possible effect of GFPBW1 on immune response bias, the levels of IgG antibody subtypes were distinguished on Day 14 after the second immunization. Compared to those in the OVA group, remarkable increases in anti-OVA-specific IgG1, IgG2a, IgG2b, and IgG3 levels were observed in the OVA + GFPBW1 group (Fig. 2f–i), indicating that GFPBW1 amplified both Th1 and Th2 immune responses. In particular, GFPBW1 was more effective at inducing IgG1 and IgG2b than at inducing IgG2a and IgG3 (Fig. 2f–i). In addition, we also asked whether GFPBW1, a larger polysaccharide molecule, could promote the local retention of antigen at the injection site, thereby achieving antigen retardation. We observed the retention of Cy5.5-labeled OVA antigen at the injection site by small animal in vivo imaging, and at seven consecutive time points from 0 to 24 h, GFPBW1 did not retard OVA antigen release, suggesting that the immune response-promoting effect of GFPBW1 is not dependent on this mechanism (Supplementary Fig. S3). These results suggested that GFPBW1 could be used as a vaccine adjuvant to induce both Th1 and Th2 immune responses and thus may promote both cellular and humoral immune responses.
Safety evaluation of GFPBW1 as a vaccine adjuvant
For clinical application, the safety of an adjuvant is vital. First, we performed a preliminary evaluation of the safety of GFPBW1 in C57BL/6 and BALB/c mice (Fig. 3a–d). We immunized C57BL/6 or BALB/c mice by intramuscular injection on Days 0 and 14 in the following groups: saline, OVA protein, OVA + GFPBW1 (10 μg per mouse), OVA + GFPBW1 (50 μg per mouse), and OVA + GFPBW1 (300 μg per mouse). According to the body weight change curves, there was no significant difference in the body weight of the mice among the groups, and none of them experienced weight loss (Fig. 3a, c). In the three groups of GFPBW1-treated mice, even in the OVA + GFPBW1 (300 μg per mouse) group, the temperature fluctuations were within the normal range and were not significantly different from those in the saline group or the OVA group, as shown by the body temperature measurements (Fig. 3b, d). Cage-side observation of immunized mice did not reveal any abnormalities.
Body weight and body temperature curves of C57BL/6 mice (a, b; n = 5) and BALB/c mice (c, d; n = 5) twice immunized with saline, OVA, or OVA + GFPBW1 (10, 50 or 300 µg/mouse). e H&E staining of the hearts, livers, spleens, lungs, kidneys, and injection sites of C57BL/6 mice (n = 5) immunized with saline, OVA, or OVA + GFPBW1 (300 µg per mouse). The data are presented as the mean ± SEM.
A slight increase in the number of neutrophils according to hematology analysis was observed in the OVA + GFPBW1 (50 and 300 μg per mouse) groups (Supplementary Fig. S4), which was within the normal background range and showed no dose-dependent response; therefore, this change was considered to be a non-adverse effect. None of the other hematological parameters was significantly altered compared to those of the saline injection control group.
Furthermore, the major organs of the immunized mice, including the heart, liver, spleen, lungs, and kidneys, as well as the injection site, were dissected and collected after the mice were sacrificed, after which H&E staining was conducted (Fig. 3e). Histopathological analysis revealed no obvious pathological injury or abnormality in the major organs or injection sites of the mice after immunization with OVA + GFPBW1 (300 μg per mouse) compared with saline injection. Moreover, the hematological parameters, such as leukocyte and erythrocyte counts, of the mice in the OVA + GFPBW1 (300 μg per mouse) group were within the normal range for each of the 20 indices (Supplementary Fig. S4). In addition, to evaluate the safety of a higher dose and a greater frequency of immunization, C57BL/6 mice were immunized with saline, OVA, or OVA + GFPBW1 (1000 µg per mouse) on Days 0, 3, 6, and 13 and euthanized on Day 14 for microscopic diagnosis, and the results revealed no histologic lesions in major organs (heart, liver, spleen, lung, kidney) or the injection site (Supplementary Fig. S5).
These results suggest that GFPBW1 has a favorable safety profile in vivo and could be further evaluated for adjuvant development and application.
GFPBW1 could be used to enhance the efficacy of aluminum adjuvants
Aluminum adjuvant has been used clinically for nearly a century, and it is still the most widely used adjuvant in vaccines. However, aluminum adjuvants usually induce Th2-type immune responses in vivo, with less induction of Th1-type immunity. Consequently, we evaluated the coadjuvant effect of GFPBW1 and aluminum adjuvant. We immunized C57BL/6 mice (Fig. 4a) with the model antigen OVA protein via intramuscular injection on Days 0 and 14 in the saline, OVA protein, OVA + alum, OVA + alum + GFPBW1 (50 μg per mouse), and OVA + alum + GFPBW1 (300 μg per mouse) groups. The levels of anti-OVA IgG in mouse serum were detected by ELISA from 7 days after the first immunization to 21 days after the second immunization. Compared with those in the OVA + alum group, anti-OVA IgG antibody levels increased in a dose-dependent manner in the OVA + alum + GFPBW1 group at 14 days after the first immunization (Fig. 4b, e).
a Immunization schedule. C57BL/6 mice were immunized twice with saline, OVA, OVA + alum, or OVA + alum + GFPBW1 (50 or 300 µg per mouse) at two-week intervals between the 1st and 2nd immunizations (n = 5). Serum collection was conducted on Days 7, 14, 21, 28, and 35. b–e IgG antibody levels in all serum samples collected on Days 14, 21, 28, and 35 were determined by ELISA. f–h IgG1, IgG2a, and IgG2b levels in the serum of immunized mice on Day 14 after the second immunization were also determined by ELISA. IgG1, IgG2a, and IgG2b antibody levels were measured in 400-fold diluted serum. The data are presented as the mean ± SEM. **P < 0.01; ***P < 0.001; determined by one-way ANOVA followed by Tukey’s multiple comparisons test.
Furthermore, anti-OVA antibody subtypes from immunized mice were analyzed. The aluminum adjuvant had significant IgG1 antibody induction but little induction of anti-OVA IgG2a and IgG2b antibody levels (Fig. 4g, h), which is also consistent with previous studies involving aluminum adjuvant. Notably, GFPBW1 did not increase anti-OVA IgG1 serum levels, whereas anti-OVA IgG2a and IgG2b serum levels increased substantially (Fig. 4f–h). The OD values of anti-OVA IgG2a and IgG2b were 3.89-fold and 7.94-fold greater, respectively, in the OVA + alum + GFPBW1 (300 μg per mouse) group than in the OVA + alum group. These data suggest that GFPBW1 acts as a coadjuvant for aluminum and that GFPBW1 can achieve the immunopotentiation of aluminum adjuvant and compensate for the lack of Th1 immunoinduction.
OVA + GFPBW1 prophylactic immunization completely inhibits B16-OVA tumor growth in mice
To further investigate GFPBW1 as an adjuvant in vaccine development, we assessed its immunopotentiating efficacy in a B16-OVA tumor model (Fig. 5a). Tumor size was examined, and tumor growth kinetics showed only weak tumor growth inhibition by OVA immunization compared to that in the saline group (Fig. 5b). All three groups immunized with OVA + GFPBW1 exhibited substantial dose-dependent inhibition of tumor growth. Specifically, OVA + GFPBW1 (10 μg per mouse) immunization prevented 80% of tumorigenesis in mice, whereas both OVA + GFPBW1 (50 μg per mouse) and OVA + GFPBW1 (300 μg per mouse) immunization achieved 100% tumorigenesis prevention (Fig. 5b). Consistent results were also observed from the mouse survival curves (Fig. 5c).
a Schematic diagram of animal immunizations. C57BL/6 mice were immunized twice in advance, with an interval of two weeks. After 6 weeks, the right subcutaneous inoculation of 5 × 105 B16-OVA melanoma tumor cells was performed. b Tumor growth curves are shown (n = 8). c Survival curves are shown (n = 8). d, e After 5 weeks, animals without tumors were again inoculated with 5 × 105 B16-OVA cells on the left side. Tumor growth curves (n = 7 or 8) and survival curves (n = 7 or 8) are shown. The data are presented as the mean ± SEM.
Furthermore, we performed tumor rechallenge experiments on mice with nongrowing tumors. Again, from the tumor growth kinetics and mouse survival curves (Fig. 5d, e), 100% tumor growth inhibition was achieved with all OVA + GFPBW1 (10 μg per mouse), OVA + GFPBW1 (50 μg per mouse), and OVA + GFPBW1 (300 μg per mouse), demonstrating that GFPBW1 as an adjuvant induced long-lasting immune memory and potent immune responses against the OVA antigen. These results suggest the potential of GFPBW1 as a prophylactic tumor vaccine adjuvant.
OVA + GFPBW1 therapeutic immunization suppresses B16-OVA tumor growth in mice
In contrast to preventive tumor vaccines, therapeutic tumor vaccines are associated with greater tumor burdens in patients. Currently, the development of therapeutic tumor vaccines is one of the hotspots in cancer immunotherapy. We therefore further investigated the efficacy of GFPBW1 as adjuvant in a therapeutic tumor vaccine using the B16-OVA tumor model (Fig. 6a). At 6 and 13 days after tumor bearing, the mice were immunized with saline, OVA protein, OVA + GFPBW1 (10 μg per mouse), OVA + GFPBW1 (50 μg per mouse), or OVA + GFPBW1 (300 μg per mouse). Tumor growth kinetics showed that OVA protein immunization had little effect on tumor growth, whereas all three OVA + GFPBW1 immunization groups exhibited dramatic tumor growth impediments in a significant dose-dependent manner (Fig. 6b). The tumor inhibition rate of OVA + GFPBW1 (300 μg per mouse) immunization was 47.73%. Analysis of mouse survival and individual tumor growth curves revealed that the adjuvant GFPBW1 halted tumor growth and increased mouse survival (Fig. 6c, d). These findings support the feasibility of GFPBW1 as an adjuvant for therapeutic cancer vaccines.
a Scheme of animal immunizations. C57BL/6 mice were inoculated with 5 × 105 B16-OVA tumor cells subcutaneously on the right side and immunized on Days 6 and 13. b Tumor growth curves are shown (n = 8). c Survival curves are shown (n = 8). d Individual growth curves are shown (n = 8). The data are presented as the mean ± SEM.
OVA + GFPBW1 treatment systematically alter the tumor immune microenvironment in the B16-OVA tumor model
To investigate the changes in the gene expression of GFPBW1 as an adjuvant, RNA sequencing of B16-OVA tumor tissues from C57BL/6 mice immunized with saline, OVA protein, GFPBW1 (300 μg per mouse), OVA + GFPBW1 (300 μg per mouse), or OVA + alum was also performed. Compared to the saline group, the GFPBW1 group exhibited differential gene expression, which was mainly enriched in immune system-related pathways, such as antigen processing and presentation, and interferon-gamma-mediated pathways (Supplementary Fig. S6a). This finding highlights the activating effect of GFPBW1 on APCs. Compared with the OVA protein-immunized group, the OVA + GFPBW1 group also exhibited similar pathways, especially pathways related to cytotoxic cell functions (Supplementary Fig. S6b). From the GO enrichment analysis between the OVA + GFPBW1 and OVA + alum groups, Th1-type immune response pathway genes were among the differentially expressed genes (Supplementary Fig. S6c) and were more abundant in the OVA + GFPBW1 group, which is consistent with the above findings, suggesting that GFPBW1 may be a promising adjuvant to alum for improving the balance between Th1 and Th2 immune responses.
Subsequent heatmap analysis of the immune pathways showed that antigen and adjuvant treatment systematically altered the tumor immune microenvironment. Compared to those in the OVA group, the genes highly expressed in the OVA + GFPBW1 group were enriched in antigen processing, MHC, B-cell functions, T-cell functions, NK cell functions, interferon and complement, among others (Fig. 7a). These highly expressed genes suggest that OVA + GFPBW1 can induce more immune cells to infiltrate the tumor than OVA treatment alone.
RNA sequencing analysis of tumor tissue from C57BL/6 mice (4 mice per group) bearing B16-OVA tumor cells and immunized on Days 6 and 13. a Heatmap showing that expression of immunity-related genes changed after immunization [defined as the log2 (fold change)]. b, c Immune cell infiltration analysis by ImmunCellAI. The data are presented as the mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; determined by one-way ANOVA followed by multiple comparisons test.
To further elucidate the mechanism of the effect of the adjuvant GFPBW1 and its difference from that of the aluminum adjuvant, we compared the intratumoral immune cell infiltration scores among the OVA, OVA + GFPBW1 and OVA + alum groups using the ImmuCellAI method. Compared to the OVA and OVA + alum groups, the OVA + GFPBW1 group had the highest overall infiltration score, indicating that GFPBW1 induced a “hot” tumor microenvironment (Fig. 7b). Among innate immune cells, the infiltration levels of DCs, including cDC2s and pDCs (Fig. 7b), were significantly increased in the OVA + GFPBW1 group, suggesting that GFPBW1 can activate APCs, thereby promoting their intratumoral accumulation and function. Among the T-cell subsets, the OVA + GFPBW1 group showed a significant increase in intratumoral T-cell infiltration, particularly of cytotoxic CD8+ T cells and memory CD8+ T cells (Fig. 7c), which is also consistent with the good antitumour activity observed with OVA + GFPBW1 administration. In addition, GFPBW1 significantly improved the intratumoral infiltration of plasma cells (Fig. 7c), suggesting a significant increase in the humoral immune response against tumor antigens.
Discussion
Cancer vaccines are promising tools for cancer immunotherapy. Adjuvants are needed to enhance the immune response to tumor-associated antigens (TAAs) and tumor-specific antigens (TSAs). Although several adjuvants and delivery systems are currently being investigated or have been approved, cancer vaccine development has been disappointing in clinical translation [1, 2]. A growing understanding of the tumor microenvironment and immune mechanism indicates the importance of improving not only immunogenicity but also overcoming the suppression of the innate immune system. Therefore, new generations of adjuvants should have promising abilities to activate the immune system, especially antigen-presenting cells (APCs). In recent years, continuous research on polysaccharides has revealed that many plant polysaccharides have good immunomodulatory effects and low toxicity [17]. In our previous research, we demonstrated that GFPBW1 (an extract of β-glucan from Grifola frondosa) exerts antitumour effects by activating macrophages through Dectin-1/Syk/NF-κB signaling [10]. Therefore, combined with our previous results, these findings indicate that the use of GFPBW1 as a cancer vaccine adjuvant has important research value.
To confirm the ability of GFPBW1 to induce the maturation and activation of APCs, spleen cells extracted from C57BL/6 mice were treated with serial concentrations of GFPBW1, and the activation or maturation markers CD80, CD86, and major histocompatibility complex II (MHC II) on DCs, macrophages and B cells were detected. Our experimental results indicated that in vitro, GFPBW1 could increase the expression of CD80, CD86 and MHC II on APCs in a dose-dependent manner. Moreover, GFPBW1 induced high levels of TNF-α and IL-6 in THP-1 cells, indicating that it can modulate Th1 and Th2 immune responses. According to the above results, we conclude that stimulating APC activation and maturation is one way for GFPBW1 to be used as an adjuvant.
Compared with OVA alone, OVA + GFPBW1 induced significantly greater OVA-specific antibody titers and antibody subtypes, including IgG1, IgG2a, IgG2b and IgG3, after two intramuscular immunizations. Interestingly, the significantly increased serum levels of IgG1 and IgG2b in the 50 μg per mouse group were comparable to those in the 300 μg per mouse group, while significantly increased serum levels of IgG2a and IgG3 were detected only in the 300 μg per mouse group, which is consistent with the dose-dependent effect of APC activation in vitro (at 50 μg/mL and 500 μg/mL dose levels). Additionally, in the subsequent in vivo tumor vaccine model, the efficacy in the 300 μg per mouse group was better than that in the 50 μg per mouse group. These results suggest that GFPBW1 can increase both Th1 and Th2 responses. Th1 and Th2 cells are responsible for controlling and eliminating intracellular and extracellular pathogens, respectively [18]. Adjuvants influence humoral and cellular immunity by inducing Th1/Th2-associated cytokines, which subsequently produce IgG2a, IgG2b and IgG3/IgG1 antibody isoforms [19]. Several polysaccharides have been proven to increase Th1/Th2 antigen-specific cellular and humoral responses [20]. These results are consistent with the in vitro results showing that GFPBW1 induces Th1/Th2 cytokines (TNF-α/IL-6) in THP-1 cells in vitro.
Interestingly, compared with the aluminum adjuvant, GFPBW1 in combination with aluminum significantly increased the levels of OVA-specific IgG2a and IgG2b but not those of IgG1. Aluminum is the earliest approved adjuvant in the clinic and induces a Th2-biased immune response [21]. GFPBW1 may compensate for the lack of Th1 immunoinduction by the aluminum adjuvant, making it a promising candidate as a coadjuvant to aluminum.
Polysaccharides, a class of natural polymers composed of glycosidically linked monosaccharides, are effective and biodegradable biomolecules with relatively low toxicity. In the present study, GFPBW1 was tolerated by immunized mice. A slight increase in the number of neutrophils according to the hematology analysis was observed in the OVA + GFPBW1 group, which was within the normal background range. These results suggested that GFPBW1 is safe in vivo and could be further evaluated for adjuvant development and application.
Various studies have emphasized the immune-modulating effects of natural polysaccharides from herbal medicine [22,23,24]. Interestingly, many crude polysaccharide extracts have been reported to have adjuvant effects [20, 25]. Acetylated dextran (Ac-DEX) has been used as an antigen adjuvant carrier to enhance humoral and cellular responses by inducing MHC I/MHC II antigen presentation [26] and enhancing humoral and cellular responses [27]. β-glucan can bind to Dectin-1 (a C-type lectin receptor, CRR) and other PRRs, such as complement receptor 3 and TLR4, on immune cells (NK cells, lymphocytes and myeloid cells) to promote B lymphocyte or T lymphocyte activation and cytokine production, thereby enhancing humoral and cellular responses [28,29,30]. In this study, we found that GFPBW1 (a β-glucan) promoted the activation of DCs and macrophages and enhanced OVA-specific humoral and cellular responses. The adjuvant effect of GFPBW1 is considered to be mediated by the Dectin-1 pathway; according to our previous work, GFPBW1 exerts antitumour effects via Dectin-1/Syk/NF-κB signaling [10].
Furthermore, we used model antigen OVA and B16-OVA melanoma cells to verify that GFPBW1, which completely inhibited tumor growth and prolonged animal survival, had positive effects on preventive tumor vaccines. Preventive vaccines for human papilloma virus (HPV) and hepatitis B virus (HBV) have received regulatory approval, and the incidence of related cancers has declined dramatically over the past few years [31, 32]. Preventive vaccines for other viruses are in the preclinical experimental stage. However, for cancer vaccines, therapeutic cancer vaccines are even more urgently needed in the clinic.
The development of therapeutic cancer vaccines has been disappointing, and many early-stage cancer vaccines show promise in preclinical models but often fail to translate into clinical efficacy [33]. The development of therapeutic cancer vaccines faces three major challenges: (1) low immunogenicity; (2) an established disease burden; and (3) an immunosuppressive tumor microenvironment [1, 34]. Appropriate immunocostimulants are required to deliver antigens, activate APCs, and increase the amplitude and function of T-cell responses [16]. In our previous study, GFPBW1 mediated tumor regression in ICR mice but not in immunodeficient nude mice [14], which indicates that the immune status of individuals significantly affects its modulatory effect. CD8+ T-cell and CD4+ T-cell responses are as important as adjuvants in assisting vaccine function. There are relatively few adjuvants capable of inducing strong CD8+ T-cell responses in humans, but this is critical to the protective effects of therapeutic cancer vaccines. Our previous research indicated that GFPBW1 can bind to dectin-1 to trigger NF-κB, which leads to the maturation of DCs and promotes downstream Th1, Th17 and CTL responses [29, 35]. Our results indicate that GFPBW1 exerts an adjuvant effect on a therapeutic vaccine by promoting the activation and maturation of APCs and subsequently increasing the infiltration of cytotoxic immune cells into the tumor environment, which contributes to its antitumour efficacy in a B16-OVA tumor-bearing mouse model. These findings suggest that GFPBW1 could be a promising candidate for further cancer vaccine development. In addition, the effectiveness of GFPBW1 in a therapeutic tumor vaccine model is not as good as that in a preventive tumor vaccine model. The possible reason is that the antitumour functions of DCs are severely impaired within an immunosuppressive tumor microenvironment [36]. GFPBW1 exerts an adjuvant effect by activating and maturing APCs; therefore, a state of tumor carriage prior to vaccination may significantly reduce the antitumour immune response to the OVA + GFPBW1 vaccine due to functional impairment of the APCs. However, further mechanisms need to be explored, which is also a limitation of our present results. We believe that further strategies, such as improved targeting of the tumor microenvironment and the construction of novel delivery systems, will improve the adjuvant effect of GFPBW1.
In conclusion, in this study, we reported for the first time that GFPBW1 could promote the maturation of APCs, enhance Th1/Th2 immune responses, support aluminum adjuvants, and act as an adjuvant for tumor vaccines. Moreover, this study also provides evidence that GFPBW1 could be applied in preventive and therapeutic tumor vaccines, which is a new application of this polysaccharide, rather than a direct tumor inhibitor. This work sheds light on the exploration of polysaccharides from herbal medicines or plants for adjuvant development.
Data availability
All data are available in the main text, the Supporting Information materials, or upon request from the corresponding author. Correspondence and requests for materials should be addressed to YFY, LKG, JHS, and KD.
References
Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21:360–78.
Lin MJ, Svensson-Arvelund J, Lubitz GS, Marabelle A, Melero I, Brown BD, et al. Cancer vaccines: the next immunotherapy frontier. Nat Cancer. 2022;3:911–26.
Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang Z, et al. The advantages of using traditional Chinese medicine as an adjunctive therapy in the whole course of cancer treatment instead of only terminal stage of cancer. Biosci Trends. 2015;9:16–34.
Zhang Y, Gu P, Wusiman A, Xu S, Ni H, Qiu T, et al. The immunoenhancement effects of polyethylenimine-modified Chinese Yam polysaccharide-encapsulated PLGA nanoparticles as an adjuvant. Int J Nanomed. 2020;15:5527–43.
Wang QJ, Meng XY, Zhu L, Xu YL, Cui WP, He XH, et al. A polysaccharide found in flowers can enhance cellular and humoral immunity in chickens. Int J Biol Macromol. 2019;130:213–9.
Wang YQ, Mao JB, Zhou MQ, Jin YW, Lou CH, Dong Y, et al. Polysaccharide from activates TLR4-mediated signaling pathways in macrophages and shows immune adjuvant activity in mice. Int J Biol Macromol. 2019;123:157–66.
Feng HB, Fan J, Song ZH, Du XG, Chen Y, Wang JS, et al. Characterization and immunoenhancement activities of polysaccharides. Carbohydr Polym. 2016;136:803–11.
Hwang SH, Shin MS, Yoon TJ, Shin KS. Immunoadjuvant activity in mice of polysaccharides isolated from the leaves of CA Meyer. Int J Biol Macromol. 2018;107:2695–700.
Liu X, Chen S, Liu H, Xie J, Hasan KMF, Zeng Q, et al. Structural properties and anti-inflammatory activity of purified polysaccharides from Hen-of-the-woods mushrooms (Grifola frondosa). Front Nutr. 2023;10:1078868.
Fang J, Wang Y, Lv X, Shen X, Ni X, Ding K. Structure of a β-glucan from Grifola frondosa and its antitumor effect by activating Dectin-1/Syk/NF-κB signaling. Glycoconj J. 2012;29:365–77.
Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, et al. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–8.
Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, et al. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med. 2002;196:407–12.
Mata-Martínez P, Bergón-Gutiérrez M, del Fresno C. Dectin-1 signaling update: new perspectives for trained immunity. Front Immunol. 2022;13:812148.
Carvalho A, Giovannini G, De Luca A, D’Angelo C, Casagrande A, Iannitti RG, et al. Dectin-1 isoforms contribute to distinct Th1/Th17 cell activation in mucosal candidiasis. Cell Mol Immunol. 2012;9:276–86.
Leibundgut-Landmann S, Osorio F, Brown GD, Reis e Sousa C. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood. 2008;112:4971–80.
Yu X, Long Y, Chen B, Tong Y, Shan M, Jia X, et al. PD-L1/TLR7 dual-targeting nanobody-drug conjugate mediates potent tumor regression via elevating tumor immunogenicity in a host-expressed PD-L1 bias-dependent way. J Immunother Cancer. 2022;10:e004590.
Sun B, Yu S, Zhao D, Guo S, Wang X, Zhao K. Polysaccharides as vaccine adjuvants. Vaccine. 2018;36:5226–34.
Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–93.
Romagnani S. Th1/Th2 cells. Inflamm Bowel Dis. 1999;5:285–94.
Wan X, Yin Y, Zhou C, Hou L, Cui Q, Zhang X, et al. Polysaccharides derived from Chinese medicinal herbs: A promising choice of vaccine adjuvants. Carbohydr Polym. 2022;276:118739.
Hogenesch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. 2012;3:406.
Zhang J, Chen Y, Zhang J, Wang Y, Liu Y. The Regulation of micro-organisms’ extra-cellular polysaccharides on immunity: a meta-analysis. Foods. 2022;11:1949.
Yang K, Chen J, Chen J, Wang Z, Song B, Li R, et al. The effect mechanism of polysaccharides inhibit tumor immune escape: A review. J Funct Foods. 2023;107:105638.
Kikete S, Luo L, Jia B, Wang L, Ondieki G, Bian Y. Plant-derived polysaccharides activate dendritic cell-based anti-cancer immunity. Cytotechnology. 2018;70:1097–110.
Zhang W, Zheng X, Cheng N, Gai W, Xue X, Wang Y, et al. Isatis indigotica root polysaccharides as adjuvants for an inactivated rabies virus vaccine. Int J Biol Macromolecules. 2016;87:7–15.
Broaders KE, Cohen JA, Beaudette TT, Bachelder EM, Fréchet JM. Acetalated dextran is a chemically and biologically tunable material for particulate immunotherapy. Proc Natl Acad Sci USA. 2009;106:5497–502.
Chen N, Gallovic MD, Tiet P, Ting JP, Ainslie KM, Bachelder EM. Investigation of tunable acetalated dextran microparticle platform to optimize M2e-based influenza vaccine efficacy. J Controlled Rel. 2018;289:114–24.
Brown GD, Gordon S. Fungal beta-glucans and mammalian immunity. Immunity. 2003;19:311–5.
Sahasrabudhe NM, Dokter-Fokkens J, de Vos P. Particulate beta-glucans synergistically activate TLR4 and Dectin-1 in human dendritic cells. Mol Nutr Food Res. 2016;60:2514–22.
Li N, Zhang Y, Han M, Liu T, Wu J, Xiong Y, et al. Self-adjuvant Astragalus polysaccharide-based nanovaccines for enhanced tumor immunotherapy: a novel delivery system candidate for tumor vaccines. Sci China Life Sci. 2024;67:680–97.
Buonaguro L, Cavalluzzo B, Mauriello A, Ragone C, Tornesello AL, Buonaguro FM, et al. Microorganisms-derived antigens for preventive anti-cancer vaccines. Mol Asp Med. 2023;92:101192.
Enokida T, Moreira A, Bhardwaj N. Vaccines for immunoprevention of cancer. J Clin Invest. 2021;131:e146956.
Paston SJ, Brentville VA, Symonds P, Durrant LG. Cancer vaccines, adjuvants, and delivery systems. Front Immunol. 2021;12:627932.
Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. npj Vaccines. 2019;4:7.
Baert K, Sonck E, Goddeeris BM, Devriendt B, Cox E. Cell type-specific differences in beta-glucan recognition and signalling in porcine innate immune cells. Dev Comp Immunol. 2015;48:192–203.
Del Prete A, Salvi V, Soriani A, Laffranchi M, Sozio F, Bosisio D, et al. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell Mol Immunol. 2023;20:432–47.
Acknowledgements
This work is supported by the National Natural Science Foundation of China (82341039), the National Key Research and Development Plan (2022YFC2304105), Shanghai Municipal Science and Technology Major Project, the Science and Technology Innovation Action Plan of Shanghai (22S11902100, 22140900500), and the National Science Foundation for Distinguished Young Scholars of China (32271332). This work was also partial supported by the Zhongshan Municipal Bureau of Science and Technology (CXTD2023010) and Department of Science and Technology of Guangdong Province (High-level Innovative Research Institute 2021B0909050003). We would like to thank colleagues from the Experiment Center for Science and Technology, Shanghai University of Traditional Chinese Medicine, and Shanghai Institute of Materia Medica, Chinese Academy of Sciences, who provided advice and technical support, thanks for the support of Anling Biomed (SuZhou) Co., Ltd for the animal experiment.
Author information
Authors and Affiliations
Contributions
YFY, LKG, JHS, KD, XH, YRL, WFL and JLL designed the experiments and analyzed the data. XH, JLL, and YRL performed the experiments and prepared the paper. XZ, QZ, WFL, HLL and GYH assisted in performing the experiments. All authors approved the final draft of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, X., Lu, Jl., Liao, Wf. et al. GFPBW1, a β-glucan from Grifola frondosa as vaccine adjuvant: APCs activation and maturation. Acta Pharmacol Sin (2024). https://doi.org/10.1038/s41401-024-01330-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-024-01330-8
- Springer Nature Singapore Pte Ltd.