Abstract
Purpose
To report the effects of a 12-week high-intensity interval training (HIIT) program on cardiometabolic biomarkers in patients with prostate cancer on active surveillance (AS) from the Exercise During Active Surveillance for Prostate Cancer (ERASE) Trial.
Methods
Fifty-two men with prostate cancer on AS were randomized to either an exercise (HIIT; n = 26) or usual care (UC; n = 26) group. The HIIT intervention consisted of progressive, supervised, aerobic HIIT at an intensity of 85 to 95% VO2peak for 28 to 40 min per session performed three times/week for 12 weeks. Blood samples were collected at baseline and postintervention to analyze cardiometabolic biomarkers. Analysis of covariance was used to examine between-group mean differences.
Results
Blood data were obtained from 49/52 (94%) participants at postintervention. Participants were aged 63.4 ± 7.1 years and 40% were obese. The HIIT group attended 96% of the planned exercise sessions. No significant between-group changes in weight were observed after the intervention. Compared to UC, HIIT significantly improved total cholesterol (−0.40 mmol/L; 95% confidence interval[CI], −0.70 to −0.10; p = 0.011), non-high-density lipoprotein-c (−0.35 mmol/L; 95% CI, −0.60 to −0.11; p = 0.006), insulin (−13.6 pmol/L; 95% CI, −25.3 to −1.8; p = 0.025), insulin-like growth factor (IGF)-1 (−15.0 ng/mL; 95% CI, −29.9 to −0.1; p = 0.048), and IGF binding protein (IGFBP)-3 (152.3 ng/mL; 95% CI, 12.6 to 292.1; p = 0.033). No significant differences were observed for fasting glucose, HbA1c, other lipid markers, IGFBP-1, adiponectin, and leptin.
Conclusions
The ERASE Trial showed that a 12-week aerobic HIIT program improved several cardiometabolic biomarkers in patients with prostate cancer on AS that may contribute to cardiovascular health benefits and potentially influence signaling pathways in the progression of prostate cancer. Further research is needed to confirm the effects of exercise on cardiometabolic markers in men with prostate cancer on AS and determine if these effects are associated with improved long-term clinical outcomes.
Similar content being viewed by others
Introduction
Low-to-moderate grade prostate cancers are often indolent and managed by active surveillance where the prostate tumors are not immediately treated with invasive modalities (e.g., surgery, radiation/hormonal therapies) but are regularly monitored for any sign of disease progression [1]. Active surveillance offers a multitude of advantages for patients diagnosed with prostate cancer, such as circumventing the deleterious side effects induced by treatment (e.g., urinary/bowel symptoms, sexual dysfunction). A subset of these patients may live decades without progression of their prostate cancers. Nevertheless, patients with early-stage prostate cancer have a high risk of developing comorbidities, where the most common cause of death among these patients is cardiovascular disease (CVD), followed by secondary cancers and other metabolic diseases [2, 3].
It is well established that increased levels of certain systemic blood biomarkers, such as concentrations of blood lipids and insulin, are associated with risks of cardiometabolic diseases and can be modified by physical activity [4]. Several meta-analyses of randomized controlled trials have proven the effects of exercise on positively modulating cardiometabolic biomarkers in cancer patients [5,6,7,8]. However, most of the previous trials focused on patients with breast cancer [6,7,8] or prostate cancer during androgen deprivation therapy (ADT) [5]. To date, several exercise studies have been conducted in the active surveillance setting [9,10,11,12], and one reported some preliminary effects of exercise on cardiometabolic biomarkers such as lowered serum lipid levels [9]. Our research group previously conducted the Exercise During Active Surveillance for Prostate Cancer (ERASE) trial [13], where high-intensity interval training (HIIT) improved cardiopulmonary fitness and biochemical progression of tumor [14] as well as psychological [15] and motivational [16] outcomes in patients with prostate cancer on active surveillance. Here, we report the effects of HIIT on cardiometabolic biomarkers. We hypothesized that, compared to usual care (UC), HIIT would positively modulate biomarkers related to cardiometabolic diseases in patients with prostate cancer undergoing active surveillance.
Methods
Study design and population
Detailed study methods of the ERASE Trial have been published elsewhere [13]. In brief, the ERASE Trial was a randomized controlled trial conducted at the University of Alberta, Edmonton, AB, Canada, examining the effects of a 12-week aerobic HIIT intervention in patients with prostate cancer on active surveillance. The trial was registered to clinicaltrials.gov (NCT03203460) and approved by the Health Research Ethics Board of Alberta–Cancer Committee (HREBA.CC-17-0248). All participants provided written consent for study participation and blood banking. Participants were recruited from the Kaye Edmonton Clinic in Edmonton, Alberta, Canada. Eligibility criteria included 18 years of age or above, having been diagnosed with prostate cancer and undergoing active surveillance, having no plan for any curative treatment at the time of recruitment, being medically cleared for performing cardiopulmonary fitness test and high-intensity aerobic training, and no participation in any structured vigorous-intensity exercise. Once patients agreed to participate in the study, they completed baseline assessments and were randomized either to HIIT or UC group. Following baseline testing, patients were randomly assigned to either the HIIT group or the UC group in a 1:1 ratio, utilizing a block randomization design with blocks of 4 or 6. The allocation sequence was created using computer-generated numbers and was concealed from the staff responsible for recruitment and initial assessments. Participants and those administering the interventions were aware of the group assignments. However, while outcome assessors knew the group assignments for health-related fitness evaluations, they were kept blinded to the biochemical progression outcomes.
Exercise intervention
Details of the study interventions have been described elsewhere [13]. The HIIT group received a supervised, three times a week, aerobic HIIT program for 12 weeks. Each HIIT session comprised 2 min of high-intensity exercise (workload corresponding to 85–95% VO2peak) followed by 2 min of light-intensity exercise recovery (workload corresponding to 40% VO2peak), with progression from 5 to 8 intervals resulting in 28 min to 40 min of exercise (including warm-up and cool-down for 5 min each), which has been found to be effective in improving cardiovascular outcomes in patients with chronic diseases [17,18,19]. Participants in the UC group were asked not to begin any structured high-intensity exercise during the intervention period (i.e., 12 weeks), and they were offered a 4-week HIIT program at our facility or a 12-week community-based exercise program after the intervention period [20].
Outcome measures
Fasting (≥12 h) blood samples were collected at the Kaye Edmonton Clinic Laboratory Services. Serum blood was drawn into 6.0 mL red top tubes, clotted for 30-60 min, and spun for 20 min at 2860 rpm with 0.75 mL of serum transferred into eight cryovials. Fasting glucose, HbA1c, triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and non-HDL-C were analyzed on fresh serum at the central processing laboratory and the results were available through medical records. Two additional 6.0 mL samples were collected in EDTA tubes for inflammatory cytokines and sent to the biochemistry lab in the Li Ka Shing Center for Health Research Innovation at the University of Alberta. Plasma and buffy coat samples were spun for 10 min at 2860 rpm within one hour of collection, and 1.0 mL of plasma was transferred to a secondary tube to each of the eight cryovials. Samples collected from all participants during baseline and postintervention were stored at −70 C. To minimize measurement error, all the samples were analysed simultaneously using paired assays plated in random order. Insulin, insulin-like growth factors (IGF)-1, IGF binding proteins (IGFBP)-1 and -3, adiponectin, and leptin were analyzed using a sandwich immunoassay on the Quantikine® ELISA Kits (R&D Systems, Minneapolis, MN, USA). Duplicate testing was performed with <10% coefficients of variation for all samples. The lab technicians were blinded to group assignment and appropriate standard and reference samples were included in each assay. Exercise behavior was assessed using the modified Godin Leisure-Time Exercise Questionnaire (GLTEQ) to obtain the amount of aerobic and resistance exercise per week [21].
Statistical analyses
The planned sample size was 66 (33 participants per group) to detect a between-group mean difference of 3.5 mL·kg−1·min−1 with an SD of 5.6 mL/kg/min on our primary outcome of VO2peak with 80% power using a two-tailed α < 0.05. This power was also sufficient for detecting standardized effect sizes of approximately d = 0.5 for the blood outcomes of the study. Missing values were not replaced due to low missing data ( < 5%). Extreme outliers that were greater than three times the interquartile range were removed [22]. Data normality was explored using skewness and kurtosis. Analysis of covariance was performed to determine significant between-group mean differences at postintervention adjusting for resistance exercise behavior. There was significantly different between groups and the baseline values of each outcome (HIIT group: 18 (42) min/week; usual care group: 44 (62) min/week). SPSS version 26 (SPSS Inc., Chicago, IL, USA) was used for statistical analyses.
Results
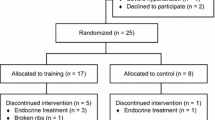
Participant flow in the ERASE trial has been reported elsewhere [14]. In brief, the recruitment was conducted from July 2018 to February 2020, and a total of 52 patients with prostate cancer undergoing active surveillance were randomized either to the HIIT (n = 26) or UC (n = 26) group. Post-intervention biomarker data were obtained from 24/26 (92%) participants in the HIIT group and 25/26 (96%) participants in the UC group. The HIIT group attended 96% of planned exercise sessions with 100% compliance with the exercise protocol.
Demographic, behavioral, and medical profiles of the ERASE participants are summarized in Table 1. Briefly, mean age was 63.4 ± 7.1 years, mean body mass index was 29.0 ± 4.7 kg/m2, 83% had one or more comorbidities including 60% with arthritis/arthralgia, 31% hypertension (systolic blood pressure≥140 mmHg or diastolic blood pressure≥90 mmHg), 17% dyslipidemia (LDL-C > 6.0 mmol/L), and 4% diabetes (fasting glucose≥7.0 mmol/L or HbA1c ≥ 6.5%). Moreover, 90% were T1c stage prostate cancer, 96% Gleason grade of 6, mean prostate-specific antigen (PSA) level was 7.3 ± 3.2, and mean time since starting active surveillance was 1.9 ± 2.2 years.
The changes in serum levels of fasting glucose, HbA1c, and lipid profiles from baseline to postintervention by randomized group are presented in Table 2. There were significant between-group differences in TC (adjusted between-group mean difference, –0.40 mmol/L; 95% confidence interval[CI], −0.70 to −0.10; p = 0.011) and non-HDL-C (adjusted between-group mean difference, −0.35 mmol/L; 95% CI, −0.60 to −0.11; p = 0.006). No significant between-group differences were found in fasting glucose, HbA1c, TG, HDL-C, and LDL-C. There was no significant difference in body weight changes between groups [14].
The changes in insulin, IGF-1, IGFBP-1, and IGFBP-3 by randomized group are presented in Table 3. Significant between-group differences were found for insulin (adjusted between-group mean difference, −13.6 pmol/L; 95% CI, −25.3 to −1.8; p = 0.025), IGF-1 (adjusted between-group mean difference, −15.0 ng/mL; 95% CI, −29.9 to −0.1; p = 0.048), and IGFBP-3 (adjusted between-group mean difference, -152.3 ng/mL; 95% CI, 12.6 to 292.1; p = 0.033). No significant between-group differences were found for IGFBP-1. The changes in adiponectin and leptin are presented in Table 4. No significant between-group differences were found in adiponectin and leptin.
Discussion
Our study examined the impact of HIIT on cardiometabolic biomarkers in patients with prostate cancer undergoing active surveillance. We found that HIIT improved certain lipid profiles, including TC, non-HDL-C, insulin, IGF-1, and IGFBP-3 levels compared to UC, whereas no significant differences were observed in fasting glucose, HbA1c, TG, HDL-C, LDL-C, IGFBP-1, adiponectin, and leptin levels.
Patients with prostate cancer, regardless of their treatment plan, have a 2.8 times higher risk of CVD mortality compared to the healthy male population [23]. Within the active surveillance group, the CVD mortality rate is three times higher than that of prostate cancer mortality [3]. The impacts of exercise on blood lipid metabolism, which are strong predictors of CVD [24], have been studied among patients with prostate cancer, mostly in hormonal treatment settings [5, 9]. However, a recent meta-analysis did not find significant improvements in any lipid parameters after exercise training in patients with prostate cancer [5]. Similarly, one preliminary study focusing on patients with prostate cancer on active surveillance (Prostate Cancer Novel Therapy; PANTERA) did not observe a trend towards a positive effect of the physical activity intervention on lipid biomarkers [9]. Our study, in contrast, found significant reductions in TC and non-HDL-C in the exercise group compared to the UC group. Particularly, the mean between-group difference of −0.40 mmol/L in TC in our study exceeded the minimal clinically important difference (MCID; 0.26 mmol/L).[REF] Although the MCID for non-HDL-C has not been established, the mean difference of −0.35 mmol/L in non-HDL-C, which is calculated by subtracting HDL from TC, may be deemed meaningful, considering the MCIDs for TC (0.26 mmol/L) and HDL (0.10 mmol/L).
A possible explanation for these discrepant findings is the mode and intensity of exercise regimens. Changes in systemic cholesterol levels are more susceptible to higher intensity aerobic exercise [25], whereas a low-dose community-based physical activity program might not have exerted significant changes in blood lipids compared to the HIIT aerobic intervention in our study. Other recent randomized controlled trials conducted in breast [26,27,28] and testicular [29] cancer survivors that incorporated aerobic HIIT also reported significant improvements in serum cholesterol levels. Our findings imply that higher-intensity aerobic exercise training (e.g., HIIT) may have a greater contribution to lowering risks of CVD by improving TC and non-HDL-C in patients with prostate cancer during active surveillance. It is important to note that the lipid profiles of the patients in our trial were largely within the normal or desirable ranges and there might have been a ceiling or floor effects on changes in these outcomes. Therefore, our findings may only apply to relatively healthy men with a low-to-moderate cardiovascular risk profile. It is possible that the intervention effects in our study might have been stronger if these men were at a higher risk of CVD, however, further research is needed to focus on patients with higher risks of CVD. Moreover, research is needed to establish which exercise mode and dose is most effective for improving blood lipids and whether the improvements in such biomarkers could lower the long-term incidences of CVD in this cancer population.
We did not observe differences in glucose and HbA1c between the HIIT and UC groups. Previous exercise trials found improvements in fasting blood glucose in men with prostate cancer undergoing ADT; [5] however, consistent with our study findings, the PANTERA study in active surveillance patients did not find an improvement in HbA1c [9]. It is unclear why our exercise intervention did not improve metabolic markers of glucose regulation as metabolic markers [30], although it may be partially due to the study population with generally normal glucose levels. Patients with prostate cancer on active surveillance do not experience hormonal treatment-induced metabolic dysregulation, where exercise may not be expected to further improve its outcomes.
Our study found significant between-group differences in insulin and certain IGF-axis biomarkers (i.e., IGF-1 and IGFBP-3) following the HIIT intervention. Specifically, the reduction in insulin levels in our study is consistent with meta-analyses in patients with breast cancer [7, 31] or any type of cancer [8]. However, unlike the previous reports suggesting weight reductions may play a key role in improving insulin levels [7], our intervention did not yield weight changes. Consequently, reductions in insulin levels in our study might be attributed directly to the HIIT intervention, independent of weight loss.
On another note, in comparison with studies conducted in prostate cancer patients, no significant effects of exercise on insulin levels was found in a recent meta-analysis in patients with prostate cancer on ADT [5]. Although this non-significant result is likely due to inconsistent findings from different studies and the heterogeneity of intervention details and treatment status, the authors underscored that the improvements in insulin sensitivity were found mostly in studies that provided high-intensity exercise training, which shows a similar trend to other cardiometabolic biomarkers.
Furthermore, the reduction in insulin levels induced by exercise may have contributed to the observed decrease in IGF-1 and increase in IGFBP-3 in our study. Insulin plays a critical role in stimulating the production of IGF-I at a cellular level and is a major regulator of IGFBP synthesis [32]. Importantly, it is established that IGF-axis is involved in prostate cancer development and its signaling pathways [33, 34], which suggests that the decrease in IGF-1 and the increase in IGFBP-3 may have contributed to the suppression of biochemical progression of prostate cancer (i.e., the LNCaP cell line growth) in our trial [14]. In support of this theory, in the study by Ngo et al., a short-term (i.e., 11 days) exercise and dietary intervention in men who were overweight or obese also modulated IGF-1 and IGFBP-1, which contributed to the suppression of LNCaP cell growth in serum [35, 36]. It is notable that the non-significant changes in IGFBP-1 in our study were understandable given that IGFBP-1 is nutritionally regulated [37], where our intervention did not have dietary components and did not induce weight changes. Similarly, it is plausible that we did not find significant changes in adiponectin and leptin in our study because they are largely regulated by fat tissue or nutritional metabolism [38]. Therefore, it is possible that our higher-intensity exercise intervention (i.e., HIIT) modulated circulating levels of insulin, IGF-1, and IGFBP-3 which might have played a role in the reduction in prostate cancer cell growth. This question warrants further investigation.
Strengths of our study include the randomized controlled trial design in a novel cancer population, the high-intensity exercise intervention that might have exerted greater physiological responses and thereby greater changes in cardiometabolic biomarkers, and the high study follow-up and intervention adherence rates. Limitations include potential recruitment bias (selection bias), a baseline imbalance in resistance exercise participation between the groups, other medications that participants might have been taking during the intervention period, and statistical analyses on multiple biomarkers that might have increased the probability of chance findings.
In conclusion, the ERASE Trial provides the first report of the effects of HIIT on cardiometabolic biomarkers in men with prostate cancer on active surveillance. Our findings of lower values for some lipid parameters and insulin-related hormones/growth-factors suggest that a 12-week high-intensity aerobic exercise training may lower the risks of CVD and impact metabolic signaling pathways that might have suppressed prostate cancer progression. This highlights the potential clinical importance of high-intensity exercise and the implication for its translation into practice in this oncology setting. Nevertheless, larger trials are needed to further determine the long-term effects of exercise on disease-related biomarkers and clinical events in patients with prostate cancer undergoing active surveillance.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request. Access to the data will be provided following institutional and ethical guidelines, and appropriate data use agreements must be signed.
References
Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol. 2011;29:3669–76.
Riihimäki M, Thomsen H, Brandt A, Sundquist J, Hemminki K. What do prostate cancer patients die of? The Oncologist. 2011;16:175.
Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N. Engl J Med. 2016;375:1415–24.
Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:815–40.
Bigaran A, Zopf E, Gardner J, La Gerche A, Murphy DG, Howden EJ, et al. The effect of exercise training on cardiometabolic health in men with prostate cancer receiving androgen deprivation therapy: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2021;24:35–48.
Meneses-Echavez JF, Correa-Bautista JE, Gonzalez-Jimenez E, Schmidt Rio-Valle J, Elkins MR, Lobelo F, et al. The effect of exercise training on mediators of inflammation in breast cancer survivors: a systematic review with meta-analysis. Cancer Epidemiol Biomark Prev. 2016;25:1009–17.
Kang DW, Lee J, Suh SH, Ligibel J, Courneya KS, Jeon JY. Effects of exercise on insulin, IGF axis, adipocytokines, and inflammatory markers in breast cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomark Prev. 2017;26:355–65.
Wang Y, Jin B, Paxton RJ, Yang W, Wang X, Jiao Y, et al. The effects of exercise on insulin, glucose, IGF-axis and CRP in cancer survivors: Meta-analysis and meta-regression of randomised controlled trials. Eur J Cancer Care (Engl). 2020;29:e13186.
Bourke L, Stevenson R, Turner R, Hooper R, Sasieni P, Greasley R, et al. Exercise training as a novel primary treatment for localised prostate cancer: a multi-site randomised controlled phase II study. Sci Rep. 2018;8:8374.
Van Blarigan EL, Kenfield SA, Olshen A, Panchal N, Encabo K, Tenggara I, et al. Effect of a home-based walking intervention on cardiopulmonary fitness and quality of life among men with prostate cancer on active surveillance: the active surveillance exercise randomized controlled trial. Eur Urol Oncol. 2024;7:519–26.
McIntosh M, Opozda M, Galvão DA, Chambers SK, Short CE. Identifying the exercise-based support needs and exercise programme preferences among men with prostate cancer during active surveillance: a qualitative study. Eur J Oncol Nurs. 2019;41:135–42.
Moon C, Gallegos AM, Sheikh B, Kumar P, Liss M, Patel DI. Pilot study on the impact of a home-based exercise program on inflammatory cytokines and quality of life in men with prostate cancer under active surveillance. Cancer Control. 2022;29:10732748221130964.
Kang DW, Fairey AS, Boule NG, Field CJ, Courneya KS. Exercise duRing active surveillance for prostatE cancer-the ERASE trial: a study protocol of a phase II randomised controlled trial. BMJ Open. 2019;9:e026438.
Kang DW, Fairey AS, Boule NG, Field CJ, Wharton SA, Courneya KS. Effects of exercise on cardiorespiratory fitness and biochemical progression in men with localized prostate cancer under active surveillance: the ERASE randomized clinical trial. JAMA Oncol. 2021;7:1487–95.
Kang DW, Fairey AS, Boule NG, Field CJ, Wharton SA, Courneya KS. A randomized trial of the effects of exercise on anxiety, fear of cancer progression and quality of life in prostate cancer patients on active surveillance. J Urol. 2022;207:814–22.
Kang DW, Boulé NG, Field CJ, Fairey AS, Courneya KS. Effects of supervised high-intensity interval training on motivational outcomes in men with prostate cancer undergoing active surveillance: results from a randomized controlled trial. Int J Behav Nutr Phys Act. 2022;19:126.
Cardozo GG, Oliveira RB, Farinatti PT. Effects of high intensity interval versus moderate continuous training on markers of ventilatory and cardiac efficiency in coronary heart disease patients. ScientificWorldJournal. 2015;2015:192479.
Nybo L, Sundstrup E, Jakobsen MD, Mohr M, Hornstrup T, Simonsen L, et al. High-intensity training versus traditional exercise interventions for promoting health. Med Sci Sports Exerc. 2010;42:1951–8.
Warburton DE, McKenzie DC, Haykowsky MJ, Taylor A, Shoemaker P, Ignaszewski AP, et al. Effectiveness of high-intensity interval training for the rehabilitation of patients with coronary artery disease. Am J Cardiol. 2005;95:1080–4.
Alberta Cancer Exercise (ACE) Program (https://www.albertacancerexercise.com).
Amireault S, Godin G, Lacombe J, Sabiston CM. The use of the Godin-Shephard Leisure-Time Physical Activity Questionnaire in oncology research: a systematic review. BMC Med Res Methodol. 2015;15:60.
Ghasemi A, Zahediasl S. Normality tests for statistical analysis: a guide for non-statisticians. Int J Endocrinol Metab. 2012;10:486–9.
Luo Z, Chi K, Zhao H, Liu L, Yang W, Luo Z, et al. Cardiovascular mortality by cancer risk stratification in patients with localized prostate cancer: a SEER-based study. Front Cardiovasc Med. 2023;10:1130691.
Emerging Risk Factors C, Di Angelantonio E, Gao P, Pennells L, Kaptoge S, Caslake M, et al. Lipid-related markers and cardiovascular disease prediction. JAMA. 2012;307:2499–506.
Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 2014;44:211–21.
Lee K, Tripathy D, Demark-Wahnefried W, Courneya KS, Sami N, Bernstein L, et al. Effect of aerobic and resistance exercise intervention on cardiovascular disease risk in women with early-stage breast cancer: a randomized clinical trial. JAMA Oncol. 2019;5:710–4.
Gonzalo-Encabo P, Christopher CN, Lee K, Normann AJ, Yunker AG, Norris MK, et al. High-intensity interval training improves metabolic syndrome in women with breast cancer receiving Anthracyclines. Scand J Med Sci Sports. 2023;33:475–84.
Isanejad A, Nazari S, Gharib B, Motlagh AG. Comparison of the effects of high-intensity interval and moderate-intensity continuous training on inflammatory markers, cardiorespiratory fitness, and quality of life in breast cancer patients. J Sport Health Sci. 2023;12:674–89.
Adams SC, DeLorey DS, Davenport MH, Stickland MK, Fairey AS, North S, et al. Effects of high-intensity aerobic interval training on cardiovascular disease risk in testicular cancer survivors: A phase 2 randomized controlled trial. Cancer. 2017;123:4057–65.
Norton L, Norton K, Lewis N. Exercise training improves fasting glucose control. Open Access J Sports Med. 2012;3:209–14.
Han JK, Kim G. Role of physical exercise in modulating the insulin-like growth factor system for improving breast cancer outcomes: A meta-analysis. Exp Gerontol. 2021;152:111435.
Phillips LS, Harp JB, Goldstein S, Klein J, Pao CI. Regulation and action of insulin-like growth factors at the cellular level. Proc Nutr Soc. 1990;49:451–8.
Gennigens C, Menetrier-Caux C, Droz J. Insulin-like growth factor (IGF) family and prostate cancer. Crit Rev Oncol/Hematol. 2006;58:124–45.
Chan JM, Stampfer MJ, Ma J, Gann P, Gaziano JM, Pollak M, et al. Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. J Natl Cancer Inst. 2002;94:1099–106.
Ngo TH, Barnard RJ, Tymchuk CN, Cohen P, Aronson WJ. Effect of diet and exercise on serum insulin, IGF-I, and IGFBP-1 levels and growth of LNCaP cells in vitro (United States). Cancer Causes Control. 2002;13:929–35.
Ngo TH, Barnard RJ, Leung PS, Cohen P, Aronson WJ. Insulin-like growth factor I (IGF-I) and IGF binding protein-1 modulate prostate cancer cell growth and apoptosis: possible mediators for the effects of diet and exercise on cancer cell survival. Endocrinology. 2003;144:2319–24.
Collett-Solberg PF, Cohen P. The role of the insulin-like growth factor binding proteins and the IGFBP proteases in modulating IGF action. Endocrinol Metab Clin North Am. 1996;25:591–614.
Stofkova A. Leptin and adiponectin: from energy and metabolic dysbalance to inflammation and autoimmunity. Endocr Regul. 2009;43:157–68.
Funding
This study was supported by the Canadian Institutes of Health Research (No. 389507) and Prostate Cancer Canada (No. D2017-1820).
Author information
Authors and Affiliations
Contributions
DK and KC conceptualized and designed the study, secured funding, and supervised the project. DK, KC, and AF were responsible for patient recruitment and data collection. DK, KC, and NB designed and implemented the intervention of the study. DP and CF were responsible for biomarker collection, assays, and the interpretation of the biomarker outcomes. DK performed the statistical analysis and interpreted the results. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The trial was registered to clinicaltrials.gov (NCT03203460) and approved by the Health Research Ethics Board of Alberta—Cancer Committee (HREBA.CC-17-0248). Informed consent was obtained from all subjects. Our research involving human subjects, human material, or human data is in accordance with the Declaration of Helsink.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, DW., Field, C.J., Patel, D. et al. Effects of high-intensity interval training on cardiometabolic biomarkers in patients with prostate cancer undergoing active surveillance: a randomized controlled trial. Prostate Cancer Prostatic Dis (2024). https://doi.org/10.1038/s41391-024-00867-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41391-024-00867-3
- Springer Nature Limited