Abstract
ST2 functions as a receptor for the cytokine IL-33. It has been implicated in carcinogenesis. In this study, we sought to mechanistically determine how ST2 and IL-33 function to support cancer stem cell (CSC) activity and drive gastric cancer (GC) pathogenesis. ST2+ subpopulation spontaneously arose during gastric tumorigenesis. A thorough evaluation of ST2 and IL-33 expression in gastric tumors revealed that they show an overlapping expression pattern, notably in poor differentiated GC and metastasis foci. Moreover, their expression levels are clinically correlated to cancer progression. Using a genetic model of CSC-driven gastric carcinogenesis, ST2+ subpopulation displays increased tumorigenicity, chemoresistance and metastatic potentials through increased survival fitness endowed by an elevated MAPK-regulated Bcl-xL. The IL-33/ST2 axis enhances the self-renewal and survival of GC stem cells and organoids. Importantly, we observed a synergistic cooperation between IL-33/ST2 and the canonical Wnt pathway in transactivating Wnt-dependent transcription and supporting CSC activity, a partnership that was abrogated by inhibiting Bcl-xL. Concordant with this, ST2+ subpopulation was targeted by MEK1/2 and Bcl-xL-specific inhibitors. These findings establish ST2 as a functional CSC marker that fortifies the Wnt signal while availing a novel therapeutic strategy to suppress GC progression by targeting the IL-33/ST2/Bcl-xL signaling axis.
Similar content being viewed by others
Introduction
Gastric cancer (GC) presents one of the most aggressive forms of cancer and is the third leading cause of cancer-related deaths worldwide [1]. While surgical resection remains the mainstay of treatment for advanced and most early-stage GCs, its limitations surface as regional recurrences and/or distant metastasis [1]. Tumors are composed of a heterogeneous population of cells. It has been postulated that only a rare subpopulation of cells, often referred to as cancer stem cells (CSC), is responsible for sustaining tumorigenesis, establishing the heterogeneity inherent in tumors, giving rise to distant metastasis, and developing chemoresistance [2]. To identify and therapeutically target CSCs, cell surface markers specifically expressed in this subpopulation of cells, such as CD44, Lgr5 and aquaporin 5 (AQP5) have been explored [3,4,5].
We established a Cdh1F/+;Trp53F/F;Smad4F/F (hereafter ChetPS) mouse model that recapitulated human GC with distant metastasis [6, 7]. Two GC cell lines were established from the ChetPS mouse model, named S1 and its metastatic cousin S1M, that could develop tumors when transplanted into syngeneic miced [8]. Although S1 is highly malignant, only a small fraction of cells eventually acquired metastatic capabilities, while S1M exhibited increased CSC-like features, including enhanced tumorigenesis, metastatic potential, and chemoresistance [9]. Amongst the genes found highly expressed in S1M cells is Il1rl1, which encodes the protein suppression of tumorigenicity 2 (ST2). ST2 functions as a receptor for Interleukin 33 (IL-33), a member of the IL-1 family of cytokines [10]. ST2 exists in two main forms as a result of alternative splicing: a membrane-bound receptor (ST2L) as well as a soluble receptor (sST2) [10]. While ST2L transduces intracellular signaling upon IL-33 binding, sST2 acts as a decoy receptor and sequesters extracellular IL-33. ST2 is mainly expressed in immune cells, including T helper 2 cells, mast cells, regulatory T cells, group 2 innate lymphoid cells, CD8+ T cells, and natural killer cells. Thus, the role of IL-33/ST2 axis has been widely studied and established in inflammatory diseases [10].
Several recent studies have reported that the IL-33/ST2 pathway may contribute to tumor growth and progression in different human cancers. A majority of these studies have focused on the role of ST2-expressing tumor-infiltrating immune cells that fashion a tumor-promoting and immunosuppressive microenvironment [11,12,13,14]. In addition, IL-33/ST2 have been implicated in the gastric carcinogenesis. IL-33 from alternatively activated macrophages promoted spasmolytic polypeptide-expressing metaplasia (SPEM) in the stomach [15]. On the other hand, tumor-derived IL-33 activated mast cells that in turn promoted GC growth and tumor burden [16]. In yet another study, cancer-associated fibroblasts (CAF)-derived IL-33 reportedly accentuated GC metastasis via ST2L-dependent induction of EMT [14]. Thus, understanding the underlying molecular mechanisms and the effects of IL-33/ST2 axis on GC development and progression may provide valuable insights for devising therapeutic interventions to control the malignant behavior of GC. In the current study, we identified an ST2-positive (ST2+) subpopulation that drives the tumorigenic and metastatic potential of S1/S1M cells. We investigate the basis for the spontaneous occurrence of the ST2+ CSC-like population and found that IL-33 is often co-expressed leading to the activation of the MAPK/ERK pathway and increased Bcl-xL expression. Importantly, the IL-33/ST2 signal strongly augmented Wnt activity to enhance cancer stemness. Clinically, ST2/IL-33 expression is strongly correlated with high-grade undifferentiated human GC and distant metastasis. Importantly, we showed that inhibition of the MAPK/Bcl-xL pathway arrested tumor progression in vivo by specifically targeting ST2-expressing cells. Our data uncover a novel therapeutic strategy against chemoresistant and highly malignant CSC.
Results
ST2-positive subpopulation spontaneously arises during tumorigenesis
In line with previously published mRNA sequencing data [8], ST2 was found to be amongst the most differentially expressed gene between the CSC-like fraction of S1M mouse GC cells and parental S1 cells (Supplementary Table 1). To investigate its significance, surface expression of ST2 in S1 and S1M cells was analyzed by flow cytometry. Surface ST2 expression was much higher in S1M cells than in S1 cells or normal stomach organoids (Fig. 1A). Moreover, we observed the expression of IL-33, the ligand for ST2, was also markedly elevated (Fig. 1B). To investigate if these two genes are co-expressed, S1M cells were fractionated by FACS based on ST2 expression. This revealed that ST2-high S1M cells expressed higher levels of IL-33 than ST2-low population (Fig. 1C). Interestingly, IL-33 treatment further enhanced the expression of IL-33 and ST2 (Fig. 1D, E). These observations suggest that ST2 and IL-33 are co-expressed and potentially maintained as part of an autocrine or paracrine loop. To investigate if this relationship is at work in vivo, we performed in situ hybridization (ISH) analyses in place of IHC due to a lack of antibodies that could reliably detect ST2 and IL-33 in mouse tissues. S1M orthotopic tumors showed elevated expression of ST2 and IL-33 in a subset of cancer cells, indicating intratumoral heterogeneity and suggesting that the ST2/IL-33 signaling axis may be aberrantly activated during tumorigenesis (Fig. 1F).
A FACS analysis of ST2+ cells in normal mouse stomach organoids, S1 cells, and S1M cells. Right, the percentage of ST2+ cells. B RT-qPCR analysis of IL-33 mRNA expression in normal mouse stomach organoids, S1 cells and S1M cells. C RT-qPCR analysis of IL-33 mRNA expression in ST2-low and ST2- high S1M cells. D, E RT-qPCR analyses of IL-33 (D) and ST2 (E) mRNA expressions in S1M cells after treatment with vehicle or IL-33 (100 ng/ml) for 48 h. F–H Representative ISH images for ST2 and IL-33 mRNA expressions in S1M orthotopic allografts (F), ChetPS mouse primary GC tissues (G), and MNU and H. pylori-induced mouse primary GC tissues (H). I, J Quantification of IL-33- (I) and ST2-positive (J) cells in mouse stomach mucosa, MNU and H. pylori-induced intestinal GC, and ChetPS diffuse GC. All experiments were repeated at least three times with comparable results. Representative data for repeated experiments are shown in panels [B–D], and [E]. Individual dots in these figures represent technical replicates. The mean ± SD from more than three independent biological replicates are displayed in panel [A]. Individual dots in this figure represent biological replicates. Where statistical analyses were performed, the lines indicate the two groups analyzed by Student’s t-tests. *p < 0.05, Student’s t-tests. Error bars indicate mean ± SD.
IL-33 and ST2 are co-expressed in poorly differentiated gastric cancer and enriched during cancer progression
Furthermore, ISH analyses of serial sections of poorly differentiated gastric tumors of the ChetPS mouse model revealed restricted and overlapping expressions of ST2 and IL-33 in the tumor (Fig. 1G, I, J) [6], providing evidence for their coordinated expression in vivo. We further analyzed mouse GC tissues with a wide range of differentiation statuses derived from N-methyl-N-nitrosourea (MNU) and Helicobacter pylori-treated mice [17]. In these tumor tissues, ST2 and IL-33 expressions were restricted to poorly differentiated regions and seldom detected in differentiated regions (Fig. 1H, I, J). The restricted but overlapping distribution of ST2 and IL-33 observed in serial section staining raises the possibility that ST2 and IL-33 are concurrently expressed in a subset of cells within a tumor. To address this possibility, we conducted high-resolution multiplexed in situ hybridization analyses in the S1M and S1 orthotopic tumors and their metastatic foci (Fig. 2A). We confirmed that tumor cells expressed ST2 whereas mesenchymal cells did not (Fig. 2B). Of note, co-expression of ST2 and IL-33 are unequivocally detected in a subset of GC tumor cells (Fig. 2C). Interestingly, although the size of the ST2+ population between S1M and S1 is significantly different when in monolayer culture in vitro (Fig. 1A), their proportions are much more comparable in primary allografts (Fig. 2D, E). This observation is consistent with the notion that ST2+ cells are the tumor-initiating subpopulation and their frequency determines the tumorigenicity of S1 and S1M cells. Moreover, these data suggest that once a tumor is initiated, ST2+ cells are maintained in a significant proportion, fueled by host niche factors to sustain tumor growth, Notably, the proportion of IL-33/ST2 double-positive cells increased significantly in poorly differentiated tumor lesions and metastatic foci (Fig. 2D, E), consistent with a more malignant and phenotypically plastic state.
A Representative gross images of S1M orthotopic tumors showing liver metastases. Red dotted line, orthotopic tumor; yellow dotted line, liver with metastatic lesions. Scale bar = 1 cm. B In situ hybridization/immunohistochemistry double staining for ST2 (ISH) and E-cadherin or vimentin (IHC) of S1M orthotopic tumors. E-cadherin, an epithelial cell marker. Vimentin, a mesenchymal cell marker. Representative images showing co-expressing (ST2/E-cadherin) cells were provided. Vimentin-positive cells did not express ST2. Left images, negative control using negative control probe. C Representative ISH images for double-positive, single-positive, and double-negative areas of ST2 and IL-33 in S1M orthotopic tumors. D Representative ISH images for ST2 and IL-33 mRNA in S1M orthotopic tumors and metastases. Right, Proportion of ST2 and/or IL-33-positive areas in S1M orthotopic tumors and corresponding metastatic tumors. Total tumor areas from 3 orthotopically transplanted mice were analyzed. E Representative ISH images for ST2 and IL-33 mRNA in S1 orthotopic tumors and metastases. Right, Proportion of ST2 and/or IL-33-positive areas in S1 orthotopic tumors and corresponding metastatic tumors. Total tumor areas from 3 orthotopically transplanted mice were analyzed.
ST2 and IL-33 expression in human GC and their clinical significances
We next surveyed the expression of ST2 and IL-33 in GC cell lines published by the Cancer Cell Line Encyclopedia (CCLE) [18] and observed low ST2 expression (Fig. 3A, B). This was further clarified by our examination of ST2 expression in human GC cell lines, which revealed only a small proportion of ST2+ tumor cells (Fig. 3C, D and Supplementary Fig. 1A), consistent with our earlier observation in murine GC tissues and cells. To investigate the clinical significance of ST2 expression in GC tumor cells, IHC analysis of ST2 expression in human GC TMA slides was performed using an antibody that was validated against ST2-overexpressing SNU-484 xenografts (Supplementary Fig. 1B). As with human GC cell lines, the human GC tissues exhibited variegated ST2 expression (Fig. 3E, F). Importantly, high-grade GC tissues showed significantly higher ST2 expression levels in tumor cells than their low-grade counterparts (Fig. 3E, F).
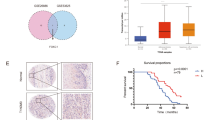
A ST2 mRNA expression levels in human GC cell lines from Cancer Cell Line Encyclopedia (CCLE) database. mRNA expression, RNA Seq RPKM. B IL-33 mRNA expression levels in human GC cell lines from Cancer Cell Line Encyclopedia (CCLE) database. mRNA expression, RNA Seq RPKM. C, D Flow cytometry analysis (D) of ST2+ cells in human GC cell lines, C the percentage of ST2+ cells; mean of 3 independent experiments. E Representative H&E and ST2 IHC images of human GC tissues using TMAs. F High ST2 expression was associated with high histological grade. Χ2 test was employed. G UMAP plot of 319,602 filtered cells in the adjacent non-cancer and gastric cancer (GC) tissue from 24 patients. H UMAP plots showing IL-33 expression of filtered cells in (G). I Correlation between ST2 and IL-33 expression levels in stomach adenocarcinoma tissues. Linear regression analyses were performed using cBioPortal on provisional datasets from TCGA, Firehose Legacy, Stomach Adenocarcinoma. J IL-33 mRNA expression levels in intestinal (n = 144), diffuse (n = 67) and signet ring (n = 8) human GC tissues from TCGA GC dataset. mRNA expression z-scores relative to diploid samples (RNA Seq V2 RSEM). K IL-33 mRNA expression levels according to metastasis stages in TCGA human GC samples. mRNA expression z-scores relative to diploid samples (RNA Seq V2 RSEM). L Kaplan–Meier plots for the overall survival of DGC patients according to IL-33 mRNA expressions in the TCGA dataset. mRNA expression z-scores relative to diploid samples (RNA Seq V2 RSEM). The survival difference between cohorts was assessed by the log-rank test. All experiments were repeated at least three times with comparable results. The mean ± SD from more than three independent biological replicates are displayed in panel [C]. Individual dots in this figure represent biological replicates. Where statistical analyses were performed, the lines indicate the two groups analyzed by Student’s t-tests. *p < 0.05, Student’s t-tests. Error bars indicate mean ± SD (J and K: means ± SEM).
In addition, we aimed to investigate IL-33 expression in clinical samples. However, due to the lack of a reliable antibody against IL-33 or low mRNA quality of commercially available TMA slides for ISH, IHC or ISH analyses could not be conducted. Therefore, we sought to establish the clinical significance of IL-33 expression via the analysis of single-cell RNA -sequencing (scRNA-seq) and bulk RNA sequencing data of human GC tissues from published datasets [18, 19]. The human GC single-cell transcriptomic profiles show that IL-33 was broadly expressed mainly in the epithelial cells of GC tissues (Fig. 3G, H and Supplementary Fig. 2). Moreover, based on the bulk RNA sequencing data from the TCGA-STAD dataset, both ST2 and IL-33 expressions showed a stronger positive correlation in poorly differentiated high-grade human GC tissues than in grade 1 and 2 GC tissues (Fig. 3I). The expression of IL-33 was significantly higher in diffuse-type GC tissues than in intestinal GC tissues, concordant with the more undifferentiated and phenotypically plastic nature of the former (Fig. 3J). Importantly, IL-33 mRNA expression level was significantly higher in patients with GC with distant metastases than in those without metastases (Fig. 3K). Moreover, high IL-33 expression is associated with lower overall survival among GC patients (Fig. 3L). These clinicopathological data provide evidence that the IL-33/ST2 signaling is a significant driver of human gastric carcinogenesis especially during malignant progression.
The IL-33/ST2 axis enhances the self-renewal and survival of GC stem cells
The elevated expression of ST2 and IL-33 in a tumorigenic subset of S1M cells and its restricted distribution in tumors implicate a role for IL-33/ST2 signaling in cancer stem cells. In support of this, ST2-high S1M cells resulted in larger and more abundant tumorspheres than ST2-low S1M cells (Fig. 4A). To investigate if the ST2+ CSCs were exclusively dependent on the specific mutations harbored by S1M cells (i.e., mutation in Cdh1, Trp53), the CT26 murine colorectal carcinoma cell line was studied as it harbors high mutation rates [20]. Flow cytometry analysis revealed that CT26 has about 3% ST2 positivity (Supplementary Fig. 3A). Concordant to our observation in S1M cells, CT26 ST2+ cells formed larger tumorspheres than ST2- cells (Supplementary Fig. 3B), suggesting that the role of ST2 in CSCs might not be restricted to GC cells harboring specific mutations.
A Sphere-forming assay using ST2-low and ST2-high S1M cells. Left, representative images of tumorspheres on 24-well low attachment plates. Right, the size of all tumorspheres (n = 3 wells) and the number of tumorspheres per well. B, C The effect of IL-33 (100 ng/ml) on S1M (B) and S1 (C) tumorsphere formation. Left, representative images of tumorspheres 5 days after treatment. Right, the size of all tumorspheres (n = 3 wells) and the number of tumorspheres per well. D Bcl-xL mRNA expressions in S1M and S1 cells after treatment with vehicle or IL-33 (100 ng/ml) for 48 h. S1M cells were starved in serum-free basal medium for 12 h prior to IL-33 treatment. E CD44 and Survivin mRNA expressions in S1M cells after treatment with vehicle or IL-33 (100 ng/ml) for 48 h. S1M cells were starved in serum-free basal medium for 12 h prior to IL-33 treatment. F Sphere-forming assay using ST2-low and ST2-high S1M cells. Left, the size of all tumorspheres (n = 3 wells). Right, the number of tumorspheres per well. G Trypan blue dye exclusion assay to measure S1M cells viability after 3 days of treatment with IL-33 (100 ng/ml) in serum-free basal medium under monolayer culture conditions. 1 × 104 cells were seeded on 24-well plates. H IL-33 and ST2 mRNA expressions in S1 tumorspheres and monolayer cells. I Representative images of p53 KO mouse stomach organoids after 3 days of treatment with vehicle or IL-33 (10 ng/ml) following ST2L or sST2 overexpression. Organoids were enzymatically dissociated into single cells and embedded in Matrigel supplemented with serum-free media containing IL-33. Right, the mean size of organoids. J Representative images of p53 KO mouse stomach organoids following ST2L overexpression after 4 and 8 days of treatment with vehicle or IL-33 (10 ng/ml). Right, the mean size at the indicated time points of all organoids. K Expression levels of stem cell markers (Lgr5, Aqp5, CD44, Sox2) and proliferation markers (c-Myc, cyclin D1) in control or ST2L-overexpressing p53 KO mouse stomach organoids. L Representative immunofluorescence staining images for Aqp5 (green), PCNA (red), and nuclei marker DAPI in control and ST2L-overexpressing p53 KO organoids represented in (G) after 8 days of treatment with IL-33 (10 ng/ml). M Representative IHC images for C-caspase-3 in organoids in (I). All experiments were repeated at least three times with comparable results. Representative data for repeated experiments are shown in panels [A–H], [J] and [K]. Individual dots in these figures represent technical replicates. The mean ± SD from more than three independent biological replicates are displayed in panel [I]. Individual dots in this figure represent biological replicates. Where statistical analyses were performed, the lines indicate the two groups analyzed by Student’s t-tests. *p < 0.05, Student’s t-tests. Error bars indicate mean ± SD.
Treatment with IL-33 increased the size of tumorspheres in S1M cells (Fig. 4B) but not in S1 cells (Fig. 4C), consistent with the low expression of ST2 in the latter, while indicating the functionality of the ST2/IL-33 pathway in the former. Bcl-xL is known to be a target gene in IL-33/ST2 signaling [21] and NF-κB pathway [22, 23]. IL-33 significantly induced Bcl-xL expression in S1M, but not in S1 cells (Fig. 4D). In S1M cells, IL-33 treatment also induced the expression of CD44 and Survivin that functionally contribute to gastric cancer stemness (Fig. 4E). However, treatment with IL-33 did not increase number of tumorspheres in S1M (Fig. 4B), which suggests that ST2 signaling may not be necessary for sphere initiation but rather for the self-renewal of CSCs within an established tumorsphere. Consistent with this, S1M cells formed larger tumorspheres than S1 cells, but there was no difference in the number of tumorspheres (Fig. 4F).
In contrast, exogenous IL-33 did not enhance the growth of S1M cells when cultured in monolayers, even in the absence of serum (Fig. 4G). In keeping with its greater relevance in the maintenance of CSC, the expression of IL-33 and ST2 mRNA were elevated when S1 cells were cultured as tumorspheres (Fig. 4H). Collectively, these results suggest that the IL-33/ST2 signaling is functionally involved in the self-renewal potential of mouse GC cells. To evaluate the effects of ST2 in untransformed gastric epithelial cells, we overexpressed ST2L or sST2 in p53 KO mouse stomach that ove organoids so as to minimize apoptosis during genetic manipulation (Supplementary Fig. 4A). Three days after single-cell dissociation and seeding in Matrigel, cells rexpressed ST2L, but not sST2, formed larger organoids than the control cells (Fig. 4H). Treatment with IL-33 further increased the sizes of ST2L-overexpressing organoids, but did not affect sST2-overexpressing organoids (Fig. 4I). Moreover, unlike control organoids that ceased to increase in size after 4 days, with increased accumulation of apoptotic cells, ST2L-overexpressing organoids continued to increase in size until day 6 post-seeding (Fig. 4J). Concordantly, ST2L-overexpressing organoids showed significantly higher expression of stem cell and proliferative markers (Fig. 4K). Of note, Aqp5 is a recently described gastric stem cell marker that is restricted to Lgr5+ stem cells in the stomach antrum [5]. In the p53 KO organoids, Aqp5+ cells were detected by immunofluorescent staining and these were more frequently stained for PCNA, compared with Aqp5- cells (Fig. 4L). Importantly, Aqp5 staining was enhanced when ST2L-overexpressing organoids were treated with IL-33 (Fig. 4L). Conversely, cleaved caspase 3-positive were less frequently detected in ST2L-overexpressing organoids than in control organoids (Fig. 4M), suggesting that ST2L may have anti-apoptotic activities. Together, these data indicate that ST2L promotes the self-renewal and survival of untransformed as well as cancerous gastric epithelial cells, which is enhanced by exogenous IL-33.
ST2-positive subpopulation possesses resistance to chemotherapy-induced cell death and anoikis
As CSCs are reportedly more resistant to chemotherapy-induced cell death and anoikis [24], we sought to determine if ST2 expression confers such resilience in GC cells. Mouse and human GC cell lines were treated with cisplatin to induce irreversible apoptosis, where an enrichment of ST2+ cells was observed post-treatment, indicating greater chemoresistance (Fig. 5A, B). Accordingly, ST2- cells were more frequently stained for Annexin V than ST2+ cells following cisplatin treatment (Fig. 5C).
A, B Flow cytometry analyses of ST2+ S1 (A) and ST2+ MKN-74 (B) cells after cisplatin exposure at indicated concentrations for 36 h in monolayer cultures. C Flow cytometry analysis of annexin V-stained ST2− and ST2+ S1 cells after 2 µM cisplatin exposure for 36 h in monolayer cultures. Dead cells were excluded with 7-AAD staining. D CellTiter-Glo® 2.0 Assay of ST2 KO and control S1M cells cultured in monolayer cultures after cisplatin treatment for 48 h. 0.5 × 104 single cells were seeded per well in 24-well plates with cisplatin at indicated concentrations. To determine ATP concentration, luminescence signal was measured using GloMax® 20/20 Luminometer. E Annexin V apoptosis assay in control and ST2 knockout (KO) S1M cells after cisplatin treatment (r 2 µM) for 36 h in monolayer cultures. D, dead cells; E, early apoptotic cells; L, late apoptotic cells. F Flow cytometry measurement of ST2+ S1 cells following 36 h culture under monolayer and low attachment conditions. G Flow cytometry measure of annexin V staining in ST2− and ST2+ S1 cells following 36 h of culture under low attachment condition. Dead cells were excluded with 7-AAD staining. H Annexin V apoptosis assay in control and ST2 KO S1M cells following 24 h of culture under a low attachment condition. All experiments were repeated at least three times with comparable results. Representative data for repeated experiments are shown in panel [D]. Individual dots in this figure represent technical replicates. Where statistical analyses were performed, the lines indicate the two groups analyzed by Student’s t-tests. *p < 0.05, Student’s t-tests. Error bars indicate mean ± SD.
We next conducted loss-of-function analyses in S1M cells through or CRISPR/Cas9 gene knockout (KO) (Supplementary Fig. 4B), as these cells show higher expression of ST2. The effectiveness of gene editing was checked by flow cytometry and Sanger sequencing, and these revealed essentially 100% KO efficiency (Supplementary Fig. 4C, D). The targeting of ST2 reduced the viability of S1M cells following cisplatin treatment (Fig. 5D). Concordantly, cisplatin treatment resulted in increased Annexin V staining in ST2 KO cells compared to the control (Fig. 5E).
Anoikis is triggered when epithelial cells detach from the extracellular matrix and is therefore considered a barrier to cancer metastasis. To induce anoikis, S1M cells were cultured in low-adherence plates following single-cell dissociation. Under these anoikis-inducing conditions, the ST2+ subpopulation in S1M cells was enriched, indicating positive selection (Fig. 5F). Accordingly, ST2− cells show greater Annexin V staining than ST2+ cells (Fig. 5G). Moreover, when cultured in suspension, ST2 KO cells showed greater staining by Annexin V/propidium iodide than in control cells (Fig. 5H). Collectively, these results further demonstrate that ST2 enhanced cell survival by inhibiting apoptosis.
IL-33/ST2 axis induces Bcl-xL via MAPK signaling activation
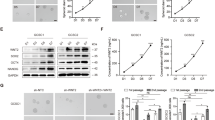
To understand the underlying mechanism through which ST2 enhances self-renewal and survival, we focused on Bcl-xL, an anti-apoptotic protein induced by IL-33/ST2 to promote mast cell survival [21]. We first examined the positive correlation between ST2 and Bcl-xL expression by IHC staining of a human GC tissues using an antibody that we empirically validated to be highly specific for ST2 (Supplementary Fig. 1B). This revealed that ST2-high GC tissues have significantly higher Bcl-xL expression than ST2-negative/low GC tissues, and that there is a strong overlap in the distribution of ST2 and Bcl-xL (Fig. 6A, B). We next examined the signaling events downstream of IL-33/ST2 that regulates Bcl-xL. We observed that ST2 KD S1M cells showed reduced ERK phosphorylation, suggesting that ST2 may have a role in maintaining the activity of MAPK/ERK pathway in GC (Fig. 6C). To test if the induction of Bcl-xL by IL-33 was mediated via MAPK activation, S1M cells were treated with trametinib, a potent MEK1/2 inhibitor. Treatment with trametinib completely abrogated the induction of Bcl-xL by IL-33 in S1M cells at both the mRNA and protein levels (Fig. 6D, E). Interestingly, trametinib also reduced basal Bcl-xL expression, suggesting that IL-33/ST2-MAPK was involved in the maintenance of steady-state Bcl-xL expression. We next examined if Bcl-xL inhibition could attenuate IL-33-induced self-renewal of tumorspheres using BH3I-1, a Bcl-xL inhibitor. Treatment with BH3I-1 effectively blocked the enhancement of S1M tumorsphere growth induced by IL-33, while only marginally reducing baseline growth (Fig. 6F, G). In contrast, BH3I-1 had no effect on the tumorsphere size of ST2 KO S1M cells (Fig. 6H). Similarly, treatment with BH3I-1 had no effect on monolayer S1M cell growth (Fig. 6I). Taken together, these data indicate that IL-33 promotes self-renewal of ST2+ GC cells by activating MAPK to induce the survival gene Bcl-xL.
A Representative IHC images of ST2 and Bcl-xL expressions in human GC tissues in a tissue microarray (TMA). B IHC scoring of Bcl-xL expression in human GC tissues in a TMA according to ST2 expression. Scoring was performed on a semi-quantitative assessment scale by multiplying the degree of intensity (0–3) and the percentage (0–5) of positive cells. C Western blot analysis of phosphorylated (p)-ERK and Bcl-xL in ST2 KD S1M cells. D Bcl-xL mRNA expression in S1M cells after treatment with IL-33 (100 ng/ml) and/or trametinib (60 nM) for 48 h. E Western blot analysis of Bcl-xL in S1M cells after treatment with IL-33 (100 ng/ml) and trametinib (60 nM) for 48 h. F, G Sphere-forming assay using S1M cells treated with IL-33 (100 ng/ml) and/or BH3I-1 (25 µM) for 5 days. F Representative images of tumorspheres. G The size of all tumorspheres (n = 3 wells) from technical replicates. H Sphere-forming assay using control and ST2 KO S1M cells treated with IL-33 (100 ng/ml) and/or BH3I-1 (25 µM) for 5 days. The graph indicates the size of all tumorspheres (n = 3 wells) from technical replicates. I Trypan blue dye exclusion assay to measure viable S1M cells after treatment with BH3I-1 for 3 days at the indicated concentrations under monolayer culture conditions. 1 × 104 cells were seeded on 24-well plates. All experiments were repeated at least three times with comparable results. Representative data for repeated experiments are shown in panels [D] and [G–I]. Individual dots in these figures represent technical replicates. Where statistical analyses were performed, the lines indicate the two groups analyzed by Student’s t-tests. *p < 0.05, Student’s t-tests. Error bars indicate mean ± SD.
ST2-high subpopulation displays increased tumorigenic and metastatic capability by survival advantage
To ascertain their tumorigenic and metastatic potentials, ST2-high and ST2-low/negative S1M cells were fractionated by FACS and injected into the subcutaneous flank tissues and spleens of immune-deficient SCID mice to perform in vivo limiting dilution assay (Fig. 7A–C). Two months post subcutaneous injection, ST2-high cells generated tumors in 6 out of 10 mice (60%), whereas ST2-negative/low cells generated tumors in 2 out of 10 mice (20%) (Fig. 7B). In addition, 2 months post splenic injection, ST2-high cells developed metastatic liver foci in 5 out of 7 mice (71.4%), whereas ST2-negative/low cells generated foci only in 1 out of 6 mice (16.7%) (Fig. 7C). The ST2-high population of S1M also demonstrated a higher frequency of tumor-initiating cells than the ST2-low/negative population both in subcutaneous and splenic transplantation models (Fig. 7B, C). To evaluate the functional contribution of ST2 on GC tumorigenesis, ST2 KO S1M and control S1M cells were transplanted into subcutaneous flank tissues of syngeneic immunocompetent mice. ST2 KO cell lines formed smaller tumors than control cells (Fig. 7D, E). Histologically, ST2 KO S1M tumors showed similar features to the control tumors, but TUNEL assay revealed a significant increase in apoptotic cells in ST2 KO allografts (Fig. 7F). In the splenic transplantation model, ST2 KO cells produced significantly fewer liver metastases (Fig. 7G). Similarly, increased apoptosis was observed in ST2 KO allografts based on IHC staining of cleaved caspase 3 (Fig. 7H).
A Flow cytometry gates for the fractionation of ST2-high and ST2-low S1M cells. B ST2 expression and tumorigenicity in subcutaneous tissues. S1M allografts generated in SCID mice through S.C. transplantation of ST2-low (left flank) and ST2-high (right flank) S1M cells at the indicated cell number. Tumors were microscopically examined 2 months post transplantation. Tumor-initiating cell (TIC) frequency was calculated and p value was measured using ELDA webtool. Right graph, tumor volume at the necropsy day when 1 × 105 cells were transplanted in a mouse. C ST2 expression and the potential for liver metastasis. Splenic transplantations of ST2-low and ST2-high S1M cells to generate liver metastases in SCID mice at the indicated cell number. Right graph, metastatic foci number at the necropsy day when 5 × 104 cells were transplanted in a mouse. Tumors were microscopically examined 2 months post transplantation. D–F S1M allografts generated in syngeneic immunocompetent mice through S.C. transplantation of 3 × 106 cells each of control and ST2 KO S1M cells. Control and ST2 KO cells were injected into the left and right flanks, respectively. D Representative gross images of control and ST2 KO S1M allografts. E Tumor volume of S1M allografts measured 4 weeks after the injection of cells. F Representative images of TUNEL assay to detect apoptotic cells in control (n = 6) and ST2 KO (n = 6) S1M allografts. Right, the number of TUNEL+ cancer cells per high power field (HPF; 200×). G, H Splenic transplantations of control#1 and ST2 KO#1 S1M cells to generate liver metastases (n = 6 for both). 2 × 106 cells were injected into the spleens of immunocompetent syngeneic mice. Liver tissues were sampled 4 weeks after the injection. G Representative gross images of control#1 and ST2 KO#1 S1M liver metastases. Red arrow heads indicate metastatic tumor foci. Right, the total number of metastatic tumor foci throughout the whole liver macroscopically counted on the surface of all liver lobes. H Representative IHC images of c-caspase-3 in control and ST2 KO S1M liver metastases. Right, the average number of c-caspase-3+ cancer cells per HPF. I RT-qPCR measurement of ST2L and sST2 mRNA expressions in S1 and S1M cells. J Control and ST2-overexpressing cells were injected into the left and right flanks, respectively. Volume of allografts measured 14 weeks after S.C. transplantation of control and ST2L- or sST2-overexpressing S1 cells in syngeneic immunocompetent mice (n = 5 in all cases; lines between data points indicate pairs of transplanted cells in the same mouse). K Representative IHC images of c-caspase-3 in S1 allografts in (J). Right, the average number of c-caspase-3+ cancer cells per HPF. L IHC analysis of Bcl-xL expression in control and ST2 KO S1M S.C. allografts in (D). Right, immunoreactivity of Bcl-xL scored by evaluating the intensity of positive cells. M IHC analysis of Bcl-xL expression in control and ST2L-overexpressing S1 S.C allografts in (J). Right, Bcl-xL IHC scoring. All experiments were repeated at least three times with comparable results. Representative data for repeated experiments are shown in panel [I]. Individual dots in this figure represent technical replicates. Where statistical analyses were performed, the lines indicate the two groups analyzed by Student’s t-tests. *p < 0.05, Student’s t-tests. Error bars indicate mean ± SD.
S1M cells expressed higher levels of both ST2L and sST2 compared to S1 cells (Fig. 7I). Thus, we next sought to determine the relative contribution of ST2L and sST2 in tumor formation. ST2L- and sST-overexpressing lines were derived from parental S1 cells that express either form (Supplementary Fig. 4E). In syngeneic transplantation assays, ectopic ST2L overexpression resulted in larger subcutaneous allografts compared to control cells. In contrast, overexpression of sST2 produced marginally smaller allografts, indicating that ST2L is the relevant isoform driving tumorigenicity in S1 cells (Fig. 7J). Moreover, this observation implies that for sST2 to functionally inhibit tumorigenesis or tumor growth, it needs to be secreted into the tumor microenvironment in sufficient quantity.
Consistent with earlier observations, IHC staining revealed decreased cleaved caspase 3 but increased Bcl-xL staining in ST2L-overexpressing allografts compared with control allografts (Fig. 7K, M). Conversely, the staining intensity for Bcl-xL in ST2 KO S1M cells is significantly lower than ST2-proficient control S1M cells (Fig. 7L). Collectively, these results from the in vivo transplantation models suggest that membrane-bound ST2 contributes to GC growth and metastasis by endowing a survival advantage.
IL-33/ST2 axis synergizes WNT signaling to enhance GC stemness through MAPK-Bcl-xL pathway
Given the well-established role of the canonical Wnt pathway in supporting normal and transformed gastric stem cells [5], we sought to clarify its relationship with the highly expressed ST2 and IL-33, especially with respect to MAPK/Bcl-xL. First, we examined their relative contribution to S1M tumorsphere formation. This revealed that individual treatment with Wnt3a or IL-33 increased S1M tumorsphere sizes. Importantly, co-treatment with Wnt3a and IL-33 further increased tumorsphere sizes and their proportions (Fig. 8A, B), with corresponding increases in CD44 and Survivin expressions (Fig. 8C, D). Through a lentiviral-based firefly reporter of Wnt activity, ST2-high S1M cells exhibited significantly higher Wnt activity than ST2-low cells in FBS-containing cultures (Fig. 8E). Based on a Gene Set Enrichment Analysis (GSEA) of genes differentially expressed between ST2-high and ST2-low S1M cells, the gene set LEF1_UP.V1_UP consisting of genes upregulated in colon cancer cells overexpressing the Wnt nuclear effecter LEF1 was enriched in ST2-high cells (Fig. 8F). Remarkably, we found that the combined treatment with IL-33 and Wnt3a synergistically increased Wnt activity, even though IL-33 treatment alone had no effect (Fig. 8G). This synergy was markedly enhanced by the co-treatment with R-spondin 3, a secreted ligand that binds to the gastrointestinal stem cell marker Lgr5 and orchestrates GI stem cell homeostasis by controlling the Wnt signal [25,26,27]. These data suggest a profound crosstalk between the canonical Wnt and IL-33/ST2 signaling axes. To investigate whether the synergistic Wnt activation is mediated by MAPK/Bcl-xL axis, we co-treated the cells with BH3I-1 and trametinib, which revealed that BH3I-1 effectively blocked the synergistic effect of IL-33 without interfering with Wnt3a (Fig. 8H). In contrast, trametinib did not block the effect of IL-33 as predicted (Fig. 8I). Rather, it marginally increased the activating effects of Wnt3a (Fig. 8I), suggesting that MEK1/2 play a more complex role, mediating both positive and negative regulation of Wnt activity, compared with the Bcl-xL axis. Hence, we concluded that IL-33/ST2 synergizes WNT signaling to enhance GC stemness through the MAPK-Bcl-xL axis.
A, B Sphere-forming assay using S1M cells treated with IL-33 (100 ng/ml) and/or Wnt3a (25 ng/ml) for 5 days. A Representative images of tumorspheres. B Percentage of spheres per well grouped according to sizes. C, D CD44 (C) and Survivin (D) mRNA expressions in S1M cells treated with IL-33 (100 ng/ml) and/or Wnt3a (25 ng/ml) for 48 h. C One-way ANOVA test, p value <0.0001, F: 75.14. D One-way ANOVA test, p value < 0.0001, F: 92.10. E TOP-Flash luciferase reporter assay to measure WNT pathway activity in ST2+ and ST2- S1 cells in serum-containing cultures. Cells were sorted based on ST2 expression by FACS. F GSEA in ST2-high and -low S1M cells. Microarray was performed using ST2-high and -low S1M cells. Changes in gene expression of ±1.5 fold were used to identify enriched sets. G TOP-Flash luciferase reporter assay to measure WNT pathway activity in S1M cells treated with IL-33 (100 ng/ml), R-spondin 3 (1 μg/ml) and/or Wnt3a (25 ng/ml) for 48 h. One-way ANOVA test, p value < 0.0001, F: 368.6. H TOP-Flash luciferase reporter assay in S1M cells treated with IL-33 (100 ng/ml) and/or Wnt3a (25 ng/ml) with/without BH3I at the indicated concentrations. One-way ANOVA test, p value < 0.0001, F: 433.7. I TOP-Flash luciferase reporter assay in S1M cells treated with IL-33 (100 ng/ml) and/or Wnt3a (25 ng/ml) with/without trametinib at the indicated concentrations. One-way ANOVA test, p value < 0.0001, F: 268.6. J, K Trametinib treatment in splenic transplantation syngeneic model using S1M cells. Mice were treated with vehicle (n = 4) or trametinib (n = 4; 1 mg/kg) by intraperitoneal (I.P.) route five times a week for 2 weeks. J Representative gross images of vehicle- and trametinib-treated groups. K Total number of visible metastatic tumor foci counted on the surface of all liver lobes for each mouse. L–N A competitive allograft assay by measuring gRNA fraction changes in allografts after drug treatment compared with vehicle-treated allografts. The allografts were generated in NSG mice by S.C. injection of control (non-target gRNA) and ST2 KO (ST2-target gRNA-expressing) S1M cells. A-1155463, I.P. route, 10 consecutive days; trametinib, I.P. route, 10 consecutive days. L Gross images of allografts 3 weeks after the transplantation. M Weight of allografts measured 3 weeks after the transplantation. One-way ANOVA test, p value: 0.0137, F: 5.426. N RT-qPCR analyses to measure each gRNA fraction in allografts. All experiments were repeated at least three times with comparable results. Representative data for repeated experiments are shown in panels [B–D], [E], [G–I] and [N]. Individual dots in these figures represent technical replicates. Where statistical analyses were performed, the lines indicate the two groups analyzed by Student’s t-tests. *p < 0.05, Student’s t-tests. Error bars indicate mean ± SD.
Exploiting the IL-33/ST2 signaling axis for cancer therapy
Lastly, to explore the therapeutic potential of MAPK/Bcl-xL inhibition in ST2-high subpopulation, we first tested if trametinib could inhibit metastasis of S1M cells in vivo and observed that it significantly reduced liver metastases in our established splenic transplantation model (Fig. 8J, K). To further investigate if the inhibition of MEK and Bcl-xL specifically targets ST2-high population, a competitive allograft assay was conducted. A cellular mixture containing equal proportions of ST2 KO and control S1M cells were subcutaneously transplanted into the flanks of NSG mice to generate heterogeneous allografts. Mice were treated with trametinib and/or A-1155463, a Bcl-xL inhibitor that was validated for in vivo treatment [28]. It was confirmed that this drug has the same effect as the BH3I-1 inhibitor in in vitro sphere formation assays (Supplementary Fig. 5). We observed that treatment with either inhibitor suppressed tumor growth (Fig. 8L, M). When the allografts were harvested and measured for the relative ratio of ST2-targetting gRNA and control gRNA, we found allografts treated with either or both inhibitors expressed lower levels of ST2-targetting gRNA relative to the control gRNA (Fig. 8N). This suggests that the drug treatment had selectively targeted the ST2-proficient control S1M cells while enriching the ST2 KO cells. These data provide functional evidence that inhibition of MEK1/2 and Bcl-xL would selectively suppress the growth of S1M cells through a mechanism that is dependent on an intact ST2. This study also illustrates the therapeutic potential of MEK1/2 and Bcl-xL inhibition in targeting highly metastatic, chemoresistant ST2-high CSCs.
Discussion
CSCs are drivers of GC progression; there is an obvious motivation to understand the molecular underpinning of the CSC phenotype, as well as the cell-intrinsic and -extrinsic cues that give rise to them. In the current study, we provide compelling in vitro and in vivo evidence that the cytokine receptor ST2 is a functional marker of CSC in GC. We demonstrated that the ST2+ subpopulation possesses an increased capacity for tumor formation and metastasis, and depletion of ST2 reduces tumor growth and metastasis. Moreover, by being resistant to apoptosis, ST2+ cells exhibited survival advantage against anoikis and chemotherapy-induced cell death. The identification of this subpopulation presented an invaluable opportunity to interrogate their molecular characteristics, through which we established that the ST2+ CSC population is supported by the co-expression of IL-33, leading to elevated MAPK/ERK signaling and the induction of Bcl-xL expression. Crucially, this leads to the synergistic induction of the canonical Wnt pathway that is essential for the maintenance of normal and transformed gastric stem cells in vivo and in vitro [5, 29, 30], The observation that CSCs exist in small numbers despite bearing essentially the same driver mutations and being maintained in the same microenvironment as non-CSCs has long puzzled researchers. The discovery of stem cell markers like Lgr5 and CD44 that concurrently serve functional roles in stem cell biology has provided important clues. Lgr5 serves as a receptor to R-spondin, through which it fortifies the canonical Wnt signal, thereby endowing Lgr5+ve stem cells with greater Wnt sensitivity than the non-stem cells. Importantly, this mechanism provides a basis for understanding the restricted distribution of stem cells, namely, they are selectively supported by a set of functional stem cell markers. These markers function as amplifiers of key signals, giving rise to a heightened state of intrinsic signaling activities, which in turn drive the expression of the aforementioned markers to sustain a self-enforcing, stem cell-restricted signal circulatory that gives rise to ST2+ CSCs. In this context, a key finding in this study is that ST2 appears a new addition to this exclusive signal modulatory that in our experimental systems marks and functionally defines cancer stem cells to drive tumorigenicity and cancer cell metastasis.
Specifically, our data show that IL-33/ST2 synergizes with Wnt3a and Rspo3 to strongly enhance Wnt-mediated transcriptional activities, leading to increased expression of stem cell markers and tumorsphere or organoid growth. In line with other reports, ST2 contributes to basal ERK1/2 activation to promote a heightened expression of Bcl-xL, an anti-apoptotic member of the Bcl-2 family of proteins [31]. Elevated expression of Bcl-xL has previously been reported in several experimental models of cancer metastasis, and its induction by IL-33/ST2 neatly explains the reduced apoptosis and increased chemoresistance associated with ST2 expression in vitro and in vivo in our experiments. The importance of Bcl-xL was underscored by the effectiveness of the Bcl-xL inhibitor BH3I-1 in curbing ST2-driven tumorigenesis and metastasis. In addition to its BH3-domain-mediated anti-apoptotic function, Bcl-xL is reported to promote cancer metastasis non-canonically through its BH4 domain [32, 33]. A recent study demonstrated that Bcl-xL activates the MAPK pathway via a physical association between its BH4 domain and KRAS to suppress its proteosomal degradation and promote cancer stemness [33]. Another study reported that Bcl-xL activates the TGFβ pathway to promote EMT and metastasis via its BH4 domain, independent of its anti-apoptotic activity [32]. As BH3I-1 inhibitor used in our study specifically targets the BH3 domain, it appears that the BH3 domain is at least partly responsible for the increased tumorigenic and metastatic potential of ST2+ cells in our models. Interestingly, the synergy between IL-33 and Wnt3a in transactivating a Wnt-responsive promoter was also selectively inhibited by BH3I-1. As apoptosis is not a feature of this assay, this observation implicates the BH3 domain to have a still obscured transcriptional role in addition to its anti-apoptotic activity. Although several transcription factors have been reported to act upstream of ST2, such as GATA2 [34], little is known of its downstream effectors. The findings of the current study will pave the way for future investigation into the cooperation of IL-33/ST2 and Wnt pathway in CSCs.
The functional contribution of IL-33/ST2 to the tumor-initiating and chemoresistant CSC phenotype in vitro is well-supported by clinical evidence, where its expression is variegated and restricted, potentially driving intratumoral heterogeneity; and strongly correlated to high-grade, undifferentiated GC. Moreover, consistent with its positive impact on cell survival, IL-33/ST2 expression is highly elevated in metastatic tumors. This presents a unique opportunity to investigate if the IL-33/ST2 axis could be targeted as a novel therapy against gastric metastasis. Encouragingly, targeting IL-33/ST2 activity with trametinib or/and BH3I-1, validated in our in vitro experiments, significantly suppressed metastasis in vivo. More importantly, through a competitive colonization approach, we functionally validated that the treatment with either or both inhibitors selectively targeted ST2-high gastric cancer cells. Collectively, the key findings of this study uncover a new therapeutic approach to selectively target CSCs for treating advanced GC and preventing metastasis. The data presented here demonstrate the potential utility of targeting Bcl-xL with BH3I-1 in attenuating the aggressive CSC subpopulation. More importantly, the identification of a cooperative crosstalk between the IL-33/ST2 and Wnt signaling axes advocates a combinatorial approach to unravel the CSC phenotype through synthetic lethality.
Methods
Cells, organoids, and mice
The genetically engineered mouse GC cells used in this study were established in earlier studies [6, 8]. Human GC cell lines were obtained from the Korean Cell Line Bank. Mouse stomach organoids were primarily cultured directly from mouse stomach tissues, as described previously [5, 35]. To generate Trp53 knockout (KO) mouse stomach organoids, organoids were established from Trp53f/f mice and Cre recombinase was delivered via lentiviral transduction. Subsequently, organoids were selected with nutlin-3 for one week. S1M allografts were generated in syngeneic immunocompetent, severe combined immune deficiency (SCID), and NOD-scid IL2rgnull (NSG) mice as previously reported [8].
Reporter assay
Wnt/β-catenin activity was quantified with a lentivirus-delivered Wnt reporter plasmid consisting a tandem repeat of seven TCF/LEF binding sites (pXL010-Wnt reporter). Firefly reporter activities were normalized against those of Renilla luciferase, from a co-transduced lentiviral pLX313-Renilla luciferase reporter. Dual-luciferase reporter assays (E1980; Promega) were performed as per manufacturer’s instructions and measured on a GloMax® 20/20 Luminometer (E5311; Promega).
Flow cytometry
To detect cell surface ST2 expression, murine GC cells were dissociated into single cells by TrypLE (12605-028; Invitrogen, Carlsbad, CA, USA) and stained with PE-rat monoclonal anti- IL-33R(ST2) antibody (12-9335-80, eBioscience, San Diego, CA, USA) or PE-rat IgG isotype control antibody (553930; BD Biosciences) for 1 h at 4 °C in the dark. To detect ST2 in human GC cells, single cell-dissociated cells using TrypLE were fixed with 4% paraformaldehyde for 15 min and incubated with goat polyclonal anti-ST2 antibody (AF523; R&D Systems, Minneapolis, MN, USA) for 1 h at room temperature. Donkey anti-goat Alexa Fluor 488 secondary antibody (ab150129; Abcam, Cambridge, UK) was next added and incubated for 30 min. Cells were analyzed on a NovoCyte flow cytometer (Agilent, Santa Clara, CA, USA). Cell sorting by FACS were performed on a BD Aria cell sorter (BD Biosciences).
In vitro cellular experiments
For tumorsphere assays, 1 × 104 cells were seeded as single-cell suspension in Advanced DMEM/F-12 medium (12634010;Gibco, MA, USA), supplemented with HEPES (Gibco, 15630-080), N-Acetylcysteine (A9165-5G; Sigma Aldrich), epidermal growth factor (R&D Systems), 2 mM GlutaMAX (35050061; Gibco), B-27™ Supplement (17504044; Gibco) and N-2 Supplement (17502048; Gibco) on low attachment 24-well plate (39724; SPL Life Sciences, Pocheon, Korea) and incubated for 5 days, as previously reported [9]. Cells were then treated with cisplatin (Sigma Aldrich), trametinib (S2673; Selleckchem, Houston, TX, USA), recombinant mouse Wnt3a (772301; BioLegend, San Diego, CA, USA), and recombinant mouse IL-33 Protein (3626-ML; R&D Systems), BH3I-1 (sc-221352; Selleckchem) at the indicated concentration.
Anoikis was induced according to a protocol previously published [7]. Briefly, cells were dissociated into single cells using TrypLE (12605-028; Invitrogen) and 5 × 104 single cells were seeded per well in cell low attachment 6-well plates (390706; SPL). After 36 h, cells were harvested. Annexin V Fluor 488 Apoptosis Detection kit (KTA0002; Abbkine, Wuhan, China) was used to detect apoptotic cells according to the manufacturer’s instructions. Annexin V-positive cells were analyzed on NovoCyte (Agilent).
Data availability
The public datasets used in this study listed in the appropriate Supplementary information section are available on Gene Expression Omnibus (GEO) database. All the other data are available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–28.
Yoon C, Park DJ, Schmidt B, Thomas NJ, Lee HJ, Kim TS, et al. CD44 expression denotes a subpopulation of gastric cancer cells in which Hedgehog signaling promotes chemotherapy resistance. Clin Cancer Res. 2014;20:3974–88.
Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–20.
Tan SH, Swathi Y, Tan S, Goh J, Seishima R, Murakami K, et al. AQP5 enriches for stem cells and cancer origins in the distal stomach. Nature. 2020;578:437.
Park JW, Jang SH, Park DM, Lim NJ, Deng C, Kim DY, et al. Cooperativity of E-cadherin and Smad4 loss to promote diffuse-type gastric adenocarcinoma and metastasis. Mol Cancer Res. 2014;12:1088–99.
Park JW, Kim MS, Voon DC, Kim SJ, Bae J, Mun DG, et al. Multi-omics analysis identifies pathways and genes involved in diffuse-type gastric carcinogenesis induced by E-cadherin, p53, and Smad4 loss in mice. Mol Carcinog. 2018;57:947–54.
Park JW, Park DM, Choi BK, Kwon BS, Seong JK, Green JE, et al. Establishment and characterization of metastatic gastric cancer cell lines from murine gastric adenocarcinoma lacking Smad4, p53, and E-cadherin. Mol Carcinog. 2015;54:1521–7.
Park JW, Park JM, Park DM, Kim DY, Kim HK. Stem cells Antigen-1 enriches for a cancer stem cell-like subpopulation in mouse gastric cancer. Stem Cells. 2016;34:1177–87.
Larsen KM, Minaya MK, Vaish V, Pena MMO. The role of IL-33/ST2 pathway in tumorigenesis. Int J Mol Sci. 2018;19:2676.
Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16:676–89.
Fournie JJ, Poupot M. The pro-tumorigenic IL-33 involved in antitumor immunity: A Yin and Yang Cytokine. Front Immunol. 2018;9:2506.
Griesenauer B, Paczesny S. The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol. 2017;8:475.
Zhou Q, Wu XY, Wang XF, Yu ZJ, Pan T, Li Z, et al. The reciprocal interaction between tumor cells and activated fibroblasts mediated by TNF-alpha/IL-33/ST2L signaling promotes gastric cancer metastasis. Oncogene. 2020;39:1414–28.
De Salvo C, Pastorelli L, Petersen CP, Butto LF, Buela KA, Omenetti S, et al. Interleukin 33 triggers early eosinophil-dependent events leading to metaplasia in a chronic model of gastritis-prone mice. Gastroenterology. 2021;160:302.
Eissmann MF, Dijkstra C, Jarnicki A, Phesse T, Brunnberg J, Poh AR, et al. IL-33-mediated mast cell activation promotes gastric cancer through macrophage mobilization. Nat Commun. 2019;10:2735.
Lee SH, Park JW, Go DM, Kim HK, Kwon HJ, Han SU, et al. Ablation of osteopontin suppresses N-methyl-N-nitrosourea and Helicobacter pylori-induced gastric cancer development in mice. Carcinogenesis. 2015;36:1550–60.
Kim J, Park C, Kim KH, Kim EH, Kim H, Woo JK, et al. Single-cell analysis of gastric pre-cancerous and cancer lesions reveals cell lineage diversity and intratumoral heterogeneity. NPJ Precis Oncol. 2022;6:9.
Bass AJ, Thorsson V, Shmulevich I, Reynolds SM, Miller M, Bernard B, et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9.
Castle JC, Loewer M, Boegel S, de Graaf J, Bender C, Tadmor AD, et al. Immunomic, genomic and transcriptomic characterization of CT26 colorectal carcinoma. BMC Genomics. 2014;15:190.
Wang JX, Kaieda S, Ameri S, Fishgal N, Dwyer D, Dellinger A, et al. IL-33/ST2 axis promotes mast cell survival via BCLXL. Proc Natl Acad Sci USA. 2014;111:10281–6.
Barroso-Gonzalez J, Auclair S, Luan S, Thomas L, Atkins KM, Aslan JE, et al. PACS-2 mediates the ATM and NF-kappa B-dependent induction of anti-apoptotic Bcl-xL in response to DNA damage. Cell Death Differ. 2016;23:1448–57.
Bernal-Mizrachi L, Lovly CM, Ratner L. The role of NF-{kappa}B-1 and NF-{kappa}B-2-mediated resistance to apoptosis in lymphomas. Proc Natl Acad Sci USA. 2006;103:9220–5.
Zhao JH. Cancer stem cells and chemoresistance: the smartest survives the raid. Pharm Therapeut. 2016;160:145–58.
de Lau W, Peng WC, Gros P, Clevers H. The R-spondin/Lgr5/Rnf43 module: regulator of Wnt signal strength. Gene Dev. 2014;28:305–16.
Sigal M, Logan CY, Kapalczynska M, Mollenkopf HJ, Berger H, Wiedenmann B, et al. Stromal R-spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature. 2017;548:451.
Yan KS, Janda CY, Chang JL, Zheng GXY, Larkin KA, Luca VC, et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5(+) intestinal stem-cell self-renewal. Nature. 2017;545:238.
Tao ZF, Hasvold L, Wang L, Wang XL, Petros AM, Park CH, et al. Discovery of a potent and selective BCL-X-L inhibitor with in vivo activity. Acs Med Chem Lett. 2014;5:1088–93.
Barker N, van Oudenaarden A, Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. 2012;11:452–60.
Oshima H, Matsunaga A, Fujimura T, Tsukamoto T, Taketo MM, Oshima M. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology. 2006;131:1086–95.
Stevens M, Oltean S. Modulation of the apoptosis gene Bcl-x function through alternative splicing. Front Genet. 2019;10:804.
Choi SY, Chen ZM, Tang LH, Fang YZ, Shin SJ, Panarelli NC, et al. Bcl-xL promotes metastasis independent of its anti-apoptotic activity. Nat Commun. 2016;7:10384.
Trecesson SD, Souaze F, Basseville A, Bernard AC, Pecot J, Lopez J, et al. BCL-X-L directly modulates RAS signalling to favour cancer cell stemness. Nat Commun. 2017;8:1123.
Baba Y, Maeda K, Yashiro T, Inage E, Kasakura K, Suzuki R, et al. GATA2 is a critical transactivator for the human IL1RL1/ST2 promoter in mast cells/basophils opposing roles for GATA2 and GATA1 in human IL1RL1/ST2 gene expression. J Biol Chem. 2012;287:32689–96.
An HW, Kim SY, Kwon JW, Seok SH, Woo SH, Kim DY, et al. In vivo CRISPR-Cas9 knockout screening using quantitative PCR identifies thymosin beta-4 X-linked that promotes diffuse-type gastric cancer metastasis. Mol Carcinog. 2021;60:597–606.
Funding
This research was supported by grants from the National Research Foundation (NRF) funded by the Korean government (2016M3A9D5A01952416, 2020R1C1C1014059, 2021M3H9A1030260). This study was also supported by 2021 Research Grant from Kangwon National University.
Author information
Authors and Affiliations
Contributions
J-WK—data curation: lead; formal analysis: lead; investigation: lead; methodology: lead; writing – original draft: equal. S-HS—investigation: supporting; methodology: supporting; resources: supporting. SK—investigation: supporting; methodology: supporting; resources: supporting. H-W—investigation: supporting; methodology: supporting. ADC—writing – review and editing: supporting. S-HW—resources: supporting. J-SO—resources: supporting. JKK—data curation: supporting; supervision: supporting. DCV—data curation: supporting; supervision: lead; writing – review and editing: lead. D-YK—funding acquisition: supporting; project administration: lead; resources: supporting; supervision: equal; writing – review and editing: supporting. JWP—conceptualization: lead; data curation: lead; formal analysis: lead; funding acquisition: lead; investigation: lead; project administration: lead; supervision: lead; writing – original draft: lead; writing – review and editing: lead.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kwon, JW., Seok, SH., Kim, S. et al. A synergistic partnership between IL-33/ST2 and Wnt pathway through Bcl-xL drives gastric cancer stemness and metastasis. Oncogene 42, 501–515 (2023). https://doi.org/10.1038/s41388-022-02575-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02575-5
- Springer Nature Limited
This article is cited by
-
The IL-33-ST2 axis plays a vital role in endometriosis via promoting epithelial–mesenchymal transition by phosphorylating β-catenin
Cell Communication and Signaling (2024)