Abstract
Long-term memory formation leads to enduring alterations in synaptic efficacy and neuronal responses that may be created by changes in neuronal morphology. We show that fear conditioning leads to a long-lasting increase in the volume of the primary and secondary dendritic branches, but not of distal branches, of neurons located at the basolateral amygdala (BLA). The length of the dendritic branches is not affected by fear conditioning. Fear conditioning leads to an enduring increase in the length and volume of dendritic spines, especially in the length of the spine neck and the volume of the spine head. Fear conditioning does not affect dendritic spine density. We further reveal that activation of Rac1 in BLA during fear conditioning impairs long-term auditory, but not contextual, fear conditioning memory. Activation of Rac1 during fear conditioning prevents the enduring increase in the dendritic primary branch volume and dendritic spines length and volume. Rac1 activation per se has no effect on neuronal morphology. These results show that fear conditioning induces changes known to reduce the inhibition of signal propagation along the dendrite and the increase in synaptic efficacy whereas preventing these changes, by Rac1 activation, impairs fear memory formation.
Similar content being viewed by others
Introduction
Evidence indicates that long-term memory (LTM) formation involves alterations in synaptic efficacy and transmission of the signal along the neuron [1,2,3,4]. These changes may be mediated by modifying the morphology of neurons in particular of dendritic spines [4, 5] but further studies need to unveil the role of these alterations in long-term memory.
In this study, we explored whether learning leads to changes in neuronal morphology and if altering such changes affects long-term memory. Toward that end, we used auditory fear conditioning. In this paradigm, an association is formed between an auditory tone (conditioned stimulus (CS)) and an aversive mild footshock (unconditioned stimulus (US)) [6,7,8,9,10]. The site of fear conditioning memory, within the basolateral amygdala (BLA; that is comprised of the lateral amygdala and the basal amygdala nuclei), is identified [6, 7, 11,12,13].
Toward elucidating whether changes in neuronal morphology are involved in memory we aimed to affect them by manipulating Rac1 GTPase activity. Rac1 GTPase is a member of the Rho GTPases family of molecular switches that cycle between an inactive GDP-bound state and an active GTP-bound form to regulate downstream effectors. Rac1 GTPase has been shown to be involved in neuronal morphogenesis and is intimately involved in the regulation of dendritic spine structure formation [14,15,16,17,18]. Rac1 is involved in memory formation and erasure [19,20,21].
To study whether Rac1 activation can affect neuronal changes induced by learning, we have employed a novel approach to photoactivate Rac1 GTPase in BLA by light with a high temporal and spatial resolution [21]. In this photoactivatable Rac1 (PA-Rac1), a complete Avena sativa Phototropin1 LOV2-Jα domain (sequence 404–547) is conjugated to the N-terminus of a constitutively active Rac1 [22]. LOV2 interacts with a C-terminal helical extension (Jα) in the dark and blocks the binding of Rac1 to its effectors. Blue light (473 nm) induces unwinding of the Jα helix and releases its steric inhibition, leading to Rac1 activation [22]. PA-Rac1 activation in the brain leads to the phosphorylation of its effector p21-activated kinase (PAK) [21]. We also revealed that activation of PA-Rac1 during fear conditioning impaired long-term fear memory but not short-term fear memory. Moreover, shining light per se or activation of PA-Rac1 after fear conditioning learning had no effect [21].
Here we study whether fear conditioning leads to morphological changes in neurons in BLA and whether activation of Rac1 in BLA affects fear conditioning memory and structural plasticity.
Materials and methods
Animals
Male C57BL6 mice (8–10 weeks) were used in this study (Harlan Laboratories). Following surgery, mice were housed separately at 22 ± 2 °C in a 12 h light/dark cycle, with ad libitum access to food and water. All experiments were done following the instructions and approval of the University of Haifa animal ethics committee for animal experiments observing National Institutes of Health guidelines and all experiments were performed in accordance with the relevant guidelines and regulations.
Fear conditioning
On the day of training, mice were placed in a training chamber (Coulbourn Instruments). Mice were allowed to acclimate in the chamber for 2 min and then subjected to 3 pairs of tone (conditioned stimulus (CS) - 20 s, 2.8 kHz, 85 dB) that co-terminated with a foot shock (unconditioned stimulus (US) - 2 s, 0.8 mA). The inter-trial interval was 120 s. Mice were tested for contextual fear conditioning in the same context 24 h after training for long-term memory (placed for 9 min in the chamber and the first 5 min were analyzed). Mice were tested for auditory fear conditioning in a different context 48 h after training for long-term memory. Behavior was recorded and the video images were transferred to a computer equipped with an analysis program (FreezeFrame). The percentage of changed pixels between two adjacent 0.25 s images was used as a measure of activity.
AAV production and microinjection
hSyn-mCherry-PARac1 containing AAVs at high titer (2.63E + 13) was produced by ELSC Vector Core Facility (Hebrew University of Jerusalem, Israel). pTriEx-mCherry-PA-Rac1 was a gift from Klaus Hahn (Addgene plasmid # 22027; http://n2t.net/addgene:22027; RRID: Addgene_22027) (Wu et al., 2009). Animals were anesthetized with Medetomidine (Domitor) 1 mg/ml and Ketamine 100 mg/ml cocktail, diluted in sterile isotonic saline (administered doses: Ketamine 50 mg/kg; Domitor 0.5 mg/kg; 100 µl/10 gm of animal body weight). Carprofen (5 mg/kg) was injected for analgesia before surgery and consecutive 3 days after surgery. AAV was injected (0.5 µl/hemisphere, 0.1 µl/min) into BLA following a stereotaxic surgery (Neurostar stereo drive). After virus injection, intracranial optic fiber (Thorlabs, Fiber Optic Cannula, Ø1.25 mm Stainless Ferrule, Ø200 µm Core, 0.39 NA) was implanted on the same line and 0.5 mm above virus injection place. After surgery, animals received the antibiotic Baytril (5 mg/kg; Enrofloxacin) for 3 consecutive days. Animals were allowed to recuperate for 4 weeks before behavioral experiments.
Light stimulation
Blue light (473 nm) from a laser was subjected at the indicated time to activate PA-Rac1. Optic fibers were connected to a 473-nm blue laser diode (Shanghai Dreamlasers) via an FC/PC adaptor. The light intensity ~15 mW/mm2 was measured at the tip of the fiber. A control group of animals got an equal amount of virus microinjection into BLA along with the fiber optic implantation but did not receive light stimulation.
ScaleS
Immediately after the auditory fear conditioning test the animals were anaesthetized using isoflurane followed by perfusion with 4% PFA in PBS. Brains were removed and post-fixed in 1% PFA and 30% sucrose. Brains were kept in −80 °C until use. After slicing, each brain slice sample (250 µm in thickness) was placed in a volume of more than 25 ml/g of tissue in each step of the protocol [23]. First, samples were placed in the S0 solution (D-(–)-sorbitol % (w/v)- 20; Glycerol % (w/v)- 5; 1 mM Methyl-ß-cyclodextrin); 1 mM α-Cyclodextrin, N-acetyl-L-hydroxyproline % (w/v)- 1, Dimethylsulfoxide % (v/v)- 3; 1X PBS (–); pH = 7.2) at 37 °C overnight for 12 h after which they were transferred to S1 solution (D-(–)-sorbitol % (w/v)-20; Glycerol % (w/v)-10; 4 M Urea; Triton X-100 % (w/v)- 0.2; pH 8.3), S2 solution (D-(–)-sorbitol % (w/v)- 27; 2.7 M Urea; Triton X-100 % (w/v)- 0.1; Dimethylsulfoxide % (v/v)-8.3; pH=8.3) and S3 solution (D-(–)-sorbitol % (w/v)- 36.4; 2.7 M Urea; Dimethylsulfoxide % (v/v)- 9.1; pH = 7.9) with an incubation period of 4 h each at 37 °C. Following these incubations, samples were placed in a PBS solution at 4 °C overnight, for an incubation period of 12 h. Finally, the samples were transferred to a S4 solution (D-(–)-sorbitol % (w/v)- 40; Glycerol % (w/v)-10; 4 M Urea; Triton X-100 % (w/v)- 0.2; Dimethylsulfoxide % (v/v)- 20; pH = 7.9) at 37 °C for 12 h. The images acquisition was made using 10× and 20× objectives for the elaboration of maps of the brain slices and region of interest and 60x objectives to visualize dendritic spine morphology. Z-stack images of 0.15 μm increments were made. The photographs were analyzed using the Imaris program (Oxford Instruments). For branch levels, the Imaris software calculates it as follows: At each branching point, the dendrite segment with a smaller mean diameter sequentially increases the branch level, while the dendrite segment with a greater diameter maintains the same branch level. In the case of two dendrite segments with the same diameter, the segment with a smaller branching angle keeps the branch level, while the dendrite segment with a greater branching angle sequentially raises its branch level.
Statistics
Repeated measure ANOVA and t-test analysis were done for the auditory or contextual fear conditioning memory tests, respectively. Kruskal–Wallis analysis that followed with Dunn’s test with Bonferroni correction was performed for the morphological experiments. The effect size was determined (Partial Eta squared (ɳp2) for ANOVA analysis and epsilon squared (ϵ2) for Kruskal–Wallis analysis). α level is 0.05 (also after Bonferonni corrections so that when multiplying the unadjusted p value by the number of comparisons we never exceeded 0.05).
Results
Activation of PA-Rac1 in the basolateral amygdala impairs auditory, but not contextual, fear memory formation
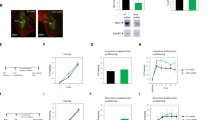
We activated PA-Rac1 in BLA during CS-US pairing in fear conditioning and examined the effects on both contextual fear conditioning 24 h after training, and auditory fear conditioning 48 h after training (see Fig. 1A for a schematic presentation of the experiment and Fig. 1B for AAV expression in BLA). Activation of PA-Rac1 during fear conditioning has no significant effect on freezing responses during training (F(1,12) = 0.024, p = 0.880; ɳp2 = 0.002). There is no treatment × tone trial interaction (F(2,24) = 0.262, p = 0.772) (Fig. 1C). Activation of PA-Rac1 (n = 7) during training does not affect contextual fear conditioning, tested 24 h after training when compared to animals that expressed PA-Rac1 in BLA but that were not activated by light (n = 8) (p = 0.66; ɳp2 = 0.015) (Fig. 1D). Activation of Rac1 GTPase during fear conditioning (n = 7) inhibited fear LTM compared to the no-light control group (n = 8) when tested 48 h after training (F(1,13) = 10.753, p = 0.006; ɳp2 = 0.453) (Fig. 1E). There is no treatment × tone trial interaction (F(1,13) = 1.022, p = 0.33). These results show that the activation of PA-Rac1 in BLA neurons had no effect on contextual fear conditioning but specifically impaired long-term auditory fear conditioning memory 48 h after training.
A. Schematic representation of the research approach. We injected AAV expressing the PA-Rac1 into the BLA. A month later the animals were trained for fear conditioning, subjected to light to activate PA-Rac1, and tested for contextual fear conditioning memory 24 h after training and auditory fear conditioning memory 48 h after training. After the last training, the brains were removed and subjected to the ScaleS clearing protocol, imaging and analysis of neuronal morphology. Controls are animals injected with PA-Rac1 and subjected to fear conditioning with no light or left naïve with light stimulation or without. B A month after injection of AAV mCherry is detected in basolateral amygdala in neurons in soma and dendrites. C Freezing during learning is not significantly different between fear conditioning with no light and light groups (F(1,12) = 0.024, p = 0.880). D Freezing during contextual fear conditioning is not different between the fear conditioning with no light (n = 8) and light groups (n = 7) (p = 0.66). E Auditory fear conditioning is impaired in Rac1 activated animals (n = 7) compared to non-activated mice (n = 8) (F(1,13) = 10.753, p = 0.006).
Fear conditioning alters dendritic shaft morphology in basolateral amygdala neurons and such changes are prevented by Rac1 GTPase activation
The results above show that activation of Rac1 GTPase in BLA impairs fear conditioning LTM. Previous studies have shown an association between Rac1 activity and alterations in dendritic morphology [14,15,16,17,18]. We were therefore interested to further study whether activation of Rac1 GTPase can affect morphological changes induced by learning. We first examined whether fear conditioning leads to alterations in dendritic shaft morphology in BLA and whether PA-Rac1 activation affects these changes. Animals were injected with AAV expressing PA-Rac1 into the BLA and divided into 4 groups: 1) Naïve animals that were subjected to the training box with no light stimulation. 2) Naïve animals that were subjected to the training box with light stimulation in the BLA (same light stimulation protocol as in fear-conditioned animals). 3) Fear-conditioned animals with no light stimulation. 4) Fear conditioning with light stimulation. Animals were perfused immediately after the last memory test (48 h after training) and subjected to ScaleS clearing procedure and imaging.
We examined the effect of the different treatments on dendritic shaft morphology. We found an effect on dendritic volume (H(3) = 14.663, p = 0.002; ϵ2 = 0.167). Posthoc analysis revealed that fear conditioning (n = 18 neurons; n = 4 animals) led to a significant increase in the total volume of the dendritic shaft when compared to the naïve group (n = 17 neurons; n = 3 animals) (p = 0.001) (Fig. 2A). We further analyzed the volume of the dendrite per dendritic branch (Fig. 2B). We revealed a significant effect in the first dendritic branch (H(3) = 19.792, p < 0.001, ϵ2 = 0.225) and the second dendritic branch (H(3) = 10.104, p = 0.018, ϵ2 = 0.117). Posthoc analysis revealed that fear conditioning led to an increase in the dendritic volume in the first (p < 0.001) and second (p = 0.02) dendritic branch when compared to naïve animals (Fig. 2C). The increase in dendritic volume was significantly blocked by PA-Rac1 activation during fear conditioning (n = 15 neurons; n = 4 animals) in the primary first branch (p = 0.035) but not in the secondary branch (p = 1) (Fig. 2C). Rac1 activation per se has no significant effect on dendritic morphology (p = 0.228) (Fig. 2C) (n = 17 neurons; n = 5 animals). There are no significant changes in other dendritic branches (p > 0.2). There is no difference in the volume of the soma between all groups (H(3) = 2.723, p = 0.436) (Fig. 2D). Next, we examined the effects of fear conditioning on the length of the dendrites. We revealed that fear conditioning does not affect the total length of the dendrites in BLA (H(3) = 2.622; p = 0.454; ϵ2 = 0.03) (Fig. 2E) (Fear conditioning- n = 18 neurons; n = 4 animals; Naïve- n = 17 neurons; n = 3 animals; Fear conditioning PA-Rac1 activation- n = 15 neurons; n = 4 animals; Naïve Rac1 activation; n = 17 neurons; n = 5 animals). Additional analysis revealed that there is a general effect on length in the first branch (H(3) = 3, p = 0.047, ϵ2 = 0.09) but an in-depth post-hoc analysis revealed that the groups are not different from each other (Fig. 2F). There are no significant changes in other branches (p > 0.3). Cumulatively, these results show that fear conditioning leads to an increase in the volume but not the length of the primary and secondary branches. In addition, Rac1 activation per se does not affect dendritic shaft morphology but prevents the morphological changes induced by fear conditioning.
A Fear conditioning leads to an increase in the total volume of the dendrite when compared to the naïve group (p = 0.001). B An example for primary and secondary branches (arrows). C Fear conditioning leads to an increase in the total volume of the primary (p < 0.001) and secondary (p = 0.02) dendritic branches in BLA neurons when compared to BLA neurons from the naïve group. Rac1 activation during fear conditioning prevents the increase in the volume of the primary dendritic branch (volume is significantly reduced when compared to fear conditioning without Rac1 activation- p = 0.035). D The volume of the soma is not different between the groups (p = 0.436). E The total length of the dendrites is not different between the groups (p = 0.454). F The dendritic length along the different branches is not different between the groups.
We further analyzed the general structure of the dendritic tree and its complexity (Fear conditioning- n = 18 neurons; n = 4 animals; Naïve- n = 17 neurons; n = 3 animals; Fear conditioning PA-Rac1 activation- n = 15 neurons; n = 4 animals; Naïve Rac1 activation; n = 17 neurons; n = 5 animals). There is no difference in the space that the neurons occupy (convex hull analysis) between the different groups (H(3) = 0.291, p = 0.962; ϵ2 = 0.161) (Fig. 3A) and in the number of dendrites extending from the neurons (H(3) = 3.2, p = 0.362; ϵ2 = 0.036) (Fig. 3B). There is an overall effect on the number of dendritic segments (H(3) = 7.896, p = 0.048; ϵ2 = 0.09) where fear conditioning showed the lower scores (Fig. 3C). However, an in-depth posthoc analysis revealed that the groups are not different from each other. There is an effect on the number of dendritic branch points (H(3) = 8.163, p = 0.043; ϵ2 = 0.093) (Fig. 3D) where neurons of fear conditioning groups showed the lowest score. Posthoc analysis reveals that activation of PA-Rac1 during fear conditioning leads to an increase in branch points compared to fear conditioning where PA-Rac1 is not activated (p = 0.04) (Fig. 3D). These results show that PA-Rac1 activation increases the number of dendritic branch points of fear-conditioned animals to the level of the naïve animals. We observed an effect on dendritic branching 65 μm, 70 μm, 75 μm and 80 μm from the soma as determined by intersections in Sholl analysis (H(3) = 11.142, p = 0.011, ϵ2 = 0.155; H(3) = 8.405, p = 0.038, ϵ2 = 0.122; H(3) = 9.859, p = 0.02, ϵ2 = 0.149; H(3) = 8.010, p = 0.046, ϵ2 = 0.075) (Fig. 3E). Posthoc analysis revealed that the effect is caused by differences between the naïve no PA-Rac1 activation and naïve PA-Rac1 activation in the 65 μm, 75 μm and 80 μm distances (p = 0.011, p = 0.019, p = 0.035, respectively). There was no significant difference between the groups at the 70 μm distance. Thus, such changes are not induced by fear conditioning or have an effect on fear conditioning.
A There is no difference in space that the neurons occupy (H(3) = 0.291, p = 0.962) between BLA neurons of different groups. B There is no effect on the number of dendrites extending from the neurons (H(3) = 3.2, p = 0.362) in BLA of the different groups. C There is an overall effect on the number of dendritic segments (H(3) = 7.896, p = 0.048). However, in-depth analysis revealed that the groups are not different from each other. D There is an effect on the number of dendritic branch points (H(3) = 8.163, p = 0.043) where neurons of fear conditioning groups showed the lowest score. Activation of PA-Rac1during fear conditioning leads to an increase in branch points compared to the fear conditioning non-activated group (p = 0.04). E We observed an effect on dendritic branching 65 μm, 70 μm, 75 μm and 80 μm from the soma as determined by the intersections in Sholl analysis (H(3) = 11.142, p = 0.011; H(3) = 8.405, 0.038; H(3) = 9.859, p = 0.02; H(3) = 8.010, p = 0.046). Posthoc analysis revealed that the effect is caused by differences between the naïve no PA-Rac1 activation and naïve PA-Rac1 activation in the 65 μm, 75 μm and 80 μm distances (p = 0.011, p = 0.019, p = 0.035, respectively). There was no significant difference between the groups at the 70 μm distance.
Cumulatively, fear conditioning leads to structural changes in dendritic shaft morphology. These morphological alterations may relieve the constraints on signal propagation in the dendrite (see discussion). Rac1 activation prevents these morphological changes and inhibits the formation of fear memory.
Fear conditioning leads to changes in spines morphology that are prevented by Rac1 GTPase activation
The aforementioned results show that fear conditioning increases dendritic shaft volume in BLA neurons and that Rac1 activity prevents this morphological change. We were interested next to examine the effects of fear conditioning on dendritic spines in BLA neurons and the response of these changes to Rac1 activity that impairs fear memory formation. Toward that end, we measured spines density along the dendrite and their morphology (see Fig. 4A for examples of labeled spines). We found that fear conditioning does not increase significantly the spine density in BLA neurons in different dendritic branches (Fig. 4B). We next explored the effect of fear conditioning on dendritic spines morphology. We found an effect on spine length (H(3) = 21.796, p < 0.001; ϵ2 = 0.248). Posthoc analysis revealed that fear conditioning led to an increase in the average length of spines measured from the dendritic shaft (n = 18; n = 4 animals) when compared to naïve animals (n = 17; n = 3 animals) (p = 0.028) (Fig. 4C). In addition, we found that Rac1 activity during fear conditioning blocked the ability of fear conditioning to increase spines length (n = 15; n = 4 animals) when compared to animals where fear conditioning was performed without Rac1 activation (p = 0.008). The spines length in Rac1 activated neurons in fear conditioning animals was kept at its basal level and was not different from spines length in the naïve no light group (p = 1). Spine length in BLA neurons in the naïve light group where Rac1 is activated (n = 17 neurons; n = 5 animals) was not different from spine length in the naïve no light group (p = 1). The spine neck in particular was increased significantly between the four groups (H(3) = 22.587, p < 0.001; ϵ2 = 0.260). Posthoc analysis found that fear conditioning (no light) increases the length of the spine neck when compared to naïve animals (no light) (p = 0.019) (Fig. 4D). The increase in the length of the spine neck following fear conditioning was prevented by Rac1 activation during fear conditioning training (p = 0.014). Spines neck length in Rac1 activation fear-conditioned mice was not different from spines neck length in naïve mice with no light (basal level) (p = 1). Rac1 activation per se in naïve mice had no effect on spines neck length when compared to naïve no light (p = 1). We next examined spines volume differences between the four groups and found an effect (H(3) = 12.887, p < 0.001; ϵ2 = 0.146) (Fear conditioning- n = 18 neurons; n = 4 animals; Naïve- n = 17 neurons; n = 3 animals; Fear conditioning PA-Rac1 activation- n = 15 neurons; n = 4 animals; Naïve Rac1 activation; n = 17 neurons; n = 5 animals). Posthoc analysis found that fear conditioning increased the volume of spines when compared with the naïve group (light and no light combined) (p < 0.006) (Fig. 4E). Rac1 activation during training abolished the ability of fear conditioning to increase spine volume and spine volume was significantly different between fear conditioning with no Rac1 activation and those with Rac1 activation (p = 0.01). Spine volume in BLA neurons in the fear conditioning light group where Rac1 is activated was not different from spine volume in the naïve no light group (basal level) (p = 1). Spine volume in BLA neurons in naïve light group where Rac1 is activated was not different from spine volume in the naïve no light group (p = 0.471). Spine head volume is different between the groups (H(3) = 10.277, p = 0.006; ϵ2 = 0.136). Posthoc analysis found that spine head volume is increased in fear conditioning when compared with the naïve group (light and no light combined) (p = 0.029) (Fig. 4F). Rac1 activation during training abolished the ability of fear conditioning to increase spine head volume and spine head volume was significantly different between fear conditioning with no Rac1 activation and those with Rac1 activation (p = 0.006). Spine head volume in BLA neurons in the fear conditioning light group where Rac1 is activated was not different from spine volume in the naïve no light group (basal level) (p = 0.602). We examined possible alteration in the mean spine diameter per dendritic branch and revealed an effect in branches 1, 2 and 3 (H(3) = 26.26, p < 0.001, ϵ2 = 0.298; H(3) = 24.743, p < 0.001, ϵ2 = 0.288; H(3) = 13.772, p = 0.003, ϵ2 = 0.203) (Fear conditioning- n = 18 neurons; n = 4 animals; Naïve- n = 17 neurons; n = 3 animals; Fear conditioning PA-Rac1 activation- n = 15 neurons; n = 4 animals; Naïve Rac1 activation; n = 17 neurons; n = 5 animals). Posthoc analysis found that fear conditioning (no light) increased the mean diameters of spines when compared to naïve no light or naïve light groups in branches 1, 2 and 3 (p < 0.001; p < 0.001; p < 0.025) (Fig. 4G). However, if fear conditioning was performed in conjunction with PA-Rac1 activation no significant increase in spine diameter was observed when compared to the basal levels in the naïve groups (p = 1). There are no significant changes in other dendritic branches. Cumulatively, these results show that fear conditioning does not lead to changes in spine density but in the length and volume of the spine in particular the length of the neck and volume of the head of the spine. Rac1 activation per se has no effect on spines morphology but if activated during fear conditioning training it prevented the fear conditioning-induced spines morphogenesis.
A Example of dendritic spines (arrows) that were analyzed in the BLA. B Fear conditioning does not lead to changes in spine density in the different dendritic branches of neurons in BLA. C Fear conditioning leads to changes in dendritic spines length when compared to naïve (p = 0.028). Rac1 activation during fear conditioning abolished the ability of fear conditioning to increase the spine length (p = 0.008) and kept the spines at a length that is not different from controls (basal level) (p = 1). D Fear conditioning leads to changes in dendritic spines neck length when compared to naïve (p = 0.019). Rac1 activation during fear conditioning abolished the ability of fear conditioning to increase the spine neck length (p = 0.014) and kept the spines at a length that is not different from controls (p = 1). E Fear conditioning increases the volume of spines when compared with the naïve group (light and no light combined) (p = 0.006). Rac1 activation during training abolished the ability of fear conditioning to increase spines volume and spines volume was significantly different between BLA neurons of fear conditioning animals with no Rac1 activation and those with Rac1 activation (p = 0.01). Spines volume in fear conditioning light group where Rac1 is activated was not different from spines volume in naïve no light group (basal level) (p = 1). F Fear conditioning increases the volume of the spine head when compared with the naïve group (light and no light combined) (p = 0.029). Rac1 activation during training abolished the ability of fear conditioning to increase spines head volume and spines head volume was significantly different between BLA neurons of fear conditioning animals with no Rac1 activation and those with Rac1 activation (p = 0.006). Spines head volume in fear conditioning light group where Rac1 is activated was not different from spines head volume in naïve no light group (basal level) (p = 0.602). G Fear conditioning (no light) increased the mean diameters of spines in branches 1–3 when compared to naïve no light or naïve light (p < 0.025). The diameter of spines in fear-conditioned animals where Rac1 was activated during fear conditioning is not different from naïve animals. H A model based on the current results shows that fear conditioning leads to an increase in proximal dendritic shaft volume and dendritic spines length and volume in neurons in the BLA and to long-term fear memory. Rac1 activation prevents the fear conditioning-induced morphological changes and impairs long-term fear conditioning. The results infer that these structural morphological changes in BLA neurons are needed for long-term memory formation.
Discussion
In this study, we explored whether fear conditioning leads to long-lasting changes in neuronal morphology in the basolateral amygdala (BLA). Moreover, we examined whether the activation of Rac1 GTPase in these neurons will affect long-term memory and such structural changes. Prevention of neuronal morphology and memory formation by Rac1 activation will strongly imply that these neuronal morphological alterations are involved in fear conditioning long-term memory formation.
We show that Rac1 GTPase activation in BLA during fear conditioning impairs auditory long-term fear memory formation but not contextual fear conditioning. This difference is not caused because of examination of the contextual and auditory fear memories at different time points (24 h and 48 h, respectively) as when we activated PA-Rac1 during fear conditioning we see impairments in auditory fear conditioning memory also when we tested it 24 h after training [21]. We observed that fear conditioning leads to an increase in dendritic shaft volume in the proximal dendrite (primary and secondary branches), but not in more distal dendritic branches. The alteration in dendritic volume is prevented in the primary dendritic branch by activation of Rac1 during fear conditioning training. Dendritic branches were not different in length. In addition, fear conditioning increased spines length and volume, especially the length of the spine neck and the volume of the spine head, and Rac1 activation prevented such alterations. Spine density was not affected by fear conditioning.
Fear conditioning affects neuronal morphology in the amygdala. Fear conditioning alters the morphology of BLA neurons that show an increase in spines and synapses size [24,25,26]. This is consistent with our observation. Alteration in spine head volume is linked to changes in synaptic transmission. For example, it was shown that spines with large postsynaptic densities (PSDs) tend to have a higher level of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) than spines with smaller PSDs [27]. Since the area of PSDs is correlated with that of the dimensions of the spine head [28] it is implied that spines with larger heads express more glutamate receptors than spines with smaller heads. In addition, a study found a correlation between the amplitudes of currents in the spine and the spine head volume showing that the distribution of functional AMPARs is approximately proportional to the spine head volume [29]. Thus, synaptic efficacy mediated by AMPA receptors is correlated with spine head volume from silent synapses in small spines to highly responsive larger spines. Thus, enduring increase in spine head volume induced by fear conditioning can increase the efficacy in transmission between neurons in BLA and be part of the memory trace. We also show that fear conditioning leads to an increase in spines length which is contributed mainly to changes in the spine’s neck. Spine neck plasticity appears to mainly affect local voltage amplification in spines and biochemical compartmentalization, such as of Ca2+, within the spine head [30] that may affect signal transduction and bidirectional diffusion of material from dendrite to spines [31, 32]. Spines with longer thinner spine necks confine more molecules. Thus, changes in the spine neck may affect synaptic efficacy and also neuronal function [33, 34]. For example, spines with long necks have small somatic voltage contributions. We found here that activation of Rac1 during learning prevents both fear memory formation and alterations in spines morphology indicating that changes in spines morphology, namely increase spines volume and length, are required for fear memory formation.
We also observed that fear conditioning leads to an increase in dendritic shaft volume at the primary and secondary branches. The length of the dendrites is not affected. The geometry of the dendrite can affect its electrical properties. For example, the diameter of the dendrite can affect the input impedance where small-diameter dendrites have a high input impedance that can affect and increase local synaptic potentials and thus increase the activation of voltage-gated conductance [35]. It was demonstrated that an increase in the diameter of proximal dendrites increased the synaptic efficiency of distal dendrites [36]. We revealed that the alteration in dendrite volume at the primary branch induced by fear conditioning is reduced by Rac1 activation which also impairs fear memory indicating that this morphological change is needed for memory formation. In addition, fear conditioning showed the smallest number of dendritic branches in BLA neurons that were increased back to the naïve level by PA-Rac1 activation during fear conditioning. Reducing the branch points in the dendritic shaft facilitates signal propagation along the dendrite [37, 38]. Thus, increasing both the volume of the dendrite and reducing its branch points can facilitate the propagation (and backpropagation) of signals along the dendrite and preventing such changes, by Rac1 activation, can reduce such propagation and impair memory. Sholl analysis did not detect fear conditioning-induced changes in intersections. However, some differences were detected between Naïve no PA-Rac1 activation and Naïve PA-Rac1 activation at distal dendritic locations from the soma. Interestingly, Rac1 inhibition activity can reduce dendritic complexity (as measured by sholl analysis) at more distal dendritic locations [39]. It could be that there are different Rac1 effectors in these distal dendritic areas.
The results of the study indicate that the morphological changes that are responsive to PA-Rac1 are specific to auditory fear conditioning. We show that fear conditioning leads to alterations in neuronal morphology. PA-Rac1 reduces the morphological alterations (in some cases up to the basal level) and impairs auditory fear conditioning long-term memory. The animals do learn contextual fear conditioning when they receive shock only (also in the context of the auditory-shock protocol). If the morphological changes are caused by shock only, leading to contextual fear conditioning, we would expect to see an effect of PA-Rac1 activation also on contextual fear conditioning memory. However, we do not see any effect of PA-Rac1 activation on contextual fear conditioning. Therefore, the changes in morphology affected by PA-Rac1 are associated with auditory fear conditioning induced by the tone-shock pairing and not contextual fear conditioning memory induced by the shock only. PA-Rac1 also cannot relieve the effects of the footshock as short-term memory is intact [21]. However, although both groups respond to the shock similarly, the PA-Rac1 activated animals show a reduction in auditory fear conditioning long-term memory and changes in morphology. Therefore, the reduction of morphological alteration by PA-Rac1 is not caused by the relief of effects of shock and reduction in stress. In addition, stress affects contextual fear conditioning memory [40]. If PA-Rac1 activation reduces stress and neuronal morphogenesis, then we would expect to see an effect on contextual fear conditioning as well but we do not see an effect. Taken together, our observations show that shock per se, stress and contextual fear learning do not contribute to the effects on PA-Rac1 responsive morphological changes.
Interestingly, we saw that Rac1 activation per se did not reduce the volume and length of the spines or led to a reduction in dendritic volume in the naïve group but rather specifically prevented the increase in spine volume and length and dendritic volume induced by fear conditioning. Thus, it seems that Rac1 activation inhibits signaling that leads to an increase in spine volume and length and dendritic shaft volume by fear conditioning. Both spines and dendritic shafts contain actin cytoskeleton and actin cytoskeleton can affect dendritic spines and dendritic shaft morphology [41,42,43,44]. Rac1 regulates actin regulatory proteins that in turn affect the actin cytoskeleton and spine morphology [45]. It is possible that fear conditioning leads to an increase in actin polymerization through the regulation of actin regulatory proteins and that Rac1 activation inhibits the actin regulatory proteins and counteracts fear conditioning-induced morphogenesis.
We think that subsequent studies should be also conducted in female mice as behavioral outcomes induced by alterations of proteins located at the Rac1 pathway may be different between males and female mice (e.g., as seen with Dock4 a Rac1 guanine nucleotide exchange factor [46]). Moreover, neuronal properties and morphology and morphological responses to inputs in the BLA can be different between males and females [47, 48].
The observations in the study show that: 1) Fear conditioning leads to specific changes in neuronal morphology in BLA neurons. 2) These alterations are long-lasting and are preserved 48 h after training. 3) Rac1 activation during learning prevents the dendritic morphological changes induced by fear conditioning. Since the activation of Rac1 is effective during fear conditioning training the cellular signaling for dendritic changes is fast. 4) The prevention of both fear conditioning long-term memory and fear conditioning-induced morphological alteration by Rac1 activation indicates that these neuronal enduring morphological alterations are needed for fear conditioning memory formation and form the memory trace.
References
Hebb DO. The organization of behavior: a neuropsychological theory. New York, NY: Wiley; 1949.
Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9.
Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–8.
Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev Neurosci. 2004;5:45–54.
Bailey CH, Kandel ER, Harris KM. Structural components of synaptic plasticity and memory consolidation. Cold Spring Harb Perspect Biol. 2015;7:a021758.
Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–32.
LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84.
Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34.
Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–34.
Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–6.
Schafe GE, Nader K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001;24:540–6.
Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91.
Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–24.
Luo L, Hensch TK, Ackerman L, Barbel S, Jan LY, Jan YN. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature. 1996;379:837–40.
Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–38.
Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–80.
Pilpel Y, Segal M. Activation of PKC induces rapid morphological plasticity in dendrites of hippocampal neurons via Rac and Rho-dependent mechanisms. Eur J Neurosci. 2004;19:3151–64.
Newey SE, Velamoor V, Govek EE, Van Aelst L. Rho GTPases, dendritic structure, and mental retardation. J Neurobiol. 2005;64:58–74.
Gao Q, Yao W, Wang J, Yang T, Liu C, Tao Y, et al. Post-training activation of Rac1 in the basolateral amygdala is required for the formation of both short-term and long-term auditory fear memory. Front Mol Neurosci. 2015;8:65.
Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, et al. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature. 2015;525:333–8.
Das A, Dines M, Alapin JM, Lamprecht R. Affecting long-term fear memory formation through optical control of Rac1 GTPase and PAK activity in lateral amygdala. Sci Rep. 2017;7:13930.
Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–8.
Hama H, Hioki H, Namiki K, Hoshida T, Kurokawa H, Ishidate F, et al. ScaleS: an optical clearing palette for biological imaging. Nat Neurosci. 2015;18:1518–29.
Lamprecht R, Farb CR, Rodrigues SM, LeDoux JE. Fear conditioning drives profilin into amygdala dendritic spines. Nat Neurosci. 2006;9:481–3.
Ostroff LE, Cain CK, Bedont J, Monfils MH, Ledoux JE. Fear and safety learning differentially affect synapse size and dendritic translation in the lateral amygdala. Proc Natl Acad Sci USA. 2010;107:9418–23.
Choi DI, Kim J, Lee H, Kim JI, Sung Y, Choi JE, et al. Synaptic correlates of associative fear memory in the lateral amygdala. Neuron. 2021;109:2717–2726.e3.
Takumi Y, Ramírez-León V, Laake P, Rinvik E, Ottersen OP. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci. 1999;2:618–24.
Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci. 1989;9:2982–97.
Noguchi J, Nagaoka A, Watanabe S, Ellis-Davies GC, Kitamura K, Kano M, et al. In vivo two-photon uncaging of glutamate revealing the structure-function relationships of dendritic spines in the neocortex of adult mice. J Physiol. 2011;589:2447–57.
Noguchi J, Matsuzaki M, Ellis-Davies GC, Kasai H. Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron. 2005;46:609–22.
Bloodgood BL, Sabatini BL. Neuronal activity regulates diffusion across the neck of dendritic spines. Science. 2005;310:866–9.
Santamaria F, Wils S, De Schutter E, Augustine GJ. Anomalous diffusion in Purkinje cell dendrites caused by spines. Neuron. 2006;52:635–48.
Araya R, Jiang J, Eisenthal KB, Yuste R. The spine neck filters membrane potentials. Proc Natl Acad Sci USA. 2006;103:17961–6.
Araya R, Vogels TP, Yuste R. Activity-dependent dendritic spine neck changes are correlated with synaptic strength. Proc Natl Acad Sci USA. 2014;111:E2895–904.
Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9:206–21.
Wolf E, Birinyi A, Székely G. Simulation of the effect of synapses: the significance of the dendritic diameter in impulse propagation. Eur J Neurosci. 1992;4:1013–21.
Williams SR, Stuart GJ. Action potential backpropagation and somato-dendritic distribution of ion channels in thalamocortical neurons. J Neurosci Off J Soc Neurosci. 2000;20:1307–17.
Vetter P, Roth A, Häusser M. Propagation of action potentials in dendrites depends on dendritic morphology. J Neurophysiol. 2001;85:926–37.
Vadodaria KC, Brakebusch C, Suter U, Jessberger S. Stage-specific functions of the small Rho GTPases Cdc42 and Rac1 for adult hippocampal neurogenesis. J Neurosci. 2013;33:1179–89.
Cordero MI, Venero C, Kruyt ND, Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats. Evidence for a role of corticosterone. Horm Behav. 2003;44:338–45.
Dillon C, Goda Y. The actin cytoskeleton: integrating form and function at the synapse. Annu Rev Neurosci. 2005;28:25–55.
Leite SC, Sousa MM. The neuronal and actin commitment: why do neurons need rings? Cytoskeleton. 2016;73:424–34.
Lanoue V, Cooper HM. Branching mechanisms shaping dendrite architecture. Dev Biol. 2019;451:16–24.
Bucher M, Fanutza T, Mikhaylova M. Cytoskeletal makeup of the synapse: shaft versus spine. Cytoskeleton. 2020;77:55–64.
Costa JF, Dines M, Lamprecht R. The role of Rac GTPase in dendritic spine morphogenesis and memory. Front Synaptic Neurosci. 2020;12:12.
Guo D, Peng Y, Wang L, Sun X, Wang X, Liang C, et al. Autism-like social deficit generated by Dock4 deficiency is rescued by restoration of Rac1 activity and NMDA receptor function. Mol Psychiatry. 2021;26:1505–19.
Guadagno A, Wong TP, Walker CD. Morphological and functional changes in the preweaning basolateral amygdala induced by early chronic stress associate with anxiety and fear behavior in adult male, but not female rats. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:25–37.
Guily P, Lassalle O, Chavis P, Manzoni OJ. Sex-specific divergent maturational trajectories in the postnatal rat basolateral amygdala. iScience. 2022;25:103815.
Acknowledgements
We would like to thank Boris Shklyar from the Bioimaging Unit, Faculty of Natural Sciences, University of Haifa, for his help with the imaging. This research was supported by a grant from the Ministry of Science and Technology, Israel and the Israel Science Foundation for RL.
Author information
Authors and Affiliations
Contributions
JFC performed the experiments and analyzed the data, MD performed part of the behavioral experiments, KA performed part of the behavioral experiments and RL performed the statistical analysis and wrote the paper with inputs from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Costa, J.F., Dines, M., Agarwal, K. et al. Rac1 GTPase activation impairs fear conditioning-induced structural changes in basolateral amygdala neurons and long-term fear memory formation. Neuropsychopharmacol. 48, 1338–1346 (2023). https://doi.org/10.1038/s41386-022-01518-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-022-01518-8
- Springer Nature Switzerland AG
This article is cited by
-
EphrinB2 in excitatory neurons and astrocytes in the basolateral amygdala controls long-term fear memory formation
Communications Biology (2024)