Abstract
The aim of this study was to provide evidence-based recommendations regarding the efficacy, safety, and tolerability of currently used pharmacological treatments for adults with acute bipolar mania. To achieve this, we conducted a systematic review and network meta-analysis (NMA) using R software and related packages. We searched primary clinical databases until February 2023 for reports of randomized controlled trials of drug treatments and adjunctive therapies for adults with acute bipolar mania, with outcomes including efficacy (mean change from baseline to endpoint in mania rating scores), safety (clinically significant adverse events from baseline to end of treatment), and tolerability (the proportion of patients who completed the whole trial to the planned endpoint). A total of 113 studies were included in our analysis, in which 23,491 participants (50.38% males; mean age = 38.6 years; mean study duration = 3.39 weeks; mean manic baseline score = 29.37) were randomly allocated to one of 51 monotherapies, adjunctive treatments, or placebo. Our results showed that tamoxifen (mean difference, −22.31 [−25.97, −18.63], N = 2, n1 = 43, n2 = 39) and tamoxifen+ lithium or valproate (LIT/VAL) (−16.37 [−22.55, −10.25], N = 1, n1 = 20, n2 = 20) had the best and second-best clinical efficacy in adults with acute bipolar mania over the placebo. Furthermore, olanzapine, paliperidone, quetiapine, ziprasidone, risperidone, divalproex, and haloperidol were significantly better tolerated than placebo. Combination therapies of antipsychotics and LIT/VAL appeared to be more effective than their corresponding monotherapies. While pharmacotherapies were associated with specific common adverse events, we found no evidence of increased incidence of headache or depression events compared to the placebo. Overall, our NMAs provided important insights into the effectiveness, safety, and tolerability of pharmacological treatments for acute bipolar mania and can help guide treatment decisions for clinicians.
Similar content being viewed by others
Introduction
Bipolar disorder (BD) poses numerous challenges, particularly in the acute phase of treatment. One of the conditions that requires immediate attention during its symptomatic phase is mania [1]. Acute mania is a manic phase of bipolar disorder marked by a heightened duration of exuberant mood, increased energy levels, and inflated self-esteem. The psychosocial implications of acute mania encompass impaired insight into one’s behavior, hazardous risk-taking behavior, social withdrawal, and legal entanglements [2, 3]. Acute mania can affect individuals of all ages and genders, and existing mental health difficulties may increase the likelihood of developing manic episodes. This intense mental state can significantly disrupt a person’s life and carry negative social and economic consequences. Research suggests that the worldwide prevalence of bipolar disorder is estimated to be ~1%, and the economic burden of bipolar disorder in the United States is estimated to exceed
Current guidelines recommend lithium, divalproex, atypical antipsychotics, and several combination therapies as clinical treatments for individuals experiencing mania [4,5,6]. Typical monotherapy for acute mania in adults involves a single medication, such as antipsychotics, or mood stabilizers. Antipsychotics such as olanzapine and risperidone are also commonly used and have shown effectiveness in short-term management of manic symptoms. Additionally, mood stabilizers, such as lithium carbonate, are often recommended to prevent future manic episodes [4,5,6]. Available evidence supports the efficacy of monotherapies in the short-term management of mania for adults [7,8,9,10,11]. Recent meta-analyses on a large scale only considered monotherapies and demonstrated that the monotherapy of tamoxifen, carbamazepine, and risperidone showed superior efficacy compared to other antipsychotics for acute mania or manic episodes in the short term [12, 13].
Combinations of antipsychotics and mood stabilizers (AP/MS) are frequently used for the acute treatment of mania in adults, in addition to monotherapy. These therapies involve multiple medications, such as antipsychotics, and/or mood stabilizers. A study has shown that AP/MS therapy is more effective than monotherapy in managing mania in the short term, but higher dropout rates have been associated with the use of multiple drugs [14]. Moreover, a network meta-analysis (NMA) revealed that the combination of aripiprazole and valproate was the most effective in reducing recurrence/relapse rates for mood and depressive episodes during the maintenance phase [15]. Though previous studies indicated the efficacy of monotherapies for acute mania treatment, the efficacy, safety, and tolerability of pharmacological therapies, including single and multiple regimens, in the management of acute mania in adults have not been systematically compared due to limited available evidence.
To aid in clinical treatment and clarify the efficacy and safety of monotherapies and combined therapies, a systematic review and NMA of the efficacy, safety, and tolerability of all currently available pharmacological therapies for adults with acute bipolar mania was conducted, building upon our previous study.
Methods
Search strategy and selection criteria
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [16] (PRISMA NMA Checklist of Items to Include When Reporting A Systematic Review Involving a Network Meta-analysis). A thorough protocol for this systematic review and NMA is accessible on the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42023387438. We conducted a comprehensive search for randomized control trials (RCTs) in various databases, including Cochrane Central Register of Controlled Trials, PubMed, Embase, MEDLINE, PsycINFO, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform, and regulatory agency websites, with no language limitations. We also contacted pharmaceutical companies and researchers to address any gaps in the original studies’ reporting and to obtain new, previously unreported data. Additionally, we manually searched international trial registrations and major scholarly articles in this field for RCTs. The flow diagram is detailed in Supplementary Appendix Sup001.
Inclusion criteria considered journal papers, conference proceedings, and sponsor publications such as trial summaries, regulatory assessments, and trials. We conducted a systematic review of relevant research studies, including those with enrichment designs, the following were the inclusion criteria for studies: (1) both published and unpublished RCTs; (2) studies comparing pharmacological treatments to placebo or other active drugs, whether administered as monotherapy or in combination, for patients with acute hypomanic/manic episodes; (3) studies of adults with an average age of 18 years or older and a baseline mania mean score of at least 20, and had to use internationally recognized diagnostic criteria, including Diagnostic and Statistical Manual of Mental Disorders (DSM)-3, DSM-3-R, DSM-4, DSM-4 TR, DSM-5, International Classification of Diseases (ICD)-10, Chinese Classification of Mental Disorders (CCMD)-2R, CCMD-3, or earlier standardised criteria; (4) double-blind, single-blind RCTs, and RCTs with any level of blinding for the network meta-analysis. We included a wide range of pharmaceutical therapies for acute bipolar mania, including conventional psychiatric pharmaceuticals, atypical psychiatric drugs, mood stabilizers, and other medicines authorized by medical device regulatory bodies for mania from various sources, including the British National Formulary, the US Food and Drug Administration, the European Medicines Agency, the Pharmaceuticals and Medical Devices Agency in Japan, the National Medical Products Administration (formerly the China Food and Drug Administration) in mainland China, and the Therapeutic Goods Administration in Australia. We only included RCTs with full data and individuals given dosages within the relevant therapeutic range or most frequently used dose, in accordance with The Maudsley Prescribing Guidelines in Psychiatry, 14th Edition [17], a professional guideline on the clinical use of psychotropic drugs for mental illnesses.
We excluded studies that: (1) contained dietary supplements (e.g., vitamins, fish oil, and omega-3 fatty acids) and botanical medications (e.g., St. John’s wort) due to the possibility of herb-drug interactions; (2) contained enrolled individuals with a combined diagnosis of BD and another mental condition or that were only partially in the maintenance phase; (3) contained child/adolescent BD patients due to significant disparities in phenomenology and diagnostic difficulties between juvenile and adult mania; (4) contained 20% or more of the participants had psychotic depression or treatment-resistant depression, in addition to those who have a serious concurrent medical issue; (5) terminated prematurely without an efficacy analysis during the research to ensure methodological rigor and comparability across studies. We additionally excluded studies with a total sample size of less than 10 participants. To select studies for inclusion in our network meta-analysis, two researchers (WH and SH) independently read main reports and additional materials, retrieving pertinent data with quantitative estimates or accurate data for outcome measures. To assess the risk of bias, we utilized the Cochrane risk of bias criteria in the Review Manager software (RevMan version 5.4.1) [18]. The authors double-verified the transmission and computation of study data. Any discrepancies were resolved through consensus and arbitration with other review team members (ML, JX and SH).
Data synthesis, outcome measures, and data extraction
The primary outcomes were efficacy and safety, and the secondary outcome was the tolerability of treatments. Efficacy was evaluated by mean change from baseline to endpoint in mania rating scores, including the Young Mania Rating Scale (YMRS), Mania Rating Scale (MRS), Mania Scale (MAS), and Manic State Rating Scale (MSRS). Safety was evaluated by all clinically significant adverse events from baseline to end of treatment, and tolerability was evaluated by the proportion of patients who completed the whole trial to the planned endpoint. We classified frequent undesirable occurrences using the Medical Dictionary for Regulatory Activities (MedDRA). The evaluation time point with three weeks later was chosen as the short-term endpoint to evaluate the effectiveness, acceptability, and tolerability of active treatments. Studies that lacked 3-week data were included if they provided data from time periods ranging from 14 days to 4 weeks. All trials with variable doses were considered, as they allowed researchers to titrate to the highest effective dose for each participant. In the analysis of trials with two or more treatment arms of the same medication at various dosages, information from treatment arms was combined within a medicinal dosage range. The intention-to-treat or modified intention-to-treat principles were applied to the retrieved data, with missing data sought from published systematic review papers when necessary. Unpublished material was given priority when there was a disagreement between published and unpublished data. To eliminate performance and detection bias for subjective results, double-blind studies were included, while single-blind studies were included for primary outcomes. Other relevant information, such as scientific features (first author, location, publication year, trial design, follow-up duration, sample size, and sponsorship), participant characteristics (baseline and endpoint scores, gender, mean age), intervention specifics (dose ranges, mean doses, treatment group, control group), and outcome measures from the included trials were extracted and filled in a prior designed form.
Data analysis
A previous literature review led us to plan a Bayesian NMA using random effects models [19]. The NMA comprised three types of studies: (1) trials that compared monotherapy of active medication with either placebo or another active medication, (2) trials that studied combinations or adjuvants of two agents that were indicated (we categorized treatments initiated simultaneously as combined therapy and those added subsequently as adjuvant therapy), and (3) trials that compared combinations of medications with lithium or valproate (LIT/VAL) to placebo-LIT/VAL. If the data was missing or unclear, we reached out to the research authors via email or social networking sites such as https://www.researchgate.net/. In situations where dichotomous outcome data was unavailable, we presumed that patients who dropped out after randomization had an unfavorable outcome. In the case of continuous outcome data, we followed the same approach as the original study to address missing data. Typically, this involved mixed model repeated measures or last observation carried forward. If neither of these approaches was employed, we only assessed data from patients who completed the trial.
We used a variety of techniques to calculate missing standard deviations (SD) from p-values, t-values, F-values, and standard errors or filled them in using a validated method. For dichotomous outcomes, we calculated the odds ratio (OR) and 95% credible interval (95% CI), and for continuous outcomes, we calculated the mean difference (MD, Cohen’s d) and their 95% CIs using pairwise and network meta-analysis. To perform the analysis, we used the “gemtc” package in R statistical software (version 4.0.5), which employs a Markov chain Monte Carlo (MCMC) approach. Each MCMC model was generated by running 10,000 adaptation and 25,000 sampling iterations. We used a model fit test to determine the degree of model fit, with models having lower deviance information criterion (DIC) values being preferred over those with higher values to reduce the difference between predicted and observed output values. We assessed the statistical heterogeneity in every comparison made in both pairwise and network meta-analyses using the t2 and I2 statistics [20]. Network meta-analyses were conducted using a random-effects model in a frequentist setting, assuming medium to significant heterogeneity across all comparisons and accounting for correlations caused by multi-arm research. We used a fixed-effect Mantel–Haenszel technique [21] for the network meta-analysis of double-blind RCTs and compared the results with a fixed-effects inverse-variance model for rare occurrences. We evaluated the convergence diagnostics for MCMC models using Gelman and Rubin’s convergence diagnostics, which computed the possible scale reduction factor for each variable in x, along with the upper and lower confidence bounds. We detected approximate convergence when the upper boundary approached 1. Bayesian approaches allowed us to rate the efficacy of directly and indirectly comparable treatment regimens using the probability and surface under the cumulative ranking (SUCRA) values [22], with a higher probability and SUCRA value indicating greater treatment effectiveness. Finally, we generated a heatmap of treatment ranking using an online NMA application called NMAstudio (https://www.nmastudioapp.com/).
To investigate whether the transitivity assumption was met, we analyzed the distribution of major study characteristics across comparative studies. To evaluate inconsistency between direct and indirect sources of information, we employed both global and local methodologies. We used the node splitting technique to assess statistical inconsistency, considering inconsistency significant if p-values were less than 0.05 [23]. We also used back-calculation and separate indirect from direct design evidence methodologies to analyze local inconsistency, comparing direct and indirect evidence for each matched treatment comparison. If we had at least 10 studies, we used a comparison-adjusted funnel plot [24] to assess the potential for small-study effects and publication bias for each treatment pair. Additionally, we used the leverage versus residual deviation plot to determine if the majority of points fell within the displayed curved parabola with a constant of 3. We assessed included studies using the Cochrane tool for assessing risk of bias [20], and also assessed confidence in the evidence across six domains using an online program called the Confidence in Network Meta-Analysis framework (CINeMA, available at https://cinema.ispm.unibe.ch/) [25].
To evaluate the possibility of differences in treatment effects and the robustness of our results, we carried out network meta-regression analyses and subgroup network meta-analyses, using confounding factors (including sample size, location, publication year, mania scale scoring, gender ratio, mania baseline, stabilizers or not, patients age, sponsorship, and mixed episodes or not) to influence study outcomes. We also conducted sensitivity analyses on studies with low overall risk of bias, trials that applied standardized diagnostic criteria for mania, and trials with imputed standard deviations. Additionally, we used league tables to demonstrate the comparison results of direct and indirect therapies in network meta-analysis.
Results
Study characteristics
A diagram illustrating the search procedure and a comprehensive description of the methodology are performed in Supplementary Appendix Sup001, Flow diagram. Of the 13,052 articles initially identified between July 1989, and February 2023, 9072 were duplicates, 3792 were eliminated after evaluating the titles and abstracts, and 12 were deemed ineligible and excluded after a thorough review of the full texts due to inclusion criteria requiring a minimum age of 18 years, 12 were excluded due to limited sample size or lack of a control group. Five RCTs were also excluded as they included complicated combination treatments (e.g., allopurinol+haloperidol+LITVAL) and these comparisons were not able to be included and calculated in NMA. In addition, two RCTs regarding tamoxifen adjuvant therapies were removed due to use of a mania rating scale that did not meet our inclusion criteria. One study comparing carbamazepine and divalproex was removed from our NMA as we confirmed a p-value less than 0.01 after node-splitting analysis.
We selected 111 eligible study articles, and 2 additional articles were identified from previous studies publications. Of the 113 eligible RCTs, the majority were double-blind, 76 reported available data for monotherapy comparisons, including drug-drug and drug-placebo, while 37 RCTs reported available data for comparisons of combined/adjuvant treatments, including active drugs and/or LIT/VAL, active drugs and/or placebo, placebo and/or LIT/VAL, and LIT/VAL.
Of the 113 studies included in this review, DSM criteria (including DSM-III, DSM IV, and DSM V criteria) were used in 105 studies, while the RDC, ICD-10, and CCMD criteria were separately used in one study. The follow-up duration for these studies ranged from 2 to 6 weeks, a substantial proportion of studies used score changes in the YMRS as the primary or secondary outcome. The remaining 15 studies used mean score changes in the MRS, the MAS, and the MSRS as the primary outcome. Of the 113 studies, 58 were conducted in North America (51.32%), 16 in Iran, 8 in the multiple centers, 6 in Spain, 5 in China, 3 in United Kingdom, 4 in India, 3 in South Africa, 2 in Israel, 2 in Japan, 1 in Brazil, Russia, New Zealand, Turkey, Taiwan, and South Korea, respectively.
113 RCTs (51 treatments, n = 23,491, Fig. 1; males = 50.38%; mean age = 38.6 years, SD = 4.4; mean study duration = 3.39 weeks, SD = 0.97; mean manic baseline score = 29.37, SD = 3.24) were included with the following 26 antipsychotic, non-psychotic, or placebo monotherapy arms (number of studies (N)/individuals (n)): aripiprazole (8/1094), asenapine (5/709), allopurinol (1/90), brexpiprazole (2/320), carbamazepine (5/271), cariprazine (4/601), divalproex (15/1094), ebselen (1/27), endoxifen (2/144), eslicarbazepine (1/57), haloperidol (6/443), lamotrigine (5/328), licarbazepine (1/218), lithium (20/1048), melatonin (1/21), olanzapine (13/924), oxcarbazepine (1/30), paliperidone (2/305), quetiapine (10/1009), risperidone (6/637), tamoxifen (2/43), topiramate (4/434), valnoctamide (1/70), verapamil (1/17), ziprasidone (2/268), placebo (56/5811), and the following 25 antipsychotic and non-psychotic combined therapy arms: allopurinol+LIT/VAL (2/90), aripiprazole+LIT/VAL (3/457), asenapine+LIT/VAL (2/205), celecoxib+LIT/VAL (2/44), clonidine+LIT/VAL (1/36), dipyridamole+LIT/VAL (1/60), donepezil+LIT/VAL (1/15), haloperidol+LIT/VAL (4/101), levetiracetam+LIT/VAL (2/74), LIT/VAL (3/323), lovastatin+LIT/VAL (1/24), memantine+LIT/VAL (1/35), melatonin+LIT/VAL (1/30), olanzapine+LIT/VAL (4/405), paliperidone+LIT/VAL (2/299), placebo+asenapine (1/59), placebo+LIT/VAL (27/1934), placebo+quetiapine (1/176), placebo+ziprasidone (1/101), quetiapine+LIT/VAL (6/686), risperidone+LIT/VAL (4/175), rivastigmine+LIT/VAL (1/35), tamoxifen+LIT/VAL (1/20), topiramate+LIT/VAL (1/143), ziprasidone+LIT/VAL (1/226). The study characteristics are summarized in Supplementary Appendix Sup002, Supplementary Table 1. In addition, out of the total number of studies reviewed, 71 were sponsored by pharmaceutical corporations and industry. 15 studies included individuals with rapid cycling, 48 included individuals with mixed state/episode, while 11 excluded them. Moreover, 35 studies included individuals with psychosis, and 4 excluded them.
Although 21 studies were assessed to have a low risk of bias, many trials lacked appropriate randomization methods, which resulted in an unclear risk of bias rating. One study was deemed to have a high risk for attrition bias because of incomplete data, while one study was classified as high risk for reporting bias because of selective report. Other studies were evaluated to have a moderate risk of bias overall (Supplementary Appendix Sup002, Supplementary Fig. 3).
Mean changes of mania rating scale scores
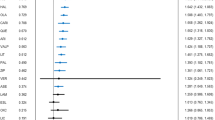
Compared with the placebo, tamoxifen, tamoxifen+LITVAL, clonidine+LITVAL, allopurinol+LITVAL, rivastigmine+LITVAL, levetiracetam+LITVAL, risperidone+LITVAL, celecoxib+LITVAL, olanzapine+LITVAL, melatonin+LITVAL, asenapine+LITVAL, quetiapine+LITVAL, dipyridamole+LITVAL, aripiprazole+LITVAL, haloperidol+LITVAL, placebo+quetiapine, memantine+LITVAL, risperidone, ziprasidone+LITVAL, lovastatin+LITVAL, carbamazepine, olanzapine, cariprazine, ziprasidone, paliperidone, haloperidol, topiramate+LITVAL, quetiapine, placebo+LITVAL, lithium, aripiprazole, asenapine, and divalproex were substantially more effective in the reduction of manic rating scale scores (MD [95% CI] ranged from −2.86 [−4.26, −1.50] for divalproex to −22.31 [−25.97, −18.63] for tamoxifen; Figs. 2 and 3). Tamoxifen (N = 2, n1 = 43, n2 = 39) and tamoxifen+LITVAL (N = 1, n1 = 20, n2 = 20) were ranked as the best and second-best treatments with SUCRA values of 0.9988 and 0.9785 (Fig. 4). The statistical difference was demonstrated in the league table including the MD values and 95% CI of direct and indirect comparisons. Although global heterogeneity was low (I2 = 37.85%), we detected moderate to high heterogeneity between several comparisons: aripiprazole vs. placebo (I2 = 75.73%), aripiprazole vs. lithium (I2 = 79.02%), carbamazepine vs. placebo (I2 = 82.61%), divalproex vs. placebo (I2 = 71.35%), lithium vs. olanzapine (I2 = 73.41%), placebo vs. risperidone (I2 = 71.54%). There was statistical consistency between direct and indirect estimates, with the exception of the following one comparison: divalproex vs. olanzapine+LITVAL (olanzapine+LITVAL outperformed divalproex more in the direct comparison, MD: −9.0 [−13.0, −5.1], N = 1, n1 = 37, n2 = 38, but significantly less in the indirect comparison, MD: −1.4 [−6.3, 3.6], p-value = 0.02).
The analysis was performed using a random-effects model. A black node and an intersecting vertical and horizontal line indicate no significant difference between the active treatment and the placebo. A green node and a mean difference less than 0 indicate that the active treatment is significantly superior to placebo. “MD” represents mean difference, “LITVAL” represents lithium or valproate, and “RE” represents random effects.
A random effects model was used to conduct the analysis. When the vertical line intersects with the horizontal lines and the nodes are black, it suggests there is no significant difference between the active treatment and placebo. If the mean differences are less than 0 and the nodes are green, it indicates that the active treatment is significantly better than placebo. The abbreviation “MD” stands for mean difference, “LITVAL” stands for lithium or valproate, and “RE” stands for random effects.
The Y-axis shows the decreasing probability of a given therapy being ranked as the first, second, or third-best option for treating acute mania, moving gradually towards being ranked as the worst option. “SUCRA” is an abbreviation for the surface under the cumulative ranking curve. It’s worth noting that a higher SUCRA value suggests that an intervention may perform better than all additional mania therapy in network comparisons. In this plot, tamoxifen has been identified as the most effective treatment, with a SUCRA value of 0.9988. “LITVAL” refers to lithium or valproate.
We performed a network meta-regression analysis using possibly confounding parameters to derive a final relationship based on Beta-value and 95% CI. The meta-regression analyses indicated that these factors might not have affected the treatment outcomes of acute manic/hypomanic/mixed episodes in this study, except for mixed episodes. This suggests that the inclusion of patients with mixed episodes in trials could influence the final results of the NMA (Beta-value: −4.21, [−6.10, −2.10]).
Sensitivity analyses (focusing on studies without MS arm, studies without AP arm, studies without placebo arm, nonindustry-sponsored studies, studies not including individuals with rapid-cycling, non-USA-conducted studies, non-Iran-conducted studies, and studies with high-quality design) were performed by removing corresponding studies from our NMA. The findings demonstrated that the magnitude of the treatment effect on the primary analysis outcome was comparable to that of the adjusted analyses (Supplementary Appendix Sup002, Supplementary Table 7).
Safety
Compared to placebo, several antipsychotics were associated with an increased risk of various side effects in this study. Akathisia had a higher incidence with aripiprazole, brexpiprazole, cariprazine, paliperidone, risperidone, and ziprasidone (OR [95% CI] range: 1.27 [1.03–3.48] for paliperidone to 5.47 [3.44–7.81] for haloperidol, N = 20, n = 4638). Constipation was more common with aripiprazole, allopurinol+LITVAL, aripiprazole+LITVAL, cariprazine, olanzapine, olanzapine+LITVAL, quetiapine+LITVAL, and quetiapine (OR [95% CI] range: 1.72 [1.12–2.55] for aripiprazole to 2.81 [1.50–5.47] for quetiapine, N = 24, n = 4972). Dizziness had a higher incidence with asenapine, carbamazepine, haloperidol, olanzapine, olanzapine+LITVAL, paliperidone+LITVAL, quetiapine, quetiapine+LITVAL, and ziprasidone (OR [95% CI] range: 2.76 [1.81, 3.89] for olanzapine to 3.21 [2.13, 4.75] for carbamazepine, N = 26, n = 6235). Dry mouth was more common with carbamazepine, olanzapine, quetiapine+LITVAL, and quetiapine (OR [95% CI] range: 2.97 [2.02–4.16] for quetiapine to 4.74 [1.55, 17.88] for carbamazepine, N = 20, n = 4118). Extrapyramidal symptoms had a higher incidence with aripiprazole, asenapine, cariprazine, haloperidol, olanzapine, olanzapine+LITVAL, and ziprasidone (OR [95% CI] range: 1.50 [1.01, 3.07] for olanzapine to 4.82 [3.13–6.46] for haloperidol, N = 24, n = 6003). Somnolence was more common with aripiprazole, asenapine, cariprazine, olanzapine, olanzapine+LITVAL, paliperidone, placebo+asenapine, quetiapine, quetiapine+LITVAL, risperidone, and ziprasidone (OR [95% CI] range: 3.01 [1.82, 5.33] for aripiprazole to 24.18 [5.81, 130.41] for placebo+asenapine, N = 35, n = 8487). Weight gain had a higher incidence with asenapine, olanzapine, paliperidone, quetiapine, and ziprasidone (OR [95% CI] range: 1.87 [1.04–4.89] for ziprasidone to 7.87 [4.38–15.44] for olanzapine, N = 20, n = 4822).
Compared with the placebo, cariprazine and risperidone (N = 5, n = 1473) had a higher incidence of nausea (OR [95% CI] ranged from 1.72 [1.02–2.91] for cariprazine to 5.38 [1.29–22.46] for risperidone). Rivastigmine+LITVAL had a higher incidence of nausea (4.24 [1.47–12.24], N = 1, n = 70) than placebo+LITVAL.
Compared with the placebo, there were no significant differences in the incidence of headache (N = 27, n = 6486), except for brexpiprazole (0.54 [0.28–1.00]). In contrast, allopurinol+LITVAL (0.20 [0.02–0.89], N = 10, n = 1941) was associated with a lower incidence of headache compared to placebo+LITVAL.
No statistically significant difference was observed in the incidence of depression associated with any treatment compared to the placebo (N = 16, n = 5082) or the placebo+LITVAL (N = 11, n = 1951).
Tolerability
92 studies (n = 17,599), which contained 48 interventions (allopurinol, allopurinol+LITVAL, aripiprazole, aripiprazole+LITVAL, asenapine, asenapine+LITVAL, brexpiprazole, carbamazepine, cariprazine, celecoxib+LITVAL, clonidine+LITVAL, dipyridamole+LITVAL, divalproex, donepezil+LITVAL, ebselen, endoxifen, eslicarbazepine, haloperidol, haloperidol+LITVAL, lamotrigine, levetiracetam+LITVAL, licarbazepine, lithium, LITVAL, lovastatin+LITVAL, melatonin, melatonin+LITVAL, memantine+LITVAL, olanzapine, olanzapine+LITVAL, oxcarbazepine, paliperidone, paliperidone+LITVAL, placebo, placebo+asenapine, placebo+LITVAL, placebo+quetiapine, quetiapine, quetiapine+LITVAL, risperidone, risperidone+LITVAL, rivastigmine+LITVAL, tamoxifen, tamoxifen+LITVAL, topiramate, valnoctamide, verapamil, and ziprasidone) and 72 direct comparisons, were included in our NMA regarding tolerability of different treatments. Compared with the placebo, olanzapine (OR [95% CI]; 2.5 [1.8, 3.3], N = 11, n = 966), paliperidone (1.9 [1.2, 3.0], N = 2, n = 305), quetiapine (1.8 [1.4, 2.5], N = 9, n = 923), ziprasidone (1.8 [1.1, 3.0], N = 3, n = 283), risperidone (1.7 [1.2, 2.4], N = 6, n = 637), divalproex (1.6 [1.0, 2.4], N = 9, n = 580), and haloperidol (1.5 [1.0, 2.2], N = 5, n = 433) indicated the higher completion rate in the treatment of mania (Figs. 5 and 6), at the same time, these medications were also more effective than placebo for the treatment of mania/hypomania. Olanzapine and quetiapine were ranked as the best and second-best treatments with SUCRA values of 0.8390 and 0.7366 (Fig. 7). The statistical difference was demonstrated in the league table including the OR values and 95% CI of direct and indirect estimates. Asenapine significantly outperformed valnoctamide. Divalproex significantly outperformed brexpiprazole. Olanzapine significantly outperformed allopurinol, allopurinol+LITVAL, aripiprazole, aripiprazole+LITVAL, asenapine, brexpiprazole, carbamazepine, cariprazine, divalproex, eslicarbazepine, haloperidol, licarbazepine, lithium, lovastatin+LITVAL, memantine+LITVAL, placebo+LITVAL, valnoctamide, and verapamil. Paliperidone significantly outperformed valnoctamide and verapamil. Quetiapine+LITVAL significantly outperformed placebo+LITVAL. Quetiapine significantly outperformed valnoctamide and verapamil. Risperidone significantly outperformed valnoctamide. Topiramate significantly outperformed valnoctamide.
The analysis was conducted using a random effects model. If the vertical line intersects with the horizontal lines and the nodes are black, it suggests no significant difference between the active treatment and placebo. If the odds ratios are greater than 1 and the nodes are green, it indicates that active treatment is significantly better than placebo. “LITVAL” refers to lithium or valproate, while “RE” stands for random effects.
The analysis was conducted using a random effects model. If the vertical line intersected with horizontal lines and the nodes were black in color, it suggests that there is no significant difference between the active treatment and the placebo; If the odds ratios were more than 1 and the nodes were green in color, this indicates active treatment is significantly superior to placebo. The abbreviation “LITVAL” stands for lithium or valproate, while “RE” stands for random effects.
The X-axis displays the treatments, including the placebo, that were analyzed. The Y-axis indicates the decreasing probability of a given therapy being ranked as the first, second, or third best option for treating acute mania, gradually moving towards being ranked as the worst option. “SUCRA” is an abbreviation for the surface under the cumulative ranking curve. A higher SUCRA value indicates that an intervention may outperform all additional mania therapy in network comparisons. In this plot, olanzapine is identified as the best treatment, with a SUCRA value of 0.8390. “LITVAL” stands for lithium or valproate.
Despite the low level of global heterogeneity (I2 = 15.43%), we detected moderate to high heterogeneity between several comparisons: carbamazepine vs. lithium (I2 = 63.77%), divalproex vs. placebo (I2 = 65.44%), haloperidol+LITVAL vs. risperidone+LITVAL (I2 = 69.99%), paliperidone vs. placebo (I2 = 66.56%). The direct and indirect estimates displayed statistical consistency, except for the subsequent comparisons: asenapine+LITVAL vs. olanzapine+LITVAL (N = 1, n1 = 50, n2 = 48, direct comparison = 0.21, indirect comparison = 2.58, p.value = 0.0035), asenapine+LITVAL vs. placebo+LITVAL (N = 1, n1 = 155, n2 = 163, direct comparison = 2.56, indirect comparison = 0.21, p.value = 0.0035), olanzapine+LITVAL vs placebo+LITVAL (N = 2, n1 = 320, n2 = 215, direct comparison = 0.94, indirect comparison = 6.87, p.value = 0.0041), this may be due to the limited sample size of the above studies and the limited number of included studies.
Publication bias, NMA confidence evaluated using the CINeMA for the pairwise meta‑analyses outcomes
We utilized comparison-specific funnel plots in conjunction with standard statistics of mean difference and log odds ratio to detect any potential publication bias in NMA models, in comparison to placebo (Supplementary Appendix Sup002, Supplementary Fig. 4). However, these plots were insufficient to determine whether the hypothesized risk of publication bias had any effect on the results of our NMA model. Although there were some instances of comparison imprecisions and incoherence that were identified as “major concerns”, the overall evidence from the Confidence in Network Meta-Analysis (CINeMA) results was deemed to be of moderate confidence (Supplementary Appendix Sup002, Supplementary Table 9).
Discussion
This systematic review with NMA appraised the efficacy, tolerability, and safety of mono- or adjunctive therapies containing LIT/VAL (refers to the combinations of medications with lithium or valproate) and/or active drugs, placebo and/or active drugs, placebo and/or LIT/VAL, and LIT/VAL in treating acute bipolar mania in adult patients in randomized controlled trials. We extended our previous NMA by including an additional 25 adjuvant treatments, examining a wider range of adverse effects, and evaluating the efficacy and tolerability of different therapeutic combinations involving second-generation antipsychotics (SGA) and LIT/VAL. We opted for MD over SMD in our statistical calculations, we believe that “Mean Difference” provides a direct measure of the absolute effect size, which can be more interpretable and relevant in certain contexts. Our NMA indicated that most monotherapies and combination therapies exhibit superior efficacy to placebo in controlling acute manic episodes, and combination therapies appeared to be more effective than their corresponding monotherapies. Tamoxifen was found to be the most effective treatment with regards to the average change in mania score rating scale, which is consistent with previous meta-analysis [26] and NMAs [12, 13] on pharmacological monotherapies for acute mania. However, only a few treatments, including olanzapine, paliperidone, quetiapine, ziprasidone, risperidone, divalproex, and haloperidol, were significantly associated with a higher completion rate of mania treatment compared to placebo. In terms of adverse events, although certain antipsychotics (such as olanzapine, quetiapine, and ziprasidone) and adjuvant combinations (olanzapine+LITVAL) commonly result in adverse effects such as extrapyramidal symptoms and somnolence, our study has revealed that they do not contribute to a higher incidence of headache or depressive events compared to the placebo. All point estimates in the placebo-controlled forest plot agreed completely with the SUCRA probability representation of effective antimanic medicines. In the network meta-regression analysis, the significant influence of mixed states suggests that treatments may need to be tailored differently for patients experiencing mixed states compared to those with pure manic or hypomanic episodes. Our study is based on a more comprehensive literature review than prior study and yields the most substantial body of evidence to inform pharmacological intervention for acute mania in adults, furthermore, it extends beyond antipsychotic medications to explore the potential use of non-psychiatric drugs and assess adjuvant therapies. Our results provide clinical evidence for the management of acute mania and shed new light on this field.
Tamoxifen and tamoxifen+LIT/VAL have been identified as the top two medication treatments for acute mania based on three scale-limited RCTs [27,28,29] involving a total of 63 patients in tamoxifen used groups. Tamoxifen is a medication that selectively inhibits protein kinase C (PKC) within the central nervous system, and is employed as a therapeutic agent for women diagnosed with early or advanced-stage breast cancer who have estrogen receptor-positive tumors, regardless of their menopausal status [30], its therapeutic effect in bipolar disorder is believed to be related to its PKC inhibition, and mechanistic studies suggest that pathogenesis of BD is closely linked to anomalous PKC function and its associated substrates [31,32,33,34,35]. Tamoxifen’s antiestrogenic activity may indirectly decrease PKC activity, while estrogen’s ability to increase PKC activity in the brain can worsen mania and raise the risk of postpartum episodes of BD. However, the tolerability of tamoxifen and its adjuvant treatments was not significantly different from placebo, and the drug may also lead to adverse events such as an increased risk of thrombosis and hepatotoxicity for embryo-fetal health [36]. These findings underscore the need for larger-scale RCTs to evaluate the safety and acceptability of tamoxifen and its combined treatments for individuals with bipolar mania.
Allopurinol alone did not exhibit significant efficacy compared to placebo, but the combination of allopurinol with LIT/VAL demonstrated a significant efficacy compared to placebo. A similar pattern was observed with topiramate. These findings suggest that the dual treatments heighten the anti-manic effects compared to their monotherapies, with the combination treatments apparently relying on the effect of LIT/VAL within that combination. In addition, although most of the combination therapies involving antipsychotic drugs and LIT/VAL showed greater efficacy than the corresponding monotherapies in treating mania, such as asenapine+LIT/VAL vs. asenapine, quetiapine+LIT/VAL vs. quetiapine, and olanzapine+LIT/VAL vs. olanzapine, monotherapies, including olanzapine, paliperidone, quetiapine, ziprasidone, risperidone, divalproex, and haloperidol, were significantly better tolerated than placebo, indicating that these drugs may be the optimal choices in terms of both efficacy and tolerability. In our previous NMA, which considered pharmacological monotherapies for acute mania, risperidone was the second most effective treatment after tamoxifen. In the present study, risperidone (MD [95% CI]; –6.0 [–7.6, –4.4]) was still the second most effective antipsychotic after tamoxifen among the monotherapies, and risperidone+LIT/VAL (–9.1 [–13, –5.2]) was also ranked as a significantly effective treatment compared to other antipsychotic therapies. Despite risperidone being associated with some common adverse effects, our findings provide more robust clinical evidence supporting the use of risperidone in the treatment of bipolar mania. Furthermore, the decision to combine lithium or valproate into a treatment arm was influenced by network connectivity, but it may also introduce potential confounding factors.
As frequently prescribed mood stabilizers, lithium, carbamazepine, and divalproex have all demonstrated good therapeutic effects on acute mania. Although carbamazepine is significantly associated with some side effects, both lithium and divalproex remain relatively safe and effective treatment options with respect to tolerance and safety. As lithium and divalproex were always recommended as the first-line drug treatments for patients in the management of acute mania [4, 5], attention should be paid to patients during the initial stages of treatment.
Our study has some limitations. Firstly, our conclusion regarding the effectiveness of tamoxifen and its combination with mood stabilizers in treating bipolar mania, which were ranked as the most and second most effective treatments in our NMA, is based on three small randomized controlled trials (N = 3, n1 = 63, n2 = 59). These trials had diverse initial scores for mania, with mean values of 27.3, 38.6, and 32 on the mania rating scale. As a result, the confidence level of our conclusions is reduced. Two preliminary clinical trials [37, 38] evaluated tamoxifen compared with medroxyprogesterone acetate for the treatment of mania, and three randomized double-blind controlled studies [29, 39, 40] showed tamoxifen alone or in combination with lithium to be effective in treating acute mania. However, these studies were excluded due to their limited sample size or use of scales that did not meet our inclusion principles. Prior studies and a paired meta-analysis have shown tamoxifen to be an effective therapy for mania. Nevertheless, larger clinical studies are needed to explore the safety and tolerability of tamoxifen and its adjuvant treatment options.
Secondly, although we attempted to use network meta-regression to identify the sources of differences observed between the treatment groups, the number of differences among the included trials decreased the confidence of our findings, given the intricacy of the network meta-analysis. The inclusion of trials with varying baselines for mania scores, although mostly between 20 and 30, may have also affected the precision and generalizability of the findings. In addition, though most node-splitting results revealed significant consistency, we were unable to perform the inconsistency test for nausea and depression events in the NMA. As acute treatment of BD may progress to maintenance treatment [41], clinicians should closely monitor patients for adverse events, such as dizziness and weight gain, during long-term treatment. Furthermore, we did not consider trials of non-pharmacological treatment options, such as electroconvulsive therapy, although clinical trials [42,43,44,45] have shown the additional benefits of combining electroconvulsive therapy with mood stabilizers or antipsychotics for acute mania. Fourthly, this study assumes that various dosages have identical efficacy, but comparative investigations of dose-effect interactions between different doses of similar medications or different doses of distinct classes of pharmaceuticals sometimes result in different pharmacokinetic alterations [46]. In addition, different manic rating scales were included in our NMA. The differences in manic rating scales may reflect underlying clinical heterogeneity among study populations, such as differences in symptom severity or patient demographics. This could impact the generalizability of the study results and introduce bias into the NMA results. For example, the YMRS typically assesses manic symptoms based on 11 items, whereas the MRS consists of 26 items. The specific criteria and scoring thresholds for each item may also differ between the two scales. Lastly, most studies are funded by pharmaceutical companies, which may introduce bias in participant selection and publication goals, and updating resources and drug trial information might be costly and therefore unavailable, leading to potential omissions in the articles we included.
Conclusion
According to our findings, tamoxifen and tamoxifen+LIT/VAL have the best and second-best clinical efficacy in adults with acute bipolar mania. However, more large-scale randomized controlled trials are necessary to assess their safety, acceptability, and tolerability. Clonidine+LITVAL, allopurinol+LITVAL, rivastigmine+LITVAL, levetiracetam+LITVAL, risperidone+LITVAL, celecoxib+LITVAL, olanzapine+LITVAL, melatonin+LITVAL, asenapine+LITVAL, quetiapine+LITVAL, dipyridamole+LITVAL, aripiprazole+LITVAL, haloperidol+LITVAL, placebo+quetiapine, memantine+LITVAL, risperidone, ziprasidone+LITVAL, lovastatin+LITVAL, carbamazepine, olanzapine, cariprazine, ziprasidone, paliperidone, haloperidol, topiramate+LITVAL, quetiapine, placebo+LITVAL, lithium, aripiprazole, asenapine, and divalproex demonstrated significantly superior effectiveness in managing acute mania when compared to placebo. In addition, combination therapies appeared to be more effective than their corresponding monotherapies. Although olanzapine, paliperidone, quetiapine, ziprasidone, risperidone, divalproex, and haloperidol were significantly better tolerated than placebo, risperidone showed advantages in both efficacy and tolerability compared to other antipsychotics. Clinicians and patients should pay close attention to adverse events when choosing pharmaceutical treatment for the acute phase of mania.
Data availability
The original dataset and statistical coding used in this study will be made available to the author upon reasonable request.
References
Grande I, Berk M, Birmaher B, Vieta E. Bipolar disorder. Lancet. 2016;387:1561–72.
McIntyre RS, Berk M, Brietzke E, Goldstein BI, López-Jaramillo C, Kessing LV, et al. Bipolar disorders. Lancet. 2020;396:1841–56.
National Collaborating Centre for Mental H. National Institute for Health and Clinical Excellence: Guidance. Bipolar disorder: the management of bipolar disorder in adults, children and adolescents, in primary and secondary care. British Psychological Society Copyright © 2006, The British Psychological Society & The Royal College of Psychiatrists: Leicester (UK), 2006.
Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20:97–170.
Fountoulakis KN, Yatham L, Grunze H, Vieta E, Young A, Blier P, et al. The International College of Neuro-Psychopharmacology (CINP) Treatment Guidelines for Bipolar Disorder in Adults (CINP-BD-2017), part 2: review, grading of the evidence, and a precise algorithm. Int J Neuropsychopharmacol. 2017;20:121–79.
Goodwin GM, Haddad PM, Ferrier IN, Aronson JK, Barnes T, Cipriani A, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30:495–553.
Baldessarini RJ, Tondo L, Vázquez GH. Pharmacological treatment of adult bipolar disorder. Mol Psychiatry. 2019;24:198–217.
Cipriani A, Barbui C, Salanti G, Rendell J, Brown R, Stockton S, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378:1306–15.
Scherk H, Pajonk FG, Leucht S. Second-generation antipsychotic agents in the treatment of acute mania: a systematic review and meta-analysis of randomized controlled trials. Arch Gen Psychiatry. 2007;64:442–55.
Tamayo JM, Zarate CA Jr, Vieta E, Vázquez G, Tohen M. Level of response and safety of pharmacological monotherapy in the treatment of acute bipolar I disorder phases: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2010;13:813–32.
Yildiz A, Nikodem M, Vieta E, Correll CU, Baldessarini RJ. A network meta-analysis on comparative efficacy and all-cause discontinuation of antimanic treatments in acute bipolar mania. Psychol Med. 2015;45:299–317.
Hong Y, Huang W, Cao D, Xu J, Wei H, Zhang J, et al. A cumulative Bayesian network meta-analysis on the comparative efficacy of pharmacotherapies for mania over the last 40 years. Psychopharmacology. 2022;239:3367–75.
Kishi T, Ikuta T, Matsuda Y, Sakuma K, Okuya M, Nomura I, et al. Pharmacological treatment for bipolar mania: a systematic review and network meta-analysis of double-blind randomized controlled trials. Mol Psychiatry. 2022;27:1136–44.
Glue P, Herbison P. Comparative efficacy and acceptability of combined antipsychotics and mood stabilizers versus individual drug classes for acute mania: network meta-analysis. Aust N Z J Psychiatry. 2015;49:1215–20.
Kishi T, Ikuta T, Matsuda Y, Sakuma K, Okuya M, Mishima K, et al. Mood stabilizers and/or antipsychotics for bipolar disorder in the maintenance phase: a systematic review and network meta-analysis of randomized controlled trials. Mol Psychiatry. 2021;26:4146–57.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84.
Taylor DM, Barnes TR, Young AH. The Maudsley prescribing guidelines in psychiatry. John Wiley & Sons; 2021.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019.
Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making. 2013;33:607–17.
Dias S, Sutton AJ, Welton NJ, Ades AE. NICE Decision Support Unit Technical Support Documents. Heterogeneity: subgroups, meta-regression, bias and bias-adjustment. London: National Institute for Health and Care Excellence (NICE) Copyright © 2012 National Institute for Health and Clinical Excellence; 2012.
Leonard T, Duffy JC. A Bayesian fixed effects analysis of the Mantel-Haenszel model applied to meta-analysis. Stat Med. 2002;21:2295–312.
Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:1–9.
van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. 2016;7:80–93.
Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS ONE. 2013;8:e76654.
Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17:e1003082.
Palacios J, Yildiz A, Young AH, Taylor MJ. Tamoxifen for bipolar disorder: systematic review and meta-analysis. J Psychopharmacol. 2019;33:177–84.
Zarate CA Jr, Singh JB, Carlson PJ, Quiroz J, Jolkovsky L, Luckenbaugh DA, et al. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9:561–70.
Yildiz A, Guleryuz S, Ankerst DP, Öngür D, Renshaw PF. Protein kinase C inhibition in the treatment of mania: a double-blind, placebo-controlled trial of tamoxifen. Arch Gen Psychiatry. 2008;65:255–63.
Amrollahi Z, Rezaei F, Salehi B, Modabbernia AH, Maroufi A, Esfandiari GR, et al. Double-blind, randomized, placebo-controlled 6-week study on the efficacy and safety of the tamoxifen adjunctive to lithium in acute bipolar mania. J Affect Disord. 2011;129:327–31.
Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88.
Huang KP. Role of protein kinase C in cellular regulation. Biofactors. 1990;2:171–8.
Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–14.
Nishizuka Y, Nakamura S. Lipid mediators and protein kinase C for intracellular signalling. Clin Exp Pharmacol Physiol Suppl. 1995;22:S202–203.
Friedman E, Hoau Yan W, Levinson D, Connell TA, Singh H. Altered platelet protein kinase C activity in bipolar affective disorder, manic episode. Biol Psychiatry. 1993;33:520–5.
Kantor L, Gnegy ME. Protein kinase C inhibitors block amphetamine-mediated dopamine release in rat striatal slices. J Pharmacol Exp Ther. 1998;284:592–8.
Yang G, Nowsheen S, Aziz K, Georgakilas AG. Toxicity and adverse effects of Tamoxifen and other anti-estrogen drugs. Pharmacol Ther. 2013;139:392–404.
Kulkarni J, Berk M, Wang W, Mu L, Scarr E, Van Rheenen TE, et al. A four week randomised control trial of adjunctive medroxyprogesterone and tamoxifen in women with mania. Psychoneuroendocrinology. 2014;43:52–61.
Kulkarni J, Garland KA, Scaffidi A, Headey B, Anderson R, de Castella A, et al. A pilot study of hormone modulation as a new treatment for mania in women with bipolar affective disorder. Psychoneuroendocrinology. 2006;31:543–7.
Bebchuk JM, Arfken CL, Dolan-Manji S, Murphy J, Hasanat K, Manji HK. A preliminary investigation of a protein kinase C inhibitor in the treatment of acute mania. Arch Gen Psychiatry. 2000;57:95–97.
Fallah E, Arman S, Najafi M, Shayegh B. Effect of tamoxifen and lithium on treatment of acute mania symptoms in children and adolescents. Iran J Child Neurol. 2016;10:16–25.
Sachs GS, Thase ME. Bipolar disorder therapeutics: maintenance treatment. Biol Psychiatry. 2000;48:573–81.
Small JG, Klapper MH, Kellams JJ, Miller MJ, Milstein V, Sharpley PH, et al. Electroconvulsive treatment compared with lithium in the management of manic states. Arch Gen Psychiatry. 1988;45:727–32.
Rakesh G, Thirthalli J, Kumar CN, Muralidharan K, Phutane VH, Gangadhar BN. Concomitant anticonvulsants with bitemporal electroconvulsive therapy: a randomized controlled trial with clinical and neurobiological application. J ECT. 2017;33:16–21.
Mohan TS, Tharyan P, Alexander J, Raveendran NS. Effects of stimulus intensity on the efficacy and safety of twice-weekly, bilateral electroconvulsive therapy (ECT) combined with antipsychotics in acute mania: a randomised controlled trial. Bipolar Disord. 2009;11:126–34.
Sikdar S, Kulhara P, Avasthi A, Singh H. Combined chlorpromazine and electroconvulsive therapy in mania. Br J Psychiatry. 1994;164:806–10.
Del Giovane C, Vacchi L, Mavridis D, Filippini G, Salanti G. Network meta-analysis models to account for variability in treatment definitions: application to dose effects. Stat Med. 2013;32:25–39.
Acknowledgements
We would like to express our appreciation to Dr. Farzin Rezaei, Dr. Basant K Puri, and Dr. Saeed Shoja Shafti for furnishing unpublished information regarding their studies.
Funding
There is no external funding for this project. The corresponding author had complete access to all data in the study and was ultimately responsible for the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
This study was conceptualized and designed by WH. WH, SH, ML, and JX were responsible for ensuring the accuracy of the data analysis and the integrity of the original data, both of which they had complete access to during the study. The initial draft of the manuscript was written by WH and SH. Statistical analysis and clinical interpretation were conducted by WH, ML, and SH. All authors contributed to data collection, interpretation, and writing the final version of the manuscript. The writing, review, and submission for publication of the manuscript were supervised by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, W., He, S., Liu, M. et al. Comparative efficacy, safety, and tolerability of pharmacotherapies for acute mania in adults: a systematic review and network meta-analysis of randomized controlled trials. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02705-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02705-3
- Springer Nature Limited