Abstract
Dexmedetomidine is a sedative agent with limited dosing, safety, and efficacy information in the neonatal population. This comprehensive review describes the available evidence summarizing the use of dexmedetomidine in various neonatal populations. We identified 21 studies and 1 case report supporting the efficacy and short-term safety of DEX in neonates. Reported dosing ranges from 0.5–1.5 mcg/kg/h with or without loading doses. Clinically relevant adverse effects include bradycardia and hypotension. Future studies are needed to determine long-term safety and facilitate clinical applicability.
Similar content being viewed by others
Introduction
Dexmedetomidine (Precedex®), a highly selective, centrally acting alpha2 adrenergic agonist, provides sedative and analgesic-sparing effects [1, 2]. Dexmedetomidine (DEX) is approved by the Food and Drug Administration in non-intubated adults for procedural or surgical sedation <24 hours. In clinical practice, it is often utilized off-label in neonatal, pediatric, and adult patients as an adjunct to general anesthesia (GA), premedication for anesthetic procedures, and as sedation postoperatively and in mechanically ventilated patients for over 24 hours [3,4,5,6]. Unlike alternative pharmacologic options, DEX minimally affects the respiratory drive and gastric motility, making it an appealing option for use in neonates; however, neonatal data are limited [7].
The ideal management of pain and sedation for neonates in the intensive care unit (ICU) is poorly understood. Sedation during mechanical ventilation is often used to improve ventilator synchrony and decrease significant distress [8].
Prolonged opioid and benzodiazepine infusions, defined as greater than 7 cumulative days, in extremely preterm neonates have been associated with an increase in poor neurodevelopmental outcomes [9]. In preclinical studies, benzodiazepines demonstrate neuroapoptosis in the cerebral cortex and basal ganglia, and clinical studies mirror these neurodevelopmental concerns [10, 11]. Alternatively, DEX has been associated with the inhibition of neuronal apoptosis and suppression of cytokine-mediated brain injury [8]. These benefits have not yet been clinically evaluated. Clinical trials and case reports have described the off-label use of DEX in various neonatal populations. This review summarizes the available evidence of DEX use and provides practical and clinical recommendations in preterm and term neonates, neonates with hypoxic ischemic encephalopathy (HIE), and neonates undergoing surgery.
Methods
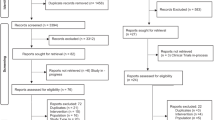
A literature search was performed in PubMed (January 1946 - September 2021) using the keyword dexmedetomidine. The results of this search were limited to the English language, humans, and “newborn: birth to 1 month”. The references of primary articles were examined to include articles not identified in the initial search. A total of 131 articles were identified. Studies were excluded if the primary patient population was >1 month old, if they examined use of DEX in pregnancy, or if they were not conducted in humans. Additionally, review articles or articles in which the primary study medication was not DEX were excluded. Case reports and series were evaluated for inclusion if findings contributed novel information (Fig. 1).
Studies were excluded if the primary patient population was > 1 month old, if they examined use of DEX in pregnancy, or if they were not conducted in humans. Additionally, review articles or articles in which the primary study medication was not DEX were excluded. Case reports and series were evaluated for inclusion if findings contributed novel information. [DEX = dexmedetomidine; HIE = hypoxic ischemic encephalopathy; NICU = neonatal intensive care unit].
Results
A total of 21 studies and one case report are included in this review. The studies were grouped by patient population, namely critically ill neonates, neonates undergoing therapeutic hypothermia (TH), and surgical neonates. Dosing information is summarized for each patient population (Tables 1–3).
Critically ill neonates
In the neonatal intensive care unit, patients frequently require sedation to facilitate mechanical ventilation synchrony and comfort. Conventional sedative and analgesic agents may contribute to negative long-term developmental effects [8]. Dexmedetomidine is an attractive alternative sedative that could be used as monotherapy or in combination with other agents. The following section describes DEX use for sedation in preterm and term neonates with dosing information summarized in Table 1.
In 2012, O’Mara et al. conducted a retrospective, observational case-control study comparing two groups of patients receiving fentanyl or DEX for sedation in mechanically ventilated neonates [12]. This study included premature neonates aged < 2 weeks at the initiation of therapy. Dexmedetomidine was started empirically or in those who met predefined criteria for continuous infusion sedation within 48 hours of life. Additional boluses with fentanyl or lorazepam were allowed. The primary efficacy outcome was the need for adjunctive analgesia or sedation during treatment. Both groups had similar bolus requirements prior to the start of DEX infusion. The mean number of sedative boluses per day was similar (3.0 fentanyl vs 1.73 DEX); however, patients on DEX had a greater percentage of treatment days requiring no additional sedation compared to the fentanyl group (54.1% and 16.5%, p < 0.0001). Total fentanyl and lorazepam exposures in the DEX group were 20.6 mcg/kg and 2.7 mg/kg, compared to patients on fentanyl who received 398 mcg/kg and 6.8 mg/kg, respectively. Mechanical ventilation was shorter in the DEX group compared to the fentanyl group (14.4 vs 28.4 days). Interestingly, patients on DEX underwent an average of 28 chest x-rays during their hospital stay compared to 49 chest x-rays in the fentanyl population. Furthermore, enteral trophic feeds were started approximately 2 days earlier in the DEX group, and the time to first meconium stool was shorter in DEX patients. The authors concluded DEX to be safe and effective in the neonates included in the study.
In 2014, Chrysostomou et al. conducted a phase II/III, open-label, multicenter safety, efficacy, and pharmacokinetic trial to evaluate DEX in preterm and term infants [13]. Patients were divided into two groups based on gestational age, Group I (patients aged ≥28 to <36 weeks) and Group II (patients aged ≥36 to ≤44 weeks). Doses were assigned to each group with three escalating dose levels (Table 1). Additional sedation with midazolam was administered in 10% of patients, all in group II. Fentanyl and morphine were administered to 17 patients (40%), with three in group I and 14 in group II. Regarding pharmacokinetics, patients in group I versus group II had lower plasma clearance (0.3 vs 0.9 L/h*kg), increased elimination (half-life of 7.6 vs 3.2 h), and increased area under the curve (2 049 vs 357 pg*h/mL). Three patients (7%) reported a total of four adverse events related to DEX, including diastolic hypotension in group I (dose level 2), hypertension in group II (dose level 1), significant agitation in group II (dose level 3), and a possible mild respiratory acidosis in group I (dose level 2).
Estkowski et al. conducted a retrospective, descriptive evaluation of DEX therapy in term neonates and infants in the pediatric ICU. Sedation for mechanical ventilation was the indication in 26 neonates (93%) [2]. The median minimum DEX infusion rate was similar for neonates and infants. Median maximum doses were higher in infants compared to neonates and maximum infusion rate was similarly higher in infants. Both neonates and infants had at least one bradycardia episode that was statistically significant. Hypotension episodes requiring dopamine for hemodynamic support occurred but information to determine whether it was associated with DEX was lacking. Three neonates (11%) received fluid boluses and four (14%) had cardiovascular adverse events with chart documentation as a reason for DEX discontinuation. Concomitant opioid therapy with morphine was present in 84% of patients and could have contributed to the cardiovascular events observed in this study. Additionally, concomitant sedative therapy was used in 71% of the entire population but was more common in infants than neonates (75/99 (76%) vs 15/28 (54%) (p = 0.02)). Despite similar dosing strategies, side effects occurred more commonly in infants than neonates.
Dersch-Mills et al. conducted a retrospective, descriptive review of neonates receiving DEX in a neonatal ICU, with all but one neonate being mechanically ventilated [14]. Preterm infants were started on DEX later in life than term infants (41 vs 3 days, p = 0.004), resulting in a similar postmenstrual age at the time of DEX initiation between the groups (37.9 vs 39.0 weeks, p = 0.163). Dexmedetomidine doses were titrated based on pain and sedation assessment scores. Concurrent opioid infusion occurred in 95% of the patients with either fentanyl, morphine, or hydromorphone. A reduction in opioids within 24 hours of DEX initiation was observed in 66.7% of patients. Thirty patients (78.9%) had DEX weaned off, while 11 (28.9%) needed clonidine, of which nine patients were on DEX > 10 days. Withdrawal was seen in 22 patients (71%) while weaning or within 72 hours of discontinuation. Postmenstrual age at start of DEX, maximum dose, and duration of therapy were not determined to contribute to the incidence of withdrawal. Although there was a high rate of adverse effects in this study (88.9% of patients), there were no significant differences between the preterm and term neonates. Approximately half of the patients that experienced hypotension during the infusion required dopamine; however, there was limited information to associate these hypotensive episodes with DEX.
Since DEX dosing variations are observed in neonates, van Dijkman et al. conducted a pilot dose finding pharmacokinetic study [15]. The study was based on a simulation of 200 patients and was then conducted in six neonates and again with an additional 11 patients for external validation in a refined model. Patients were included if they were mechanically ventilated. The targeted concentration of DEX was 0.6 mcg/L with a safety measure of <1 mcg/L. In the simulation, a continuous infusion of 0.3 mcg/kg/hour of DEX over a 24 hour period was predicted to result in appropriate exposure within 4 hours and achievement of steady state after 10 hours, resulting in a serum concentration of approximately 0.6 mcg/L. This simulation helped improve dosing recommendations.
Neonates with HIE undergoing TH
Shivering limits efficacy of TH, so sedation is recommended to mitigate this adverse effect [16]. Morphine is traditionally utilized, but safety concerns with respiratory depression and worrisome effects on long-term neurodevelopmental outcomes in preterm neonates prompted clinicians to seek alternatives. Contrary to this concern, a recent study evaluating morphine or fentanyl in TH-treated infants with HIE did not find worse neurologic outcomes at 18–24 months of age with higher cumulative opioid doses [17]. Future studies are needed to confirm long-term safety. In addition to safety concerns, altered pharmacokinetics during hypothermia may necessitate frequent dose adjustments of opioids and other agents [16]. The dosing of DEX in studies of neonates undergoing TH is summarized in Table 2.
In 2018, O’Mara et al. conducted a retrospective review assessing safety and effectiveness of DEX in patients undergoing TH for a diagnosis of HIE within 48 hours of birth [18]. Of the 19 patients evaluated, two received DEX monotherapy and 17 received a combination with fentanyl. Fentanyl was dosed at 0.5 mcg/kg/hour and increased by 0.5 mcg/kg/hour, and DEX was initiated if the dose reached 1 mcg/kg/hour. In 13 of the 17 patients receiving combination therapy, the fentanyl infusion was weaned within 4 hours of starting DEX infusion, and 14 patients were able to wean off fentanyl prior to DEX. No patients required any additional sedation agents. Eleven patients were on DEX at time of intubation, four weaned off on the day of extubation, and all were weaned within 48 hours of extubation. Regarding adverse effects, there were no significant changes in hemodynamics and only one low heart rate (HR), which resolved with weaning of fentanyl. This study introduces DEX as a safe and efficacious agent for sedation during TH.
Assessing potential effects of TH on the pharmacokinetics of DEX in neonates, McAdams et al. conducted a phase I, single center, prospective, open-label clinical study [16]. The study evaluated DEX in mechanically ventilated neonates with HIE undergoing TH. Morphine was available for patients with episodes of breakthrough shivering. Dexmedetomidine was started within 24 hours after birth, continued for the duration of TH and discontinued once normothermia was achieved. When authors compared their results to the pharmacokinetics of neonates not undergoing TH from studies by Chrysostomou et al. and Greenberg et al., clearance was lower while volume of distribution was higher [7, 13]. Mean residual time was shorter in the patients undergoing TH. None of these factors were statistically significant. Three cases of hypotension occurred, temporally associated with DEX initiation. All three cases responded to inotropic support, but study authors did not associate this as an adverse effect. No episodes of bradycardia were noted. One patient did experience a brief seizure episode 5 hours after starting DEX that did not require treatment.
In 2021, Cosnahan et al. published a retrospective, observational cohort study evaluating 70 neonates, in which 34 received DEX and 36 received scheduled morphine, all undergoing TH for HIE [19]. The primary endpoint, efficacy of DEX for pain and sedation during TH, revealed no clinically significant differences between the morphine and DEX groups. Approximately half of both groups were mechanically ventilated, and more infants in the morphine group had meconium aspiration syndrome. Ventilatory support requirements increased in the morphine group while they decreased in patients receiving DEX. Breakthrough morphine was allowed in both groups, and the DEX group received higher amounts of breakthrough morphine (0.13 ± 0.13 vs 0.04 ± 0.09 mg/kg, p = 0.001), but total morphine requirement was significantly higher in the morphine group (0.13 ± 0.13 vs 1.79 ± 0.23 mg/kg, p < 0.0001). Oxygen saturation and mean arterial pressure (MAP) did not differ between both groups. Vasopressor use was similar between the DEX and morphine groups (35.2 vs 38.8%, p = 0.81). Bradycardia occurred in the DEX group without discontinuation of the infusion. Heart rate remained within normal limits for both groups. No differences in video EEG patterns post-TH were observed, and MRI National Institute of Child Health and Human Development scores did not differ between groups. Most patients had a normal MRI in both groups. This study provided short-term neurologic outcomes of using DEX during TH.
Most recently, Elliot et al., conducted a retrospective review of 166 neonates undergoing TH for HIE to assess the effects of sedatives and gestational age on HR [20]. This study compared 120 neonates who received fentanyl monotherapy as a first-line sedative option versus 46 patients that received DEX as a first-line agent for TH, 14 as monotherapy and 32 with adjunctive fentanyl. Heart rate did not differ between the two groups at baseline, during the first 12 hours after birth, or after therapy was discontinued. Patients who received DEX had significantly lower HR from 12–36 hours after birth compared to patients receiving fentanyl monotherapy. Catecholamine use did not significantly differ between groups. Of the 46 patients that received DEX, 5 neonates required dose decreases, and 17 neonates had DEX discontinued. The most common reason was bradycardia, and others included oversedation and hypotension. Significantly more neonates in the group with DEX decrease or discontinuation were found to have normal EEG or mild encephalopathy at 48 h compared to the group that continued DEX (6/22 vs 2/24, p = 0.04). When comparing HR nadir between age groups of ≤ 35 weeks, 36–38 weeks, or >39 weeks, there was no statistical difference amongst the groups. Mean and nadir HR were lower in patients that required a decrease or discontinuation of DEX. Mean and maximum dose of DEX in groups that continued therapy did not differ from those patients that required discontinuation or decrease in DEX dose.
Surgical neonates
Dexmedetomidine is an attractive agent in the intraoperative and postoperative setting in neonatal surgical patients to facilitate sedation while minimizing respiratory depression and exposure to opioids and benzodiazepines. The following section describes utilization of DEX in a variety of surgical settings. Dosing regimen details from these studies are provided in Table 3.
Neonatal DEX use in the surgical setting was initially described in a prospective study of full-term neonates (n = 16) undergoing abdominal surgery [21]. Intraoperative DEX was utilized with sevoflurane to induce GA. Patients’ blood pressure and HR remained stable throughout the procedure, and only three patients required a ketamine bolus. This study served as a pilot for future surgical studies utilizing DEX in neonates.
Dexmedetomidine with caudal anesthesia
In a retrospective case series, Waurick et al. reviewed infants who underwent lower abdominal or lower extremity surgery and received DEX sedation with caudal anesthesia (CA) [22]. One of the ten full-term infants required escalation to GA due to hiccups after DEX dose escalation to 1.2 mcg/kg/h. Three of the 12 ex-preterm infants required conversion to GA due to prolonged surgical time or technical failure unrelated to DEX. Decreases in HR and MAP were noted in both groups with one critical MAP in an ex-preterm infant; no interventions were reported in response to this measurement. Overall, the combination of DEX sedation with CA was successful in 82% (18/22) of infants, while the remaining infants required conversion to GA.
Bong et al. completed a pilot retrospective review study of 50 neonates evaluating DEX sedation versus GA for infants undergoing inguinal hernia surgery [23]. Forty-three patients were successfully managed without additional sedatives or escalation to GA. The most commonly documented adverse effect, experienced by 36% of patients, were “irregular respiration, mimicking hiccups, or sobbing” during continuous infusion of DEX. Five patients experienced transient apnea, three patients developed mild bradycardia, and two patients experienced transient hypotension, which resolved with fluid resuscitation.
In the follow-up controlled trial, Bong et al. randomized neonates to receive DEX with CA (n = 51), or GA with CA (n = 48) for inguinal hernia surgery [24]. In the DEX with CA group, 90% of patients were successfully managed without additional sedatives or escalation to GA. Intraoperatively, patients in the DEX group had significantly lower HR and higher MAP but no significant difference in operating room time. There was no difference in hypoxemic episodes intraoperatively, but postoperatively, a significantly higher number of infants experienced episodes in the GA group compared to the DEX group. Study authors concluded that DEX with CA is an effective alternative in place of GA.
Cardiovascular surgery
Zuppa et al. published a phase 1 multicenter study evaluating the safety and pharmacokinetics of DEX in 122 full-term neonates and infants undergoing cardiac surgery with cardiopulmonary bypass (CPB) [25]. All patients received GA and fentanyl. Dexmedetomidine was initiated intraoperatively and continued postoperatively in conjunction with other continuous infusion or intermittently dosed sedatives. A dose escalation strategy was utilized to generate pharmacokinetic modeling to design dosing strategies to achieve steady state DEX plasma levels between 0.2–1 mcg/L. Five of the 122 infants experienced cardiovascular adverse effects, all of which resolved and may have had multifactorial causes. The pharmacokinetic model predicted up to a 50% reduction in DEX clearance in the immediate postoperative period and a 95% reduction while on CPB when compared with pre-CPB clearance. Based on this, study authors recommend a decreased DEX infusion rate after the first hour of CPB. Dosing recommendations were provided based on patient age and target steady state concentration (Table 3). Dosing recommendations for infants skewed higher due to maturation of hepatic metabolism in the first 3 weeks of life. Preliminary phase 1 data from this study will be expanded in a phase 3 trial.
Later in 2019, Zimmerman et al. published a single center, open-label, opportunistic, pharmacokinetic study in infants and young children ≤ 36 months of age (n = 18) undergoing CPB and receiving DEX [26]. A simulated dosing model provided preliminary dosing recommendations reflecting reduced clearance during CPB (Table 3). Hypotension was reported in eight patients (44%), but no episodes were attributed to DEX. Three patients (18%) experienced bradycardia, prompting discontinuation of DEX in one patient, but authors attributed all bradycardia events to non-DEX causes.
Postoperative sedation
In 2012, Lam et al. published a retrospective observational study in a single tertiary care university children’s hospital cardiovascular ICU [27]. Critically ill neonates and infants ≤ 12 months of age with congenital or acquired heart disease receiving DEX post-surgically for at least 24 hours were included. At the discretion of the medical team, DEX was added as a second or third agent after most patients were given morphine and/or midazolam. All patients were reported to be hemodynamically stable throughout the infusion despite statistically significant decreases in HR and MAP. The median number of sedation rescue doses decreased from two boluses prior to DEX to one bolus in the 24- to 48-hour interval, and the median number of analgesic rescue doses remained the same after DEX initiation. Of note, 74% of patients received clonidine after discontinuation of DEX infusion secondary to a withdrawal phenomenon. This study demonstrated that DEX is well-tolerated in the post-cardiac surgery neonatal population.
Subsequently, Su et al. conducted a pharmacokinetic dose escalation clinical trial in full-term neonates and infants receiving DEX after open-heart surgery [28]. Neonates were divided into three dosing cohorts, but three neonates in the highest dosing cohort experienced hypotension requiring CPR. Future patients assigned to this cohort received lower doses. Three neonates had EKG changes indicative of cardiac ischemia after DEX infusion, but none were deemed clinically significant. Compared to the studied infant population, neonates required an estimated 30–40% reduction in weight-based dosing to achieve similar steady state plasma concentrations, especially in the first 14 days after birth. The authors noted that DEX pharmacokinetics were influenced by higher total bypass time, which may decrease clearance, and presence of right-to-left intracardiac shunt, which may increase clearance. When administered equivalent weight-based doses of DEX, neonates achieve steady state concentrations at >10 hours, compared with infants who achieve steady state in 6 hours. Study authors concluded that DEX dosed at 0.3 mcg/kg/hour was well-tolerated in neonates after open heart surgery.
In 2017, Greenberg et al. conducted a single center, open-label pharmacokinetic study in infants < 12 months of age (n = 20) who were either mechanically ventilated or undergoing cardiac surgery receiving DEX [7]. Infants received at least one other sedative within 24 hours, most commonly midazolam, methadone, fentanyl, or clonidine. Fifteen infants (75%) experienced hypotension requiring vasopressors, but study authors determined severity of illness and concomitant medications were contributory. Infants with a history of cardiac surgery had approximately 40% lower clearance compared to other infants, thus requiring lower doses of DEX.
In 2019, Sellas et al. retrospectively evaluated 78 preterm and term neonates who received either DEX or an alternative sedative post-operatively, most commonly for upper gastrointestinal procedures [29]. Patients required at least 6 hours of continuous sedation post-operatively and were equally matched between groups. In the DEX group, 72% (28/39) of patients received a concomitant opioid infusion, and five patients experienced bradycardia, which was higher than the two patients in the non-DEX group (p = 0.013). No significant differences in hypotension or respiratory depression occurred. There was no significant difference in duration of opioid infusion between the two groups, but there was a lower total dose of opioids required by patients in the DEX group versus the non-DEX group (p = 0.0115).
In 2021, Sykes et al. performed a retrospective chart review of neonates who underwent open thoracic or abdominal operation [30]. Patients who received intravenous acetaminophen and DEX (n = 30) were compared with patients who received intravenous acetaminophen without DEX (n = 82) in the first ten postoperative days, and opioid exposure was evaluated in each arm. Neonates in the intravenous acetaminophen with DEX arm received significantly more morphine equivalents on average daily and had a longer median time to extubation than those in the intravenous acetaminophen without DEX arm (0.4 morphine equivalent/kg/day and 3 days versus 0.2 morphine equivalent/kg/day and 1 day). Time to full enteral feeds was not significantly different between both arms. The authors concluded that DEX was associated with increased opioid exposure in postoperative abdominal and thoracic surgery neonates.
Procedural sedation
Intranasal administration is an alternative route explored by Bua et al. [31]. In a prospective study, 3 mcg/kg intranasal DEX with midazolam rescue therapy was compared to intravenous or intranasal midazolam monotherapy. Fifty-three neonates undergoing MRI with sedation with a gestational age of ≤32 weeks or a birth weight of ≤1500 g were included. The median time to sedation for DEX alone was 10 minutes with a median time to arousal of 59 minutes. Seven neonates receiving DEX had self-resolving desaturation events, and only one received DEX as monotherapy. Overall, there was a reduction in midazolam doses needed to achieve sedation with the use of intranasal DEX.
Discussion
This review demonstrates the safety and efficacy of DEX in preterm and term neonates for a variety of disease states. In critically ill neonates, DEX proved to be an efficacious adjunctive sedative for mechanically and non-mechanically ventilated patients. Dexmedetomidine reduced the need for other sedatives, specifically the number of opioids and benzodiazepines boluses and continuous infusions [12, 14]. Dexmedetomidine was associated with a decreased time on mechanical ventilation and shortened time to full enteral feeds [12, 18].
In patients receiving TH for HIE, DEX has similarly shown to be a safe and effective agent for sedation. Due to its ability to sedate without causing respiratory depression, it is an ideal agent for patients receiving hypothermia without mechanical ventilation [18]. The neuroprotective properties identified in preclinical studies make DEX a potentially beneficial option for this patient population compared to opioids and benzodiazepines; however, none of the studies to date have evaluated long-term neurodevelopmental outcomes. Cosnahan et al. evaluated short-term benefits through video EEG monitoring and MRI at 12 to 14 days after birth but did not show a difference between the morphine and DEX groups, which may be attributed to the small sample size and minimal number of patients showing extensive HIE [18].
Dosing
In the pediatric population, DEX is used as a sedative with loading and maintenance dosing, similar to the neonatal population. In children and adolescents, recommended loading doses range from 0.5–2 mcg/kg over 10 minutes followed by a recommended maintenance dose of 0.5–1 mcg/kg/hour [32]. Dosing Tables 1–3 display varying neonatal loading doses ranging from 0.2–2 mcg/kg and maintenance doses ranging between 0.05–1.5 mcg/kg/hour. Studies with higher reported maxes up to 2.5 mcg/kg/hour included infants [7]. In general, neonatal dosing tends to be lower than infant and pediatric dosing due to immature hepatic metabolism and decreased clearance. In surgical patients (Table 3), dosing varies depending on the type of procedure, underlying patient conditions, and sedation level goal. Notably, CPB decreases clearance of DEX, so patients undergoing CPB should have infusion rates proactively reduced [24, 25, 27]. Patients undergoing TH can safely be administered similar neonatal doses of DEX with similar or slightly higher plasma concentrations based on the limited information available [16].
Adverse effects
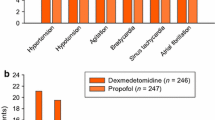
The most clinically relevant adverse effects associated with DEX in neonates include bradycardia and hypotension (Table 4). Preclinical data demonstrates potential for cerebral hypoperfusion secondary to dose-dependent hypotension [33]. Additional side effects noted include hiccups, sobbing, and irregular respirations. Seizures as an adverse effect were reported by Kubota et al. [34]. This occurred in a 41-week gestation infant receiving DEX at 0.625 mcg/kg/hour. An EEG showing ictal changes resulted in DEX discontinuation. Twelve hours later, the seizures ceased without medical intervention. At 8 months of age, the infant had normal development and no obvious neurological abnormalities. A similar adverse effect of seizure in a single patient was identified in McAdams et al. [16]. This patient experienced a seizure 5 hours after the start of DEX but did not require treatment, and the seizure did not reoccur.
Although the summarized studies provide an adequate foundation of expected adverse effects during DEX administration, no long-term studies assess developmental outcomes after human exposure during the neonatal period. Favorable neuroprotective effects have been observed in neonatal rats following induction of hyperoxia [35]. In addition, rat pups exposed to a single dose of DEX on postnatal age 7 showed no impairment of hippocampal synaptic plasticity at 9 weeks of life, supporting long-term safety in comparison with other anesthetic agents [36]. Another study demonstrated enhanced spatial learning and memory in rat pups exposed to DEX at postnatal day 7 [37]. Although limited by small sample sizes and non-human populations, these findings are promising. Long-term developmental outcomes in humans may elucidate stronger evidence to support DEX utilization in neonates over alternative sedatives.
Prolonged infusions of DEX may increase the risk of iatrogenic withdrawal, especially upon abrupt discontinuation. Although FDA approved for a 24-hour infusion, clinical studies have reported prolonged durations upwards of 10 days [14]. Strategies to avoid withdrawal symptoms include weaning of the infusion or addition of an oral alpha2-agonist, clonidine [12, 14]. Although lacking substantial data in the neonatal population, transitioning from DEX to clonidine is recommended in pediatric and adult literature [38, 39].
Future directions
Although significant progress has been made evaluating the safety and efficacy of DEX in the neonatal population, future studies would help clinical applicability. Dilutions of 2 mcg/mL and 4 mcg/mL have been reported in the literature; however, the only available dilution of DEX with stability data is 4 mcg/mL [1, 40]. Many syringe pumps limit the minimum infusion rate to 0.1 mL/hour. At an initial starting dose of 0.2 mcg/kg/hour, patients weighing < 2 kg would require infusion rates < 0.1 mL/hour. It is critical to expand stability testing for more dilute concentrations to accommodate smaller neonates.
Another challenge in the neonatal population is limited intravenous access, which presents the need for y-site administration of medications. Although recent literature has described the y-site compatibility of DEX with parenteral nutrition and fat emulsion, fish oil and plant based (SMOFlipid®), y-site compatibility with commonly used medications in neonates are lacking [41]. Availability of compatibility information in standard drug references would be helpful for using DEX in critically ill neonates.
The current literature provides evidence of the safety and efficacy of DEX in the preterm and term neonatal population. Dexmedetomidine may be an attractive option as primary sedation in TH or as adjunctive therapy in patients with additional analgesic concerns. A randomized, controlled trial evaluating the role and pharmacokinetics of DEX in TH is currently ongoing, and the results will help establish definitive place in therapy and safety [42]. Information is limited regarding the use of DEX in preterm neonates, particularly in patients < 31 weeks postmenstrual age. This review summarizes the available evidence on dosing, adverse effects, and practical considerations. Future studies can focus on determining long-term neurodevelopmental safety as well as improving ease of clinical applicability.
References
Dexmedetomidine hydrochloride [package insert]. Parmaus (NJ): HQ Specialty Pharma Cooperation; 2015.
Estkowski LM, Morris JL, Sinclair EA. Characterization of dexmedetomidine dosing and safety in neonates and infants. J Pediatr Pharm Ther. 2015;20:112–8.
Ard J, Doyle W, Bekker A. Awake craniotomy with dexmedetomidine in pediatric patients. J Neurosurg Anesthesiol. 2003;15:263–6.
Ni J, Wei J, Yao Y, Jiang X, Luo L, Luo D. Effect of dexmedetomidine on preventing postoperative agitation in children: a meta-analysis. PLoS One. 2015;10:e0128450.
Tug A, Hanci A, Turk HS, Aybey F, Isil CT, Sayin P, et al. Comparison of two different intranasal doses of dexmedetomidine in children for magnetic resonance imaging sedation. Paediatr Drugs. 2015;17:479–85.
Walker J, Maccallum M, Fischer C, Kopcha R, Saylors R, McCall J. Sedation using dexmedetomidine in pediatric burn patients. J Burn Care Res. 2006;27:206–10.
Greenberg RG, Wu H, Laughon M, Capparelli E, Rowe S, Zimmerman KO, et al. Population pharmacokinetics of dexmedetomidine in infants. J Clin Pharm. 2017;57:1174–82.
McPherson C, Ortinau CM, Vesoulis Z. Practical approaches to sedation and analgesia in the newborn. J Perinatol. 2021;41:383–95.
Puia-Dumitrescu M, Comstock BA, Li S, Heagerty PJ, Perez KM, Law JB, et al. Assessment of 2-year neurodevelopmental outcomes in extremely preterm infants receiving opioids and benzodiazepines. JAMA Netw Open. 2021;4:e2115998.
Durrmeyer X, Vutskits L, Anand KJS, Rimensberger PC. Use of analgesic and sedative drugs in the NICU: integrating clinical trials and laboratory data. Pediatr Res. 2010;67:117–27.
Ng E, Taddio A, Ohlsson A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database Syst Rev. 2017;1:CD002052.
O’Mara K, Gal P, Wimmer J, Ransom JL, Carlos RQ, Dimagulia MAVT, et al. Dexmedetomidine versus standard therapy with fentanyl for sedation in mechanically ventilated premature neonates. J Pediatr Pharm Ther. 2012;17:252–62.
Chrysostomou C, Schulman SR, Herrera Castellanos M, Cofer BE, Mitra S, Garcia da Rocha M, et al. A phase II/III, multicenter, safety, efficacy, and pharmacokinetic study of dexmedetomidine in preterm and term neonates. J Pediatr. 2014;164:276–82.
Dersch-Mills DA, Banasch HL, Yusuf K, Howlett A. Dexmedetomidine use in a tertiary care NICU: a descriptive study. Ann Pharmacother. 2019;53:464–70.
van Dijkman SC, De Cock PAJG, Smets K, Decaluwe W, Smits A, Allegaert K, et al. Dose rationale and pharmacokinetics of dexmedetomidine in mechanically ventilated newborns: impact of design optimisation. Eur J Clin Pharm. 2019;75:1393–404.
McAdams RM, Pak D, Lalovic B, Phillips B, Shen DD. Dexmedetomidine pharmacokinetics in neonates with hypoxic-ischemic encephalopathy receiving hypothermia. Anesthesiol Res. 2020;2582965.
Gundersen JK, Chakkarapani E, Jary S, Menassa DA, Scull-Brown E, Frymoyer A, et al. Morphine and fentanyl exposure during therapeutic hypothermia does not impair neurodevelopment. EClinicalMedicine. 2021;36:100892.
O’Mara K, Weiss MD. Dexmedetomidine for sedation of neonates with HIE undergoing therapeutic hypothermia: a single-center experience. AJP Rep. 2018;8:e168–e173.
Cosnahan AS, Angert RM, Jano E, Wachtel EV. Dexmedetomidine versus intermittent morphine for sedation of neonates with encephalopathy undergoing therapeutic hypothermia. J Perinatol. 2021;41:2284–91.
Elliott M, Burnsed J, Heinan K, Letzkus L, Andris R, Fairchild K, et al. Effect of dexmedetomidine on heart rate in neonates with hypoxic ischemic encephalopathy undergoing therapeutic hypothermia. J Neonatal Perinat Med. 2021;15:47–54. https://doi.org/10.3233/NPM-210737.
Dilek O, Yasemin G, Atci M. Preliminary experience with dexmedetomidine in neonatal anesthesia. J Anaesthesiol Clin Pharm. 2011;27:17–22.
Waurick K, Sauerland C, Goeters C. Dexmedetomidine sedation combined with caudal anesthesia for lower abdominal and extremity surgery in ex-preterm and full-term infants. Paediatr Anaesth. 2017;27:637–42.
Bong CL, Yeo ASH, Fabila T, Tan JS. A pilot study of dexmedetomidine sedation and caudal anesthesia for inguinal hernia repair in infants. Paediatr Anaesth. 2016;26:621–7.
Bong CL, Tan J, Lim S, Low Y, Sim SW, Rajadurai VS, et al. Randomised controlled trial of dexmedetomidine sedation vs general anaesthesia for inguinal hernia surgery on perioperative outcomes in infants. Br J Anaesth. 2019;122:662–70.
Zuppa AF, Nicolson SC, Wilder NS, Ibla JC, Gottlieb EA, Burns KM, et al. Results of a phase 1 multicentre investigation of dexmedetomidine bolus and infusion in corrective infant cardiac surgery. Br J Anaesth. 2019;123:839–52.
Zimmerman KO, Wu H, Laughon M, Greenberg RG, Walczak R, Schulman SR, et al. Dexmedetomidine pharmacokinetics and a new dosing paradigm in infants supported with cardiopulmonary bypass. Anesth Analg. 2019;129:1519–28.
Lam F, Bhutta AT, Tobias JD, Gossett JM, Morales L, Gupta P. Hemodynamic effects of dexmedetomidine in critically ill neonates and infants with heart disease. Pediatr Cardiol. 2012;33:1069–77.
Su F, Gastonguay MR, Nicolson SC, DiLiberto M, Ocampo-Pelland A, Zuppa AF. Dexmedetomidine pharmacology in neonates and infants after open heart surgery. Anesth Analg. 2016;122:1556–66.
Sellas MN, Kyllonen KC, Lepak MR, Rodriguez RJ. Dexmedetomidine for the management of postoperative pain and sedation in newborns. J Pediatr Pharm Ther. 2019;24:227–33.
Sykes AG, Oviedo P, Rooney A, Gollin G. An assessment of dexmedetomidine as an opioid-sparing agent after neonatal open thoracic and abdominal operations. J Perinatol. 2021. https://doi.org/10.1038/s41372-021-01175-7.
Bua J, Massaro M, Cossovel F, Monasta L, Brovendani P, Cozzi G, et al. Intranasal dexmedetomidine, as midazolam-sparing drug, for MRI in preterm neonates. Paediatr Anaesth. 2018;28:747–8.
Dexmedetomidine. In: Specific Lexicomp Online Database [database on the Internet]. Hudson (OH): Lexicomp Inc.: 2021 [updated 29 July 2021; cited 27 July 2021]. Available from: http://online.lexi.com. Subscription required to view.
Nakano T, Okamoto H. Dexmedetomidine-induced cerebral hypoperfusion exacerbates ischemic brain injury in rats. J Anesth. 2009;23:378–84.
Kubota T, Fukasawa T, Kitamura E, Magota M, Kato Y, Natsume J, et al. Epileptic seizures induced by dexmedetomidine in a neonate. Brain Dev. 2013;35:360–2.
Endesfelder S, Makki H, von Haefen C, Spies CD, Bührer C, Sifringer M. Neuroprotective effects of dexmedetomidine against hyperoxia-induced injury in the developing rat brain. PLoS One. 2017;12:e0171498.
Tachibana K, Hashimoto T, Kato R, Uchida Y, Ito R, Takita K, et al. Neonatal administration with dexmedetomidine does not impair the rat hippocampal synaptic plasticity later in adulthood. Paediatr Anaesth. 2012;22:713–9.
Zhang Y, Gao Q, Wu Z, Xue H, Liu B, Zhao P. Dexmedetomidine promotes hippocampal neurogenesis and improves spatial learning and memory in neonatal rats. Drug Des Devel Ther. 2019;13:4439–49.
Lee MM, Caylor K, Gockenbach N. Evaluating the transition from dexmedetomidine to clonidine for the prevention of withdrawal in critically ill pediatric patients. J Pediatr Pharm Ther. 2020;25:104–10.
Gagnon DJ, Riker RR, Glisic EK, Kelner A, Perrey HM, Fraser GL. Transition from dexmedetomidine to enteral clonidine for ICU sedation: an observational pilot study. Pharmacotherapy. 2015;35:251–9.
Murray KL, Wright D, Laxton B, Miller KM, Meyers J, Englebright J. Implementation of standardized pediatric i.v. medication concentrations. Am J Health Syst Pharm. 2014;71:1500–8.
Campos-Beata Y, Saavedra-Mitjans M, Garin N, Cardenete J, Cardona D, Riera P. Physicochemical compatibility of dexmedetomidine with parenteral nutrition. Nutr Clin Pr. 2020;35:967–72.
Baserga M, DuPont TL, Ostrander B, Minton S, Sheffield M, Balch AH, et al. Dexmedetomidine use in infants undergoing cooling due to neonatal encephalopathy (DICE Trial): a randomized controlled trial: background, aims and study protocol. Front Pain Res. 2021;2:770511.
Author information
Authors and Affiliations
Contributions
DM and PS conceived the presented idea. DM completed original literature search and methodology. All authors contributed to analyzing literature, synthesizing results, and writing manuscripts. All authors agree to the final manuscript version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McDonald, D., Palsgraf, H. & Shah, P. Dexmedetomidine – An emerging option for sedation in neonatal patients. J Perinatol 42, 845–855 (2022). https://doi.org/10.1038/s41372-022-01351-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01351-3
- Springer Nature America, Inc.