Abstract
Objective
To examine the effectiveness of soybean oil-medium chain triglycerides-olive oil-fish oil lipid emulsion (SMOF-LE) on clinical outcomes of very-low-birth-weight neonates.
Study design
We conducted a pre-post comparative study of very-low-birth-weight neonates, dividing them according to lipid emulsion received: Intralipid (soy-based; n = 680) or SMOF-LE (n = 617). Primary outcomes were mortality, chronic lung disease, severe retinopathy, infection, and necrotising enterocolitis. Secondary outcomes were cholestasis, osteopenia, time to full feeds, and time to regain birthweight.
Results
Baseline characteristics between groups were comparable. Primary outcomes did not differ significantly between groups, although any retinopathy was significantly lower in the SMOF-LE group. SMOF-LE group had lower odds of cholestasis, osteopenia, and lipid interruption, and reduced times to full feeds and to regain birthweight.
Conclusions
Compared with Intralipid, SMOF-LE was not associated with differences in mortality and major morbidities but was associated with lower odds of any retinopathy, cholestasis, and osteopenia; and improved lipid tolerance.
Similar content being viewed by others
Introduction
Very low birth weight (VLBW, <1500 g) neonates require parenteral nutrition as a source of energy in order to achieve optimum growth, and lipid emulsion (LE) is an integral part of total parenteral nutrition (TPN) [1]. Preterm birth, underdeveloped immunity, and reduced anti-oxidant defenses make VLBW neonates vulnerable to oxidative stress, which plays a significant role in the development of multiple morbidities such as chronic lung disease (CLD), retinopathy of prematurity (ROP), intraventricular hemorrhage (IVH), and necrotizing enterocolitis (NEC) [2,3,4]. Preterm birth also leads to an inadequate supply of long chain polyunsaturated fatty acids (LC-PUFA) such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) for which the majority of maternal transfer to the fetus occurs in the third trimester [5]. These LC-PUFAs are crucial for visual and cognitive development as well as to abate thrombotic and inflammatory responses [6].
Lipid emulsions (LE), in addition to providing calories, act as a rich source of essential fatty acids like linoleic acid (LA; ω-6) and alpha-linolenic acid (ALA; ω-3). These fatty acids are precursors for eicosanoids required for platelet function, immune response, inflammation, and early visual and neural development [7]. Pure soy-based LE (e.g., Intralipid) has traditionally been used in neonatal intensive care units (NICUs) worldwide. Its high ratio of ω-6:ω-3 fatty acids leads to reduced proportions of DHA and EPA since their precursor is ω-3 [8]. A multi-source LE composed of soybean oil, medium chain triglycerides (MCTs), olive oil, and fish oil (SMOF-LE) has increased in use due to the perceived advantages associated with each component. Soybean oil provides both LA and ALA; olive oil is rich in monounsaturated fatty acids, which are less susceptible to lipid peroxidation than polyunsaturated fatty acids and maintain hepatobiliary function; MCTs have a relatively fast metabolic clearance; and fish oil contains DHA [9]. It has been reported that SMOF-LE has a better tolerance, efficacy, and safety profile than soy-based LE [10, 11]. Improved clinical outcomes in, e.g., ROP or neonatal cholestasis with SMOF-LE have been shown in some studies but not in others [12]. Our NICU implemented the use of SMOF-LE as routine practice in October 2014. Our objective in this study was to compare the effects of two LEs, Intralipid and SMOF, on neonatal health outcomes. We hypothesised that the use of SMOF-LE would improve neonatal health outcomes.
Patients and methods
In this single-center, retrospective cohort study, we included preterm neonates with birth weight of <1500 grams (VLBW) or gestational age <32 weeks who received at least 7 days of LE in a level-III NICU at Mount Sinai Hospital in Toronto, Ontario, Canada. Eligible neonates were identified from our Institute’s electronic record. Neonates with major congenital anomalies or potentially lethal genetic conditions, those who died before or within the first week of initiation of lipids, and those who received both Intralipid and SMOF during transition period were excluded.
SMOF-LE usage started in our unit in October 2014. Hence, neonates were divided into two epochs of 3 years each. Epoch 1 included neonates admitted from April 1, 2011 to June 30, 2014 and given soy-based LE (Intralipid® 20%: soybean oil 200 g/L, egg phospholipids 12 g/L, glycerol 22.5 g/L). Epoch 2 included neonates admitted from October 01, 2014 to September 30, 2017 and given SMOF-LE (SMOFlipid® 20%: soybean oil 60 g/L, MCT 60 g/L, olive oil 50 g/L, fish oil 30 g/L, egg phospholipids 12 g/L, glycerol 25 g/L, vitamin E 200 mg α-TE/L). A 3-month wash-out period between two groups was used to ensure that no neonates were included who may have received both types of LE. Both LEs were supplied by Fresenius Kabi Canada Ltd (Richmond Hill, Ontario, Canada).
As a routine practice during both study periods, electrolyte-free TPN solution containing intravenous dextrose (10%) and amino acids (up to 2.5 g/kg) was started in neonates with birth weight <1500 g in the resuscitation room as soon as intravenous access was established. The LEs were administered from the next calendar day as a continuous 24 h infusion. The starting daily dose of lipid was 1 g/kg of body weight with daily increments, as tolerated, of 1 g/kg body weight to a maximum of 3.0–3.5 g of lipids/kg/day. This was consistent between the two study epochs. Neonates also received trace elements, electrolytes, minerals, and vitamins as a standard part of the TPN protocol. Enteral feeds commenced as soon as the medical team deemed the infant to be medically stable and were advanced according to a standardized feeding protocol. The standardized feeding protocol was the same for both study periods, with an exception: in June 2013, the feed advancements were slowed to every 48 h for neonates born at <650 g to reflect a growing population of extremely low birth weight (ELBW) neonates. Feeds consisted of expressed breast milk (EBM) and, if required, were supplemented with preterm formula prior to April 2013 and pasteurized human donor breast milk (DBM) thereafter according to parental choice and availability. For both study groups, human milk fortification was with a powder product and was commenced at an enteral tolerance of 160 ml/kg/day prior to 2014 and 120 ml/kg/day thereafter.
Variable definitions
Demographic and clinical data were abstracted from the hospital electronic database and patient charts. Chronic lung disease was defined as oxygen dependency or need for respiratory support at 36 weeks post-menstrual age or at discharge from our unit if transferred prior to this time [13]. Necrotizing enterocolitis was classified as stage II or stage III according to Modified Bell’s staging criteria [14]. Severe ROP was considered if ROP was stage 3 or higher in either eye or if the infant was treated with laser or anti-vascular endothelial growth factor therapy. Nosocomial infection was diagnosed if a pathogenic organism was identified in blood or cerebrospinal fluid culture in a symptomatic infant and treated with antibiotics for a minimum of 5 days. Cholestasis was defined as a serum direct bilirubin value of >18 μmol/dL (1.0 mg/dL) if the total bilirubin level was <90 μmol/dL (5.0 mg/dL) or a direct bilirubin value of >20% total bilirubin if the total bilirubin level was >90 μmol/dL (5.0 mg/dL) [15]. Osteopenia of prematurity was defined as a serum alkaline phosphatase level >600 IU/L [16].
Outcomes
Our primary outcomes were mortality prior to discharge or major morbidities defined as CLD, nosocomial infection, severe ROP, or NEC stages II or III. Secondary outcomes were ROP of any stage, neonatal cholestasis, osteopenia of prematurity, lipid interruptions due to high triglyceride level, number of days required to regain birth weight, duration of TPN and lipid administration, and length of hospital stay.
Research ethics
The data collection and analysis methods for this study were approved by the Mount Sinai Hospital Research Ethics Board.
Statistical analyses
Baseline characteristics between groups were compared using descriptive statistics. The incidences of primary and secondary outcomes between groups were compared using the Χ2 test or Fischer’s exact test for categorical variables and the Student’s t test or Kruskal–Wallis test for continuous variables, depending on their distribution. Multivariable regression analyses were conducted to adjust for possible confounding variables. Adjusted odds ratios (AORs) were calculated with 95% confidence intervals (CIs). A two-sided P value of <0.05 was considered significant. There were no adjustments for multiple comparisons.
Results
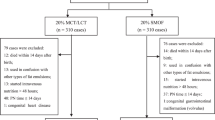
Of the total 2415 neonates who received LE during the study period in the unit, 1297 neonates were eligible for the study as they received either Intralipid or SMOF-LE for >7 days during the study period and were included in this study (Fig. 1). Of these, 680 neonates received Intralipid and 617 neonates received SMOF-LE. Comparison of baseline characteristics and demographic parameters at the time of initiation of LE revealed that the two groups were similar except for a higher proportion of caesarean births in SMOF-LE group (Table 1). Also, the age in hours when enteral feeds were initiated was earlier by 24 h in SMOF-LE group.
Primary outcome variables
The mortality rate did not differ significantly between groups (5.7% in Intralipid group vs. 6.2% in SMOF-LE group; P = 0.75) (Table 2). Similarly, rates of CLD, NEC, severe ROP, and nosocomial infections did not differ between groups (Table 2). The adjusted odds of the primary outcomes of mortality, CLD, NEC, severe ROP, and nosocomial infections also did not differ significantly between groups (Table 3).
Secondary outcome variables
Rates of lipid interruption episodes, any ROP, and osteopenia of prematurity were significantly lower in the SMOF-LE than in the Intralipid group. The duration of LE treatment in days, days to regain birth weight, and days to reach full enteral feeds were all significantly lower in the SMOF-LE group compared to the Intralipid group (Table 2). In adjusted analyses, we identified reduced odds of lipid interruptions, any ROP, osteopenia of prematurity, and neonatal cholestasis in the SMOF-LE group compared to the Intralipid group, along with a reduction in days on LE (1 day less), and days to regain birth weight (2 days less) in the SMOF-LE group (Table 3).
Discussion
In this retrospective analysis of a large cohort, we identified that the use of SMOF-LE was not associated with differences in the primary outcomes of mortality or major neonatal morbidities. However, SMOF-LE use was associated with a reduction in any-stage ROP, neonatal cholestasis, and osteopenia of prematurity. Moreover, it was associated with improved lipid tolerance and reduced time to regain birth weight.
The use of SMOF-LE has been proposed based on the composition of its components. Increases in omega-3 fatty acids, monounsaturated fatty acids, and DHA are suggested to aid immune function [17, 18] and platelet function [18], reduce inflammation [17, 18], and possibly improve neurodevelopment [19]. We hypothesized that, as a result, inflammatory-/immune-related morbidities associated with preterm birth may be modified with the use of SMOF-LE instead of soy-based LE. We did not identify any changes in CLD, NEC or treated ROP in neonates who received SMOF-LE. We did observe a reduction in any-stage ROP in the SMOF-LE-treated group, indicating the possibility of an effect on early vascular growth in the retina. Our findings are similar to those from a Cochrane review [12] that included 7 randomized trials (total participants = 469) that compared SMOF-LE vs soy-based LE in preterm neonates and reported no significant differences in major neonatal morbidities (mortality: 5 studies, 369 participants, RR: 1.26, 95% CI: 0.68–2.31; CLD: 4 studies, 314 participants, RR: 1.02, 95% CI: 0.70–1.49; nosocomial infection: 1 study, 80 participants, RR: 0.67, 95% CI: 0.12–3.78; NEC: 4 studies, 314 participants, RR: 1.35, 95% CI: 0.68–2.67; severe ROP: 3 studies, 256 participants, RR: 0.43, 95% CI: 0.06–2.85). Data for any ROP were available from only one randomised control trial, conducted by Beken et al [20]. As in the present study, they compared SMOF- and soybean-based LEs and included VLBW neonates with a gestational age <32 weeks. They found that rates of severe ROP needing treatment did not differ between groups, with only one patient needing treatment in each group. There was, however, significantly more any-stage ROP in the group that received soybean-based LE (n = 80, OR: 9.1, 95% CI: 1.9–43.8, P = 0.004 vs SMOF-based LE). It could be hypothesized that DHA, which is abundant in fish oil, may protect against the development of ROP because of its anti-inflammatory [21] or oxidative stress-reducing [9, 22] effects. Collins et al [23] randomized preterm neonates born before 29 weeks of gestation: 592 neonates in the DHA group, who received 60 mg/kg of enteral DHA; and 613 neonates in the control group, who received regular soy-based emulsion without DHA. They reported a possible increase in bronchopulmonary dysplasia (BPD) by physiological definition (relative risk 1.13, 95% CI: 1.02–1.25, P = 0.02; and clinical BPD: RR: 1.09, 95% CI: 1.00–1.18, P = 0.06). However, their supplementation differed only in DHA content and it was given enterally, whereas our focus was on supplementation by intravenous route only and our LE contained more components than DHA.
We further identified improved lipid tolerance associated with SMOF-LE use. Careful monitoring of blood triglyceride levels during parental lipid administration is recommended. Preterm neonates are at higher risk for hypertriacylglycerolemia than term neonates due to lack of adequate muscle and fat mass, which accounts for a reduced hydrolytic capacity of the lipoprotein lipase enzyme [11]. Even though there is no specific indicator of lipid intolerance, many studies have measured serum triglyceride levels as a surrogate marker for lipid tolerance [24]. In our NICU, we regularly use plasma triglyceride level as an indicator of tolerance. Similar to our study, two trials in the adult population [25, 26] have shown that SMOF-LE has better clearance and tolerance than soy-based LE. This can be explained by the presence in SMOF-LE of MCT oil, which is rapidly metabolised by lipase and cleared from the circulation faster than pure long chain fatty acids [25].
We identified reduced rates of cholestasis in the SMOF-LE group. Others have similarly reported improvement in neonatal cholestasis, but the results are inconsistent. Gura et al [27] reported that reversal of cholestasis was 4.8 times faster in neonates who received fish oil-based LE (9 weeks) vs. soy-based LE (44 weeks). In a systematic review of randomized and non-randomized studies (6 studies, total participants: 807) among preterm neonates, Vayalthrikkovil et al [28] showed that fish oil-based LE reduced cholestasis significantly when compared to soy-based LE (4 randomized trials, n = 386, RR 0.40, 95% CI 0.22–0.76, number needed to treat = 11; and 2 observational studies, n = 421, RR 0.1, 95% CI: 0.02–0.60, number needed to treat = 17). Skouroliakou et al [29] (study participants; i.e., preterm infants: 54 in SMOF group, 75 in Intralipid group) reported a trend of improving cholestasis towards the date of discharge, but this was not statistically significant. Soy-based lipids have higher amounts of phytosterols, which are associated with impaired biliary secretion; [30] and contain proinflammatory mediators, which account for hepatotoxicity and increased cholestasis [31]. It is possible that SMOF-LE, by virtue of having a high quantity of omega-3 fatty acids (including DHA) and arachidonic acid and vitamin E to counterbalance the pro-inflammatory effect of omega-6 fatty acids [32], has a protective effect on the liver that reduces cholestasis. Our finding of a reduced rate of cholestasis in the SMOF-LE groups compared with the Intralipid group could also be due to a reduced duration of TPN administration, as full feeds were reached earlier in the SMOF-LE group.
We identified that SMOF-LE use was associated with reduced rates of osteopenia of prematurity. The fact that DHA and arachidonic acid are important modulators of bone cell differentiation and bone matrix deposition may account for this improvement [33]. However, previous studies have not explored this association and would need to be explored in future studies.
We identified that the days required to reach full enteral feeds and regain birth weight were significantly lower in the SMOF-LE group (years 2014–2017) compared to the Intralipid group (2011–2014). This may be due to changes in NICU practice that favoured earlier administration of enteral feeding during the study period. We acknowledge that local practice has changed to promote earlier enteral feeding with early initiation of feeds, reduced time on trophic feeds, and earlier fortification of feeds. These changes may explain the difference we observed and could be unrelated to LE.
Our study has several strengths. To our knowledge, this is the largest study to date comparing these two soy-based and SMOF-based LEs. Apart from changes in the feeding protocol and the timing of human milk fortification, lipid administration protocols were maintained throughout the transition from Intralipid to SMOF-LE in our NICU. The similarity in baseline characteristics lends significant credibility to our findings despite the fact that this study was not a randomized trial. The information on this topic from randomized trials is limited to 469 neonates from 7 trials. Our sample size of nearly 1300 neonates could help to strengthen our evidence base such as no difference in clinically important outcomes; however, there may be some benefits in secondary outcomes. Nevertheless, our study has some limitations. First, this was a retrospective cohort analysis. As such, the study design did not allow us precise control over unknown confounders in the study groups. Second, we do not have serum measurements of any lipid components that would provide data on the uptake of the various components of SMOF-LE. Third, our results may have been affected by changes in clinical practices over the 6-year study period; for instance, adjustments in the feeding protocol or respiratory management with greater use of non-invasive ventilation.
Conclusion
In this large cohort study, practice change to routine use of SMOF-based rather than soy-based LE was not associated with changes in mortality, CLD, nosocomial infection, NEC, or severe ROP – but was associated with improved lipid tolerance and lower odds of any ROP, cholestasis, and osteopenia. However, a large randomized controlled trial adequately powered to examine safety and efficacy of LE is warranted.
References
Uauy R, Hoffman DR, Peirano P, Birch DG, Birch EE. Essential fatty acids in visual and brain development. Lipids. 2001;36:885–95.
Saugstad OD. Update on oxygen radical disease in neonatology. Curr Opin Obstet Gynecol. 2001;13:147–53.
Thibeault DW. The precarious antioxidant defenses of the preterm infant. Am J Perinatol. 2000;17:167–82.
Sharda B. Free radicals: emerging challenge in environmental health research in childhood and neonatal disorders. Int J Environ Res Public Health. 2006;3:286–91.
Haggarty P. Effect of placental function on fatty acid requirements during pregnancy. Eur J Clin Nutr. 2004;58:1559–70.
Grimm H, Mertes N, Goeters C, Schlotzer E, Mayer K, Grimminger F, et al. Improved fatty acid and leukotriene pattern with a novel lipid emulsion in surgical patients. Eur J Nutr. 2006;45:55–60.
Lapillonne A, Groh-Wargo S, Gonzalez CHL, Uauy R. Lipid needs of preterm infants: updated recommendations. J Pedia. 2013;162:S37–S47.
Choudhary N, Tan K, Malhotra A. Inpatient outcomes of preterm infants receiving ω-3 enriched lipid emulsion (SMOFlipid): an observational study. Eur J Pedia. 2018;177:723–31.
Calder PC, Adolph M, Deutz NE, Grau T, Innes JK, Klek S, et al. Lipids in the intensive care unit: recommendations from the ESPEN Expert Group. Clin Nutr. 2018;37:1–18.
Vlaardingerbroek H, Vermeulen MJ, Carnielli VP, Vaz FM, van den Akker CH, van Goudoever JB. Growth and fatty acid profiles of VLBW infants receiving a multicomponent lipid emulsion from birth. J Pedia Gastroenterol Nutr. 2014;58:417–27.
Tomsits E, Pataki M, Tölgyesi A, Fekete G, Rischak K, Szollár L. Safety and efficacy of a lipid emulsion containing a mixture of soybean oil, medium-chain triglycerides, olive oil, and fish oil: a randomised, double-blind clinical trial in premature infants requiring parenteral nutrition. J Pedia Gastroenterol Nutr. 2010;51:514–21.
Kapoor V, Glover R, Malviya MN. Alternative lipid emulsions versus pure soy oil based lipid emulsions for parenterally fed preterm infants. Cochrane Database Syst Rev. 2015:CD009172.
Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114:1305–11.
Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pedia. 1987;17:213–88.
Feldman AG, Sokol RJ. Neonatal Cholestasis. Neoreviews. 2013;14:e63.
Rustico SE, Calabria AC, Garber SJ. Metabolic bone disease of prematurity. J Clin Transl Endocrinol. 2014;1:85–91.
Driscoll DF, Bistrian BR, Demmelmair H, Koletzko B. Pharmaceutical and clinical aspects of parenteral lipid emulsions in neonatology. Clin Nutr. 2008;27:497–503.
Lapillonne A, Moltu SJ. Long-Chain polyunsaturated fatty acids and clinical outcomes of preterm infants. Ann Nutr Metab. 2016;69(Suppl 1):35–44.
Makrides M, Gibson RA, McPhee AJ, Collins CT, Davis PG, Doyle LW, et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: a randomized controlled trial. JAMA. 2009;301:175–82.
Beken S, Dilli D, Fettah ND, Kabatas EU, Zenciroglu A, Okumus N. The influence of fish-oil lipid emulsions on retinopathy of prematurity in very low birth weight infants: a randomized controlled trial. Early Hum Dev. 2014;90:27–31.
Pawlik D, Lauterbach R, Turyk E. Fish-oil fat emulsion supplementation may reduce the risk of severe retinopathy in VLBW infants. Pediatrics. 2011;127:223–8.
Deshpande G, Simmer K, Deshmukh M, Mori TA, Croft KD, Kristensen J. Fish Oil (SMOFlipid) and olive oil lipid (Clinoleic) in very preterm neonates. J Pedia Gastroenterol Nutr. 2014;58:177–82.
Collins CT, Makrides M, McPhee AJ, Sullivan TR, Davis PG, Thio M, et al. Docosahexaenoic acid and bronchopulmonary dysplasia in preterm infants. N Engl J Med. 2017;376:1245–55.
Vlaardingerbroek H, van Goudoever JB. Intravenous lipids in preterm infants: impact on laboratory and clinical outcomes and long-term consequences. World Rev Nutr Diet. 2015;112:71–80.
Schlotzer E, Kanning U. Elimination and tolerance of a new parenteral lipid emulsion (SMOF)–a double-blind cross-over study in healthy male volunteers. Ann Nutr Metab. 2004;48:263–8.
Simoens CM, Deckelbaum RJ, Massaut JJ, Carpentier YA. Inclusion of 10% fish oil in mixed medium-chain triacylglycerol-long-chain triacylglycerol emulsions increases plasma triacylglycerol clearance and induces rapid eicosapentaenoic acid (20:5n-3) incorporation into blood cell phospholipids. Am J Clin Nutr. 2008;88:282–8.
Gura KM, Lee S, Valim C, Zhou J, Kim S, Modi BP, et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 2008;121:e678–86.
Vayalthrikkovil S, Bashir RA, Rabi Y, Amin H, Spence JM, Robertson HL, et al. Parenteral fish-oil lipid emulsions in the prevention of severe retinopathy of prematurity: a systematic review and meta-analysis. Am J Perinatol. 2017;34:705–15.
Skouroliakou M, Konstantinou D, Agakidis C, Delikou N, Koutri K, Antoniadi M, et al. Cholestasis, bronchopulmonary dysplasia, and lipid profile in preterm infants receiving MCT/omega-3-PUFA-containing or soybean-based lipid emulsions. Nutr Clin Pract. 2012;27:817–24.
Clayton PT, Whitfield P, Iyer K. The role of phytosterols in the pathogenesis of liver complications of pediatric parenteral nutrition. Nutrition. 1998;14:158–64.
Park HW, Lee NM, Kim JH, Kim KS, Kim SN. Parenteral fish oil-containing lipid emulsions may reverse parenteral nutrition-associated cholestasis in neonates: a systematic review and meta-analysis. J Nutr. 2015;145:277–83.
Repa A, Binder C, Thanhaeuser M, Kreissl A, Pablik E, Huber-Dangl M, et al. A mixed lipid emulsion for prevention of parenteral nutrition associated cholestasis in extremely low birth weight infants: a randomized clinical trial. J Pedia. 2018;194:87–93 e81.
Bridges KM, Pereira-da-Silva L, Tou JC, Ziegler J, Brunetti L. Bone metabolism in very preterm infants receiving total parenteral nutrition: do intravenous fat emulsions have an impact? Nutr Rev. 2015;73:823–36.
Acknowledgements
We thank Junmin Yang, MSc for help in statistical analyses and Sarah Hutchinson, PhD and Heather McDonald Kinkaid, PhD at the Maternal-Infant Care (MiCare) Research Centre at Mount Sinai Hospital in Toronto, Ontario, Canada, for providing editorial assistance in preparing this manuscript. We also thank the Canadian Institutes of Health Research and the Ontario Ministry of Health and Long-Term Care for providing financial support to the MiCare Research Centre. PS is supported by an Applied Chair in Reproductive and Child Health Services and Policy Research from the Canadian Institutes of Health Research.
Author information
Authors and Affiliations
Contributions
RT and PS conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. SD, JS, SU, NO and KK participated in design, interpreted data, and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Torgalkar, R., Dave, S., Shah, J. et al. Multi-component lipid emulsion vs soy-based lipid emulsion for very low birth weight preterm neonates: A pre-post comparative study. J Perinatol 39, 1118–1124 (2019). https://doi.org/10.1038/s41372-019-0425-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-019-0425-7
- Springer Nature America, Inc.
This article is cited by
-
Effects of mixed oil emulsion on short-term clinical outcomes in premature infants: A prospective, multicenter, randomized controlled trial
European Journal of Clinical Nutrition (2023)
-
Hospital change to mixed lipid emulsion from soybean oil-based lipid emulsion for parenteral nutrition in hospitalized and critically ill adults improves outcomes: a pre–post-comparative study
Critical Care (2022)
-
Fish oil-containing lipid emulsions prevention on parenteral nutrition-associated cholestasis in very low birth weight infants: a meta-analysis
World Journal of Pediatrics (2022)
-
Response to Letter to the Editor from Kunal Gupta MBBS, MD: Is SMOF lipid emulsion better than soy-based lipid emulsion for low birth weight preterm neonates?
Journal of Perinatology (2020)
-
Fish oil-containing multicomponent lipid emulsion vs soy-based lipid emulsion and neurodevelopmental outcomes of children born < 29 weeks’ gestation
Journal of Perinatology (2020)