Abstract
Background
Mercury is a widespread persistent environmental pollutant associated with adverse health effects.
Objective
This first national biomonitoring survey of blood total mercury (tHg) conducted in New Zealand aimed to provide baseline data and identify exposure determinants.
Methods
Blood was collected from 191 children (age 5–18 years) and 304 adults (20–65) in 2014–2016 and analysed for tHg using inductively coupled plasma mass spectrometry. Linear regression was used to assess associations with demographic and lifestyle factors.
Results
Blood mercury was detected in 93% of children and 99% of adults, with geometric means (GMs) of 0.86 and 1.65 µg/L, respectively. The 60–65-year olds had the highest GM (2.34 µg/L). Regression indicated that tHg was 40% higher in boys compared to girls. Eating fish ≥ 3 times/week (compared to ≤once/week) was associated with 2.7 and 1.7 times higher tHg in children and adults, respectively. Shellfish consumption was also associated with higher tHg. High daily tap water consumption (≥2 L for children, ≥3 L for adults) was associated with lower tHg. In adults, smoking and milk consumption were associated with higher tHg.
Significance
Fish and shellfish consumption is a strong determinant of New Zealanders blood tHg levels, with water and milk consumption possibly acting as modulating factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Mercury (Hg) is a naturally occurring element in the earth’s crust that is released into the environment by natural processes such as volcanic activity. Since the start of the industrial revolution anthropogenic sources such as the burning of fossil fuels and industrial processes have become significant contributors of mercury to the environment. Additional sources include artisanal small-scale gold mining, currently the largest anthropogenic source of mercury emissions in the environment [1]. Consequently, mercury is now widespread and persistent in the environment and considered a major environmental pollutant. Mercury is a potent neurotoxin and exposure can adversely affect neurological, cardiovascular, reproductive, endocrine, and immune systems [2] with the developing foetus and young children particularly vulnerable due to mercury’s disturbance of many aspects of development, particularly brain maturation [3].
Mercury exists in three basic forms: elemental mercury and inorganic and organic mercury compounds, all of which are toxic to humans, although they differ with respect to absorption, how they are metabolised, and principal target organs. Mercury can be measured in human blood, urine, hair and nails, with urine mercury primarily reflecting the body’s excretion of elemental and inorganic mercury, and blood total mercury (tHg) reflecting all forms of mercury circulating in the blood, including methylmercury (MeHg), the most common form of organic mercury.
In studies of mercury in the general population, the average proportion of methylmercury to tHg in blood ranges from 57 to 79% [4], with fish consumption being the principal exposure source. Total blood mercury therefore reflects, in large part, dietary exposure to methylmercury, which has a half-life of ~50 days [5]. Methylmercury is formed by microorganisms, and is rapidly accumulated by aquatic organisms and biomagnified up the food chain, resulting in particularly high levels in predatory fish and marine mammals. Following absorption (>90%) by the gastrointestinal tract, methylmercury readily crosses cell membranes, including the blood–brain barrier and the placenta [6]. Elemental mercury on the other hand, from, for example, atmospheric pollution or dental amalgams, is mainly absorbed through the respiratory route (80%), while gastrointestinal tract absorption is less than 0.01% [7]. Like methylmercury, elemental mercury readily passes through the blood–brain barrier and the placenta. Exposure to inorganic mercury compounds, which do not readily cross the blood–brain barrier or placenta [7], may result from the diet (~7–15% of ingested inorganic mercury is absorbed by the gastrointestinal tract) and can also be formed in the human body from biotransformation of elemental and organic mercury.
The Minamata Convention on Mercury, which came into force in 2017 and to which New Zealand is a signatory, aims to protect human health and the environment from anthropogenic emissions of mercury and mercury compounds [8]. The biomonitoring of mercury in the general population is an important tool to identify the main contributors of mercury exposure, to inform intervention strategies to reduce exposure, to evaluate the effectiveness of these interventions, and to compare populations. A recent review of mercury biomarkers in human populations [9] indicated great variability in exposures within and across countries and regions, with data gaps remaining for many populations.
In New Zealand, mercury levels in hair of children and their mothers have been reported [10,11,12], but national human mercury biomonitoring had not been conducted.
Here, we report on the first biomonitoring study of blood tHg in a sample of New Zealand children and adults. Levels are compared with geometric means (GMs) and reference values from other countries, and we identify demographic and lifestyle factors associated with tHG.
Methods
Recruitment
The biological monitoring programme included a cross-section of adult and school-age New Zealanders between the ages of 5 and 65 years, as described previously [13, 14]. This cross-sectional study used stratified random sampling, stratifying on age, gender, region, and ethnicity, to ensure that the main demographic groups were represented. For the recruitment of adult participants, the 2014 New Zealand Electoral Roll was used to randomly invite, by mail, men and women in four age groups (i.e. 19–24, 25–34, 35–49, 50–64 years), from four geographic regions (Northland/Auckland, Waikato/Bay of Plenty, Lower North Island, South Island), and of Māori (the indigenous population of New Zealand) and non-Māori (predominantly European) ethnicity. For the recruitment of children, we contacted primary, intermediate, and high schools located in the same geographic regions. We asked schools to put a poster on their noticeboard with contact details for the study and provided information packs for parents to pick up from the school office. While the majority of children were recruited through schools, additional recruitment was undertaken through enrolled parents and public events such as science fairs and sports clubs, where we approached children and parents and provided information packs. The study aimed for a total of 300 adult and 200 children participants with approximately equal numbers in each of the gender/age/geographic/ethnic subgroups.
Study participants provided a blood sample, a urine sample (results not reported here), and completed a short questionnaire. The questionnaire for adults included questions on demographics (e.g. ethnicity, education, height and weight), lifestyle factors (e.g. smoking, alcohol), dwelling (e.g. age, location, type of water supply), dietary items (number of times per week eating fish, shellfish, wild game, milk, cheese, rice, tofu, potatoes, canned food), number of amalgam fillings, other aspects that may affect exposure (e.g. menopause), and occupation. To limit the length of the questionnaire, dietary items were limited to those that had been associated in the literature with the chemicals covered by the biomonitoring survey [14]. The questionnaire for children included the same questions, excluding those on occupation and including questions of number of smokers in the household. The questionnaires were completed at home by the study participants, or by the parents of the child participants, and were designed to take no more than 15 min.
Blood sampling and analyses
Blood samples were collected between September 2014 and December 2016 by a trained phlebotomist at a private pathology laboratory, medical clinic, or at the school (in the case of some children). Blood was collected in 10 mL K2EDTA-containing plastic vacutainer tubes and stored frozen (−20 °C) by the pathology laboratory until they were sent (frozen) to the study centre. Blood samples were shipped in their vacutainers on dry ice to International Accreditation New Zealand accredited Canterbury Health Laboratories in Christchurch (www.chl.co.nz/) for analyses.
tHg concentrations in whole blood were determined using inductively coupled plasma mass spectrometry, following external QC (RCPA quality Assurance Programs) and internal QC (Lyphochek metal control) protocols. Blood was diluted in an ammonia EDTA solution and aspirated into an argon plasma torch at 2700 °C for ion formation which was subsequently focused into an octopole reaction system where it collides with helium gas. The ions then passed into the quadrupole for detection by the electron multiplier. The lower limit of detection was 0.2 μg/L. Analyses were completed between May 2016 and June 2017.
Statistical analyses
Summary statistics of blood mercury concentrations, including GMs and 95% confidence interval, were calculated separately for adults and children, and by age group, gender, ethnicity (Māori/non-Māori), and geographic region. For samples below the limit of detection, half the limit of detection was used in further calculations. Linear regression on log-transformed blood mercury concentrations was used to assess associations between a range of demographic and lifestyle factors as collected via questionnaire and blood lead, separately for children and adults. For adults, these included ethnicity (Māori, Pacific, other), highest achieved education, body mass index, smoking, living with a smoker, alcohol consumption (beer, wine, spirits), age of dwelling, type of water supply of dwelling, water filter at dwelling, presence of peeling paint at dwelling, dietary items (times per week eating fish, shellfish, wild game, milk, cheese, rice, tofu, potatoes, canned food, categorised into three or four categories depending on the consumption frequency distribution in the study population), stroke, metal joints, dental fillings, occupation, and for women only: menopause, osteoporosis, number of births, and hormone replacement therapy. For children, these variables included: ethnicity, living with a smoker, age of dwelling, type of water supply of dwelling, water filter at dwelling, presence of peeling paint at dwelling, dental fillings, and diet (items the same as for adults). All variables were first run individually in models adjusting for age and gender and stratified by gender. If the variable was associated with blood tHg (p < 0.05) in any of these models, the variable was then included in a model fitting all variables significantly (p < 0.05) associated with total blood mercury, providing ORs adjusting for age and gender as well as other variables associated with blood mercury levels. Regression coefficients were exponentiated and thus reflect an exposure ratio (e.g. an exponentiated coefficient of 2 for male gender indicates a two times higher GM for tHg in males compared to females).
The study protocol was evaluated and approved by the Central Health and Disability Ethics Committee (14/CEN/44). Informed consent was provided by all adult participants and a parent or guardian of the participating children.
Results
For adults, a total of 5908 invitations were posted to addresses on the Electoral Roll and we received a reply for 1859 of those 228 were marked returned to sender, 672 refused, 441 were not eligible (did not have a phone, no longer living in New Zealand, illness or impairment or deceased), and 518 indicated to be interested in the study. Of those 518,304 (mean age 42.4, Std Dev 13.7) provided a blood sample within the time frame of the study. For children, 150 schools were invited to participate of which 40 replied positively. This resulted in 113 children recruited through schools with another 80 recruited through other means. Of those, 193 children provided a blood sample. Two participants recruited as children were older than 18 at time of blood sampling, leaving 191 children (mean age 10.1, Std Dev 3.0) participants.
Blood tHg detection frequencies were 93% for children and 99% for adults. The levels were lower for children (GM 0.86 µg/L) compared to adults (1.65 µg/L), and the highest levels were observed for the oldest age group (age 60–65: GM 2.34 µg/L; Table 1).
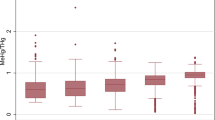
Higher blood tHg levels were found in boys compared to girls, while for adults we did not observe consistent differences by gender (Fig. 1).
Boys had 41% higher levels than girls, as shown in the regression models (Table 2). The age of the child was not associated with Hg levels. A doubling of levels was associated with weekly fish consumption, as well as fish consumption in the 48 h before blood collection; shellfish consumption was also associated with higher levels. Consumption of ≥2 L of tap water per day (self-estimated) was associated with lower blood tHg levels (Table 2).
In adults, gender was not associated with blood mercury, while older age was associated with higher levels (2% for each year of increased age; Table 3). Adults living in the Northland/Auckland region had higher mercury levels. Fish consumption, shellfish consumption, and fish consumption in the 48 h before blood taking were all associated with higher levels. Wild game consumption was associated with higher Hg levels, but only for women. Tobacco smoking was also associated with higher blood tHg levels. Milk consumption was associated with higher levels but not in a clear dose-dependent way. Tap water consumption of ≥3 L/day and consumption of food from cans ≥5 times/week were associated with lower blood mercury levels (Table 3).
Discussion
This study provides the first assessment of blood tHg concentrations in a sample of the New Zealand general population. With blood mercury GMs of 0.86 µg/L for children and 1.65 µg/L for adults, total blood mercury in New Zealand levels are twice that reported for the USA and Canada, but considerably lower than those reported for countries where human exposure to mercury in the environment is higher, such as Korea [15] (Table 4). Substantially higher blood mercury levels have been reported for certain populations of concern, including communities near artisanal and small-scale gold mining sites, and populations dependent on fish and marine mammal consumption [9].
As there is currently no New Zealand blood mercury notification level (the blood mercury concentration at which medical practitioners must notify government health authorities for action or intervention), we compared our results with the reference and guidance values for blood tHg used by some other countries to identify levels that require action or intervention (Table 5). This showed that none of the participants in this survey had blood tHg levels above Health Canada’s reference value of 20 µg/L, while Germany’s HBM I reference level of 5 µg/L was exceeded for 9% of adults, 4% of children, and 6% of women of childbearing age, signalling the potential of adverse health effects at current blood mercury levels in New Zealand, with neurodevelopmental effects in children of primary concern. A study conducted in New Zealand [11] correlated scholastic and psychological test performance of 237 children aged 6–7 years with their mothers’ hair mercury concentration during pregnancy and estimated that the lower bound of the benchmark dose (interpretable as an acceptable human exposure level) ranged from 7.4 to 10 mg/kg mercury in the mothers’ hair, which equates to 30–40 µg/L blood mercury (using the 1:250 blood:hair conversion factor [16]). None of the participants in this study had blood mercury levels exceeding 30 µg/L (the highest measured level was 19 µg/L).
Fish consumption was the strongest determinant of tHg blood levels in both children and adults. While consumption of marine mammals, which can lead to high mercury exposure in some populations [9], is not part of the New Zealand diet, fish is a frequent food item, i.e. in our sample 39% of children and 43% of adults ate fish at least once a week. Although we did not collect information on the type of fish consumed (methylmercury concentrations can vary within and between fish species by more than 100-fold [17]), some fish species with high mercury levels are common in New Zealand diets (e.g. school shark, southern bluefin tuna).
As there have been no previous population studies of blood mercury in New Zealand, a time trend for blood mercury could not be established, but two previous studies conducted in Auckland New Zealand reported hair mercury levels. The first study from the 1980s [10] included 415 school children and reported GMs ranging between 0.61 and 2.0 µg/g hair, which equates to 2.4–8.0 µg/L blood mercury (using the 1:250 blood:hair conversion factor [16]), 3–9 times higher than the blood mercury levels in children who participating in the current study. A later study from 2007/2008 included 46 Pacific mother-child pairs [12] living in Auckland and reported a median of 0.32 µg/g hair for the children at age 6 (equating to 1.3 µg/L), which is twice the level reported for children in the current study, while the median for 46 mothers (0.43 µg/g hair; equating to 1.7 µg/L blood) was similar to the GM reported here for adults. These data suggest that over the past 30 years mercury exposure in New Zealand children has likely reduced by ~3% per year, a decline comparable to that reported for other countries. A study combining data from the USA, Canada, and the Czech Republic showed annual decreases in blood tHg of ~2.25% [9]. A study among Swedish children also showed a decrease of tHg, by about 3% per year [18] between 1986 and 2013. Reductions in blood tHg over time have also been observed for women of reproductive age after public health interventions to limit their exposure to mercury in food. In particular, since 2004 women of reproductive age in the USA have been given advice to avoid fish species that contain higher levels of MeHg [17] and mean tHg levels for women of reproductive age have dropped from 1.96 µg/L in 1999–2000 to 1.39 µg/L in 2009–2010. A much larger 80% drop in blood tHg was observed after a similar public health intervention in Bermuda [19, 20], where the arithmetic mean of blood tHg in mothers reduced from 8.3 µg/L in 2003 to 1.3 µg/L in 2010.
We found that on average boys had higher blood tHg than girls, while among adults we found no gender difference. Results from the New Zealand Total Diet Survey 2003/2004 indicated that boys age 11–14 years have an estimated weekly dietary exposures of tHg of 0.74 µg/kg compared to 0.46 µg/kg/bw/week in girls of the same age group [21], suggesting that diet, in particular fish consumption, may explain the observed gender differences in blood tHg. Gender differences in children’s elimination of mercury may also play a role [22].
Consistent with our study, most studies have shown that blood mercury levels in adults are approximately two times higher than in children [9]. The positive association with age we found for adults may be a reflection of mercury accumulation with age, due to exposure exceeding elimination.
Higher income and higher education have both been associated with higher tHg in studies from Canada [23] and UK women [24], likely the result of higher fish consumption in this demographic. In our study, women with a qualification also had higher blood tHg, but no association with education was observed for men.
Although dental amalgam fillings are a known contributor to urinary Hg [22, 25], data on the relationship between dental amalgam and blood tHg levels are limited and inconsistent [23]. In the present study, we did not observe an association between the number of dental amalgams and blood tHg for children, while for adults the association was limited to men, although the association lost statistical significance after adjustment for other variables including fish consumption. We therefore limited the analysis to participants in the lowest fish consumption stratum and who did not eat fish in the 48 h prior to blood collection (results not shown in tables), which did reveal an association between the number of amalgam fillings and tHg for adults [OR for 0/1–3/4–7/>=8 metallic fillings (p value): 1.0 (reference)/1.3 (0.18)/1.6 (0.05)/ 1.4 (0.19)] and children [OR for 0/1–3/4–7 metallic fillings (p value): 1.0 (reference)/0.6 (0.86)/2.6 (0.20)], although statistical significance for the highest category was not reached.
Tobacco smoking is a potential source of exposure to inorganic mercury. In a study from Canada, the association between blood tHg and smoking status remained statistically significant after controlling for fish and shellfish consumption [23]. The same was observed in our study, in which both ex- and current smokers had elevated tHg compared to never smokers (statistically significant only for ex-smokers), suggesting that smoking contributes to mercury accumulation in New Zealanders. Living with smokers in the household was not associated with children’s blood mercury levels.
In this study, milk consumption was associated with higher blood Hg, but only for adults. While milk is not considered an important contributor of mercury exposure, milk has been suggested to increase mercury absorption [26, 27] and decrease elimination [28], possibly through a mechanism of intestinal microflora modulated by a milk diet being less able to demethylate methylmercury, thereby reducing its excretion rate [28]. Several studies have not observed an association between milk consumption and blood tHg [29, 30], while others observed negative [24, 31] as well as positive [24] associations.
For both children and adults, the consumption of tap water was associated with lower levels of tHg. While drinking water can be a source of mercury in some populations, it is an insignificant mercury source for the New Zealand population [21], which this study confirms. However, the consumption of water may facilitate mercury elimination via urine, although research in this area is lacking. We did not observe a positive association between alcohol consumption and tHg, as has been reported for Canada [23], Korea [32], and the UK [24].
We found that participants who regularly consumed food from cans had lower tHg, but information on the type of canned food was not collected. Baked beans are a common canned food product in New Zealand, and baked beans are particularly high in selenium. An antagonistic influence of selenium on the bioaccumulation of Hg in experimental animals has been observed [33], which may explain the inverse association between canned food consumption and tHg, which would be in line with another study that reported a negative association between baked beans and blood mercury levels in UK women [24].
Conclusions
This study showed that in the New Zealand population, fish consumption is an important contributor to total blood mercury, and that exceedances of international health-based reference values do occur in New Zealand children and adults. Public health messaging such as provided by the Ministry for Primary Industries [34] in combination with greater transparency in fish species’ mercury levels at the point of sale may help the consumer, pregnant women in particular, to reduce their mercury exposure by selecting low-mercury fish species [8].
References
Steckling N, Tobollik M, Plass D, Hornberg C, Ericson B, Fuller R, et al. Global burden of disease of mercury used in artisanal small-scale gold mining. Ann Glob Health. 2017;83:234–47.
Rice KM, Walker EM Jr, Wu M, Gillette C, Blough ER. Environmental mercury and its toxic effects. J Prev Med Public Health. 2014;47:74–83.
Goldman LR, Shannon MW. American Academy of Pediatrics: committee on environmental health technical report: mercury in the environment: implications for pediatricians. Pediatrics. 2001;108:197–205.
Donohue A, Wagner CL, Burch JB, Rothenberg SE. Blood total mercury and methylmercury among pregnant mothers in Charleston, South Carolina, USA. J Expo Sci Environ Epidemiol. 2018;28:494–504.
Smith JC, Farris FF. Methyl mercury pharmacokinetics in man: a reevaluation. Toxicol Appl Pharm. 1996;137:245–52.
Hoffmeyer RE, Singh SP, Doonan CJ, Ross AR, Hughes RJ, Pickering IJ, et al. Molecular mimicry in mercury toxicology. Chem Res Toxicol. 2006;19:753–9.
Park JD, Zheng W. Human exposure and health effects of inorganic and elemental mercury. J Prev Med Public Health. 2012;45:344–52.
Sharma BM, Sanka O, Kalina J, Scheringer M. An overview of worldwide and regional time trends in total mercury levels in human blood and breast milk from 1966 to 2015 and their associations with health effects. Environ Int. 2019;125:300–19.
Basu N, Horvat M, Evers DC, Zastenskaya I, Weihe P, Tempowski J. A state-of-the-science review of mercury biomarkers in human populations worldwide between 2000 and 2018. Environ Health Perspect. 2018;126:106001.
Kjellstrom TE, Reeves RL, Mitchell JW. Comparison of mercury in hair with fish eating habits of children in Auckland. Community Health Stud. 1982;6:57–63.
Crump KS, Kjellstrom T, Shipp AM, Silvers A, Stewart A. Influence of prenatal mercury exposure upon scholastic and psychological test performance: benchmark analysis of a New Zealand cohort. Risk Anal. 1998;18:701–13.
Karatela S, Ward N, Paterson J. Mercury exposure in mother-children pairs in a seafood eating population: body burden and related factors. Int J Environ Res Public Health. 2019;16:2238.
’t Mannetje A, Coakley J, Douwes J. Where are we at with lead? Current levels, time trend, and determinants of blood lead in New Zealand children and adults. Int J Hyg Environ Health. 2020;225:113468.
’t Mannetje A, Coakley J, Douwes J. Report on the biological monitoring of selected chemicals of concern results of the New Zealand biological monitoring programme, 2014-2016. Wellington, New Zealand: Centre for Public Health Research Massey University; 2018. Technical report number 2017-1. Prepared as part of a Ministry of Health contract for scientific services.
Hong YS, Kim YM, Lee KE. Methylmercury exposure and health effects. J Prev Med Public Health. 2012;45:353–63.
Clarkson TW, Vyas JB, Ballatori N. Mechanisms of mercury disposition in the body. Am J Ind Med. 2007;50:757–64.
Cusack LK, Smit E, Kile ML, Harding AK. Regional and temporal trends in blood mercury concentrations and fish consumption in women of child bearing Age in the united states using NHANES data from 1999-2010. Environ Health. 2017;16:10.
Lundh T, Axmon A, Skerfving S, Broberg K. Cadmium and mercury exposure over time in Swedish children. Environ Res. 2016;150:600–5.
Dewailly E, Rouja P, Forde M, Peek-Ball C, Cote S, Smith E, et al. Evaluation of a public health intervention to lower mercury exposure from fish consumption in Bermuda. PLoS ONE. 2012;7:e47388.
McLean Pirkle C, Peek-Ball C, Outerbridge E, Rouja PM. Examining the impact of a public health message on fish consumption in Bermuda. PLoS ONE. 2015;10:e0139459.
Chrystal L, Rumsby A. Mercury inventory for New Zealand 2008. Technical report prepared for the Ministry for the Environment. Pattle Delamore Partners Limited; 2009. https://www.mfe.govt.nz/sites/default/files/mercury-inventory-new-zealand-2008.pdf.
Woods JS, Martin MD, Leroux BG, DeRouen TA, Leitao JG, Bernardo MF, et al. The contribution of dental amalgam to urinary mercury excretion in children. Environ Health Perspect. 2007;115:1527–31.
Lye E, Legrand M, Clarke J, Probert A. Blood total mercury concentrations in the Canadian population: Canadian Health Measures Survey cycle 1, 2007-2009. Can J Public Health. 2013;104:e246–251.
Golding J, Steer CD, Hibbeln JR, Emmett PM, Lowery T, Jones R. Dietary predictors of maternal prenatal blood mercury levels in the ALSPAC birth cohort study. Environ Health Perspect. 2013;121:1214–8.
Abraham JE, Svare CW, Frank CW. The effect of dental amalgam restorations on blood mercury levels. J Dent Res. 1984;63:71–73.
Kostial K, Rabar I, Ciganovic M, Simonovic I. Effect of milk on mercury absorption and gut retention in rats. Bull Environ Contam Toxicol. 1979;23:566–71.
Chapman L, Chan HM. The influence of nutrition on methyl mercury intoxication. Environ Health Perspect. 2000;108:29–56.
Rowland IR, Robinson RD, Doherty RA. Effects of diet on mercury metabolism and excretion in mice given methylmercury: role of gut flora. Arch Environ Health. 1984;39:401–8.
Kim SA, Kwon Y, Kim S, Joung H. Assessment of dietary mercury intake and blood mercury levels in the Korean population: results from the Korean National Environmental Health Survey 2012-2014. Int J Environ Res Public Health. 2016;13:877.
Davis MA, Gilbert-Diamond D, Karagas MR, Li Z, Moore JH, Williams SM, et al. A dietary-wide association study (DWAS) of environmental metal exposure in US children and adults. PLoS ONE. 2014;9:e104768.
Bates CJ, Prentice A, Birch MC, Delves HT. Dependence of blood indices of selenium and mercury on estimated fish intake in a national survey of British adults. Public Health Nutr. 2007;10:508–17.
Park H, Kim K. Association of blood mercury concentrations with atopic dermatitis in adults: a population-based study in Korea. Environ Res. 2011;111:573–8.
Kuras R, Janasik B, Wasowicz W, Stanislawska M. Revision of reciprocal action of mercury and selenium. Int J Occup Med Environ Health. 2018;31:575–92.
Ministry for Primary Industries. List of safe food in pregnancy. Ministry for Primary Industries. https://www.mpi.govt.nz/food-safety-home/food-pregnancy/list-safe-food-pregnancy/. Accessed Nov 2020.
CDC U.S. Fourth national report on human exposure to environmental chemicals, updated tables, January 2017. Vol 1. CDC U.S.; 2017. https://www.cdc.gov/exposurereport/.
CDC U.S. Department of Health and Human Services, Centres for Disease Control and Prevention. Fourth national report on human exposure to environmental chemicals, updated tables, Centers for Disease Control and Prevention; Atlanta, Georgia; January 2019. 2019.
Health Canada. Fourth report on human biomonitoring of environmental chemicals in Canada. Results of the Canadian Health Measures Survey Cycle 4. Health Canada; 2017.
Schulz C, Conrad A, Becker K, Kolossa-Gehring M, Seiwert M, Seifert B. Twenty years of the German Environmental Survey (GerES): human biomonitoring–temporal and spatial (West Germany/East Germany) differences in population exposure. Int J Hyg Environ Health. 2007;210:271–97.
Cho S, Jacobs DR Jr, Park K. Population correlates of circulating mercury levels in Korean adults: the Korea National Health and Nutrition Examination Survey IV. BMC Public Health. 2014;14:527.
Pino A, Amato A, Alimonti A, Mattei D, Bocca B. Human biomonitoring for metals in Italian urban adolescents: data from Latium region. Int J Hyg Environ Health. 2012;215:185–90.
Haines DA, Saravanabhavan G, Werry K, Khoury C. An overview of human biomonitoring of environmental chemicals in the Canadian Health Measures Survey: 2007-2019. Int J Hyg Environ Health. 2017;220:13–28.
Choi W, Kim S, Baek YW, Choi K, Lee K, Yu SD. Exposure to environmental chemicals among Korean adults-updates from the second Korean National Environmental Health Survey (2012-2014). Int J Hyg Environ Health. 2017;220:29–35.
Nisse C, Tagne-Fotso R, Howsam M, Richeval C, Labat L, Leroyer A. Blood and urinary levels of metals and metalloids in the general adult population of Northern France: the IMEPOGE study, 2008-2010. Int J Hyg Environ Health. 2017;220:341–63.
Kuno R, Roquetti MH, Becker K, Seiwert M, Gouveia N. Reference values for lead, cadmium and mercury in the blood of adults from the metropolitan area of Sao Paulo, Brazil. Int J Hyg Environ Health. 2013;216:243–9.
McKelvey W, Gwynn RC, Jeffery N, Kass D, Thorpe LE, Garg RK, et al. A biomonitoring study of lead, cadmium, and mercury in the blood of New York city adults. Environ Health Perspect. 2007;115:1435–41.
Schulz C, Wilhelm M, Heudorf U, Kolossa-Gehring M. Update of the reference and HBM values derived by the German Human Biomonitoring Commission. Int J Hyg Environ Health. 2011;215:26–35.
Schober SE, Sinks TH, Jones RL, Bolger PM, McDowell M, Osterloh J, et al. Blood mercury levels in US children and women of childbearing age, 1999-2000. JAMA. 2003;289:1667–74.
Acknowledgements
The authors thank all study participants for their support.
Funding
This biomonitoring survey was funded by the New Zealand Ministry of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
’t Mannetje, A., Coakley, J. & Douwes, J. Total blood mercury and its determinants in New Zealand children and adults. J Expo Sci Environ Epidemiol 31, 289–298 (2021). https://doi.org/10.1038/s41370-021-00296-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41370-021-00296-7
- Springer Nature America, Inc.
Keywords
This article is cited by
-
Factors associated with blood mercury concentrations and their interactions with three glutathione S-transferase genes (GSTT1, GSTM1, and GSTP1): an exposure assessment study of typically developing Jamaican children
BMC Pediatrics (2024)
-
Determinants affecting the blood mercury levels of preschool children in Shanghai, China: A cross-sectional study
Environmental Science and Pollution Research (2023)