Abstract
Objective
Rapid infant weight gain is a key risk factor for paediatric obesity, yet there is very little evidence on how healthy behaviours in childhood might modify this association. We aimed to examine how the association of infant weight gain with adolescent adiposity might be attenuated by moderate-to-vigorous physical activity (MVPA) in childhood.
Methods
The sample comprised 4666 children in the UK Millennium Cohort Study. The two outcomes were BMI Z-score and % fat at 14 years. Sex-stratified regression models were developed testing for interactions between infant weight Z-score gain between 0 and 3 years (continuous or categorical) and MVPA at 7 years (continuous or binary). Models were sequentially adjusted for basic covariates, socioeconomic variables, and parental BMI levels.
Results
Effect modification was observed in boys but not girls and, among boys, was stronger for % fat than BMI. In a fully adjusted model for boys, the association between infant weight Z-score gain and adolescent % fat was 1.883 (1.444, 2.322) if MVPA < 60 min/day and 1.305 (0.920, 1.689) if MVPA ≥ 60 min/day; the difference between these two estimates being −0.578 (−1.070, −0.087). Similarly, % fat was 2.981 (1.596, 4.367) units higher among boys who demonstrated rapid infant weight gain (+0.67 to +1.34 Z-score) compared to normal weight gain (−0.67 to +0.67 Z-scores), but having MVPA ≥ 60 min/day reduced this effect size by −2.259 (−3.989, −0.535) units.
Conclusions
In boys, ~75% of the excess % fat at 14 years associated with rapid infant weight gain was attenuated by meeting the MVPA guideline. In boys known to have demonstrated rapid infant weight gain, increasing childhood MVPA levels, with the target of ≥60 min/day, might therefore go a long way to towards offsetting their increased risk for adolescent obesity. The lack of effect modification in girls is likely due to lower MVPA levels.
Similar content being viewed by others
Introduction
The NCD Risk Factor Collaboration has estimated that, between 1975 and 2016, the worldwide prevalence of childhood obesity (5–19 years) increased from 5 to 50 million in girls and 6 to 74 million in boys [1]. In the United Kingdom (UK), using harmonised data from a series of national birth cohort studies, we have demonstrated a secular trend toward higher body mass index (BMI) at increasingly younger ages [2]. By adolescence, overweight or obesity prevalence is already 2–3 times higher in cohorts born into the obesity epidemic era (>1970) compared to those born before the obesity epidemic era (<1970). More recent data from the 2018–2019 National Child Measurement Programme for England (NCMP; N > 1,000,000) show that 9.7% of 4–5-year olds and 20.2% of 10–11-year olds are obese [3]. The prevalence of obesity, therefore, approximately doubles between early childhood and adolescence, highlighting how critical the intervening period is for obesity prevention.
Weight gain during infancy is arguably the strongest early-life determinant of paediatric BMI. Rapid infant weight gain, defined as upward crossing through one UK centile band in the first few years of life, has consistently been found to be associated with increased obesity risk [4]. In a recent systematic review, Zheng et al. found that 15 out of 17 studies reported a significant positive association between rapid infant weight gain and risk of future overweight/obesity [5]. The pooled odds ratio for paediatric overweight/obesity of 4.16 (3.26, 5.32) is larger than the meta-analyzed estimates for other early-life risk factors (e.g. maternal smoking during pregnancy and not breastfeeding) [6], including maternal obesity and birth weight. For example, in a meta-analysis of 20 studies, Heslehurst et al. reported that maternal obesity before conception was associated with a 2.69 (2.10, 3.46) times increased odds of paediatric obesity in the offspring [7]. Similarly, Yu et al. reported that high birth weight (>4.0 kg) was associated with a 2.23 (1.91, 2.61) times increased odds of obesity compared to normal birth weight (2.5–4.0 kg) [8].
The prevention of rapid infant weight gain is clearly important, and this is a new and evolving field [9], but we also need to understand how the adverse relationship of rapid infant weight gain with adolescent obesity can be modified. Literature on this topic has tended to focus on prenatal and infancy factors, mainly birth weight and breastfeeding [10,11,12,13], and there is real dearth of evidence regarding the potentially important role of healthy lifestyle behaviours in childhood. Greater physical activity, especially at high intensity, is a key candidate because of its strong relationship with lower adiposity [14, 15], yet only two publications have investigated moderate-to-vigorous physical activity (MVPA). Using data on 1874 participants in the 1982 Pelotas birth cohort study, Kolle et al. found no evidence that the association of infant weight gain between 0 and 2 years and fat mass index at 30 years was modified by MVPA levels, although modification for weight gain between 2 and 4 years was observed [16]. Bernhardsen et al. had a much earlier outcome at 9–12 years and showed that the association of weight gain between 0 and 1 years with BMI was attenuated by greater MVPA levels, but only in boys [17]. This study was limited, however, by a small sample size of just 445 children (186 with fat mass outcomes) from the Norwegian Mother and Child Cohort Study (MoBa). Further, the physical activity data were collected at the same time point as the outcomes, thereby limiting causal inference due to possible reverse causation. As discussed by Bernhardsen et al. [17], the lack of evidence for effect modification in girls is most likely because they are not meeting levels of MVPA high enough to attenuate the adverse relationship of rapid infant weight gain with obesity risk [18,19,20]. This sex difference does, however, require further investigation.
Using data from the large UK Millennium Cohort Study (MCS), we recently reported strong positive relationships of infant weight gain between 0 and 3 years with adolescent BMI at 14 years [21]. The aim of the present study is to examine the extent to which MVPA levels at age 7 years might attenuate these deleterious associations. We hypothesise that any effect modification will be stronger in boys than girls. Given the known limitations of BMI [22], percentage body fat (% fat) at 14 years is also investigated.
Methods
Sample
The MCS is based on 18,818 people born between September 2000 and January 2002 who were living in England, Scotland, Wales, or Northern Ireland at age 9 months [23]. Subsequent sweeps have occurred when the children were aged 3, 5, 7, 11, and 14 years. The study has received ethical approval and obtained informed parental and participant consent.
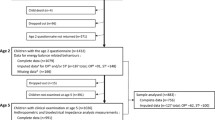
Our sample comprised 4666 singleton children (2267 males, 2399 females) with complete data on the exposures, outcomes, and effect modifiers. The sample represents 74% (4666/6309) of the singletons with reliable accelerometer data and 85% (4666/5478) of the singletons with reliable accelerometer data who attended the 14-year sweep.
Exposures
Birth weights were collected from the main carer at the 9-month sweep and have been shown to demonstrate a high level of agreement with registration data [24]. Weight was measured at 3 years of age. Both measurements were converted to Z-scores according to the World Health Organisation (WHO) Child Growth Standards [25]. A continuous exposure was calculated as change in infant weight Z-score between ages 0 and 3 years. A categorical exposure was also computed to identify infants with slow (<−0.67 Z-scores), normal (−0.67 to +0.67), rapid (+0.67 to +1.34), and very rapid weight gain (>+1.34). A change of 0.67 or 1.34 Z-scores represents shifting upward/downward through one or two, respectively, UK centile bands [26].
Outcomes
Weight and height were measured at age 14 years; details of the measurement protocols are available in the user guide [27]. BMI was calculated as kg/m2 and Z-scores (sex and age specific) were computed according to the WHO Child Growth References [28]. Overweight and obesity were defined according to the International Obesity Task Force cut-offs [29]. % fat was assessed via bioelectrical impedance using electronic Tanita scales (Tanita UK Ltd, Middlesex, UK).
Effect modifiers
Accelerometers (Actigraph GT1M, Pensacola, Florida) were used to measure physical activity levels (15 s sampling epochs) at age 7 years. Participants were instructed to wear the accelerometers, on an elastic belt around the waist, during waking time (excluding bathing or during other aquatic activities) for 7 consecutive days. Details of the data processing have been published elsewhere [30]. The cut-points developed by Pulsford et al. were used to define sedentary, light, moderate, and vigorous physical activity (VPA) [31]. Participants were required to have ≥10 h wear time on at ≥2 days for their data to be considered reliable or useable. Average time per day in MVPA was computed, as was a binary variable indicating whether or not the participant met the guideline of ≥60 min per day in MVPA [32]. Within our sample, the correlation of MVPA with wear time (0.09) was negligible. Sedentary time was not considered due to its much stronger correlation (0.67) with wear time.
Potential confounders
Set 1 comprised ethnicity, birth weight Z-score, and gestational age in weeks estimated using mother’s report of her expected due date; this has been shown to correlate highly with routine hospital records of gestational age [33].
Set 2 comprised seven measures of socioeconomic position. Family income and tenure were reported at the 9-month sweep. Mother’s and father’s age of leaving full-time education, as well as highest qualification according to the National Qualifications Framework (NQF), were based on data collected up until the 11-year sweep. Father’s or mother’s (if no father figure was present; N = 693) occupation was assessed at the 11-year sweep and was classified according to the Registrar General’s Social Class [34].
Set 3 comprised maternal and paternal BMI values, based on weights and heights reported by the mother at the 9-month sweep.
Statistical analysis
Based on the literature [17], an a priori decision was made to stratify all analyses according to sex. Descriptive statistics for all variables were produced. To further describe the sample and data, infant weight gain status was tabulated against MVPA guidelines status at 7 years and IOTF weight status at 14 years.
General linear regression (within a structural equation modelling framework) was used for inferential analyses, with full-information maximum likelihood (FIML) to handle missing confounder data under a missing at random assumption [35, 36]. All independent variables were grand mean centred and, for parsimony, all socioeconomic position variables (except tenure which is binary) were modelled as ridit scores [37]. In exploratory work, no evidence was found that the associations of infant weight gain Z-scores and MVPA with the outcomes were non-linear. BMI Z-score and % fat at age 14 years were each regressed on infant weight gain Z-scores, MVPA at age 7 years (scaled so that effect sizes represent a change of 10 min/day), and the interaction between these two independent variables. After running completely unadjusted analyses, models were sequentially adjusted for confounder sets 1–3. Sensitivity analyses were conducted investigating VPA and moderate physical activity (MPA) separately.
Analyses were re-run using the binary (instead of the continuous) MVPA variable. For each model, we obtained and present an effect size (of infant weight gain Z-score) for children who met the guideline of ≥60 min per day, an effect size (of infant weight gain Z-score) for children who did not meet the guideline of ≥60 min per day, and the difference between these two estimates (i.e. the interaction term). Finally, fully adjusted regression models were develop using the binary MVPA variable and the categorical infant weight gain variable. A figure was produced to illustrate the extent to which the association of rapid infant weight gain with % fat in boys was modified by meeting the MVPA guidelines.
All procedures were performed in Stata 14 (StataCorp LP, College Station, TX, USA).
Results
As shown in Table 1, average infant weight Z-score gain between 0 and 3 years was higher in boys than in girls. Approximately 36% of boys demonstrated infant weight gain greater than 0.67 Z-scores (equivalent to one UK centile band) compared to 32% of girls. Boys, however, had lower BMI Z-score and % fat at 14 years than girls. At age 7 years, average time in MVPA was 69 min/day in boys and 55 min/day in girls, with 63% of boys meeting the guideline of ≥60 min/day in MVPA compared to just 36% of girls. Approximately 10% of children with very rapid infant weight gain developed obesity compared to 5% of children with rapid infant weight gain, but here was little difference across the infant weight gain groups in terms of meeting the MVPA guidelines (Supplementary Table 1).
Table 2 presents regression models for BMI Z-score and % fat at 14 years using continuous exposure and effect modifier variables (the unadjusted estimates are shown in Supplementary Table 2). As hypothesised, all the infant weight Z-score gain estimates were positive, all the MVPA estimates were negative, and all the interaction estimates were negative. There were also clear patterns in the strength of the estimates. For boys, in the baseline models adjusted for confounder set 1, each 10 min/day increase in MVPA attenuated the association of infant weight gain with BMI Z-score by −0.015 (−0.032, 0.003) units and with % fat by −0.118 (−0.233, −0.004) units. Additional adjustment for the SEP variables in confounder set 2 only attenuated the interaction estimates slightly, while additional adjustment for the parental BMI variables in confounder set 3 attenuated the interaction estimates more substantially. For example, the −0.118 (−0.233, −0.004) interaction estimate for % fat was reduced to −0.065 (−0.173, 0.043) in the fully adjusted model. For girls, there was no strong evidence of effect modification in any of the models, with the P values for all interaction terms being >0.6. Note also that the main effects of MVPA in girls were small, with all but one set of 95% confidence intervals crossing zero. As shown in Supplementary Tables 3 and 4, the observed patterns of effect modification were driven more by vigorous than MPA.
The pattern of results was the same in regression models replacing the continuous MVPA variable with the binary MVPA variable (Table 3; the unadjusted estimates are shown in Supplementary Table 5). Evidence of effect modification was present in boys but not girls and, among boys, was stronger for % fat than BMI. In model adjusted for all confounders, the association between infant weight Z-score gain and adolescent % fat was 1.883 (1.444, 2.322) among boys with MVPA < 60 min/day and 1.305 (0.920, 1.689) among boys with MVPA ≥ 60 min/day; the difference between these two estimates being −0.578 (−1.070, −0.087).
The results in Table 4 build on the fully adjusted models in Table 3 and examines a categorical infant weight gain variable. As expected, for both boys and girls, children with rapid or very rapid infant weight gain had higher BMI Z-score and % fat than children with normal weight gain. Again, however, it is only in boys that we found evidence that meeting the MVPA guidelines attenuated these associations. For example, % fat was estimated to be 2.981 (1.596, 4.367) units higher among boys who demonstrated rapid infant weight gain compared to boys who had normal weight gain, but meeting (as opposed to not meeting) the MVPA guideline reduced this difference by −2.259 (−3.989, −0.535) units. As shown in Fig. 1, this meant that boys with rapid weight gain who meet the MVPA guideline had comparable % fat at 14 years to boys whose infant weight gain was normal. Evidence of effect modification for very rapid infant weight gain was weaker.
Dots are esimated associations (with 95% CIs) from the model for boys in Table 4.
Discussion
The association of rapid infant weight gain with obesity has been extensively documented [4,5,6], but there is very little empirical evidence on what healthy lifestyle behaviours might help a child attenuate their increased obesity risk related to rapid infant weight gain. The key finding of the present paper is that higher childhood MVPA was consistently found to reduce the effect size of infant weight gain on adolescent adiposity outcomes in boys, but not girls. As shown in sensitivity analyses, this pattern of effect modification in boys was driven more by vigorous than MPA. This is in agreement with previous MCS research showing that VPA, as a percentage of MVPA, is important in preventing excessive weight gain in young people [38].
Our findings are in agreement with those of Bernhardsen et al. [17] and represent a necessary replication in a much larger sample (N = 4666 vs 445). The size of our sample allowed us to also investigate rapid infant weight gain and the modifying effect of meeting the MVPA guideline of ≥60 min/day [32]. In boys, ~75% (i.e. 2.259/2.981 from Table 4) of the excess % fat at 14 years associated with rapid infant weight gain was attenuated by meeting the MVPA guideline. This result is novel and supports a clear public health message regarding the importance of childhood physical activity levels. Evidence of effect modification for very rapid infant weight gain was weaker, suggesting that there is a level of infant weight gain above which meeting the MVPA guideline is not enough, by itself, to reduce obesity risk. Further investigation considering multiple lifestyle behaviours is warranted. The other study on this topic by Kolle et al. found no evidence that the association of infant weight gain between 0 and 2 years and fat mass index at 30 years was modified by MVPA levels at 30 years [16]. It would make sense that this lack of effect modification is because of the later outcome (and effect modifier) age, but it remains unclear why the authors observed modification for weight gain between 2 and 4 years.
The sex differences in our findings were consistent across all models. Bernhardsen et al. also found no evidence that MVPA modified the relationship of infant weight gain with % fat in girls [17]. The most likely explanation is that girls are simply not meeting levels of MVPA high enough to attenuate the adverse relationship of rapid infant weight gain with obesity risk. Indeed, childhood MVPA was not even associated with adolescent BMI in girls. In our sample, just 36% of girls meet the guideline of ≥60 min/day in MVPA at age 7 years compared to 63% of boys. Numerous other studies have similarly reported lower childhood MVPA levels in girls than boys [18, 20]. The decline in MVPA between childhood and adolescence is also greater in girls than boys [19], suggesting that sex differences in MVPA after age 7 years would become even more pronounced. Because greater age-related declines in MVPA have been reported in overweight/obese childhood compared to normal weight children [39], part of the faster decline in MVPA in girls than boys might be explained by their higher overweight/obesity prevalence [2].
Infant growth clearly reflects gains in fat and fat-free mass and infant weight gain is therefore positively associated with childhood lean mass as well as adiposity [40, 41]. The effect modification we observed was stronger for % fat than BMI, thereby providing evidence that MVPA is reducing the association of greater infant weight gain with higher adiposity not lean mass. The interaction estimates in boys attenuated most upon adjustment for parental BMI values. This is unsurprising given the known strong associations between parental and offspring BMI [7, 42], part of which are genetic and part of which are due to shared environmental factors [43, 44]. Shared environmental factors are likely to promote rapid infant weight gain and low MVPA, perhaps in addition to genetic effects related to parental BMI [45, 46]. We do not interpret our results as evidence that reductions in parental BMI would make child MVPA levels obsolete in reducing the obesity risk associated with rapid infant weight gain.
The main strengths of this paper include the large sample size and crucially the collection of device-measured physical activity in between the exposure (0–3 years) and outcomes (14 years). By properly considering the temporal order of events, our analysis is less likely to be biased by reverse causation, which is a real possibility given evidence in children that higher BMI can cause lower MVPA [47]. Having our MVPA effect modifier measured in between the exposure and outcomes could be problematic if it also acts as a mediator [48], but there is no strong evidence that infant weight gain affects childhood physical activity levels. In our sample, the correlation between infant weight Z-score gain and MVPA (min/day) was only ~0.01 in both sexes. We are similarly confident in adjusting for confounders measured during or after 0–3 years of age on the basis that there is no evidence they are “caused” by the exposure. Infant weight gain was computed as weight Z-score at 3 years minus weight Z-score at birth. It is worth noting that, with adjustment for Z-score at birth, the regression of an outcome on this change variable gives exactly the same results as using a “conditional change” or “unexplained residuals” exposure or indeed just using Z-score at 3 years as the exposure [49, 50]. Unfortunately, length is not available in the MCS before 3 years of age, which meant that we were not able to investigate infant weight-for-length change. This is a common limitation in the rapid infant weight gain literature [5] and means that infants who are just becoming large (in weight and length) are grouped with those who are most at risk of obesity, due to rapid weight but not length gain. We suspect that our effect sizes may have been larger if we were able to use infant weight-for-length gain instead of weight gain. While BMI and % fat are also available in the MCS at 11 years of age, we decided to focus on the 14-year data, because by this age most girls and boys will have reached at least Tanner stage 4 of pubertal development and many will have reached full maturity [51,52,53]. Our results are less likely to be confounded by pubertal timing than those in the Bernhardsen et al.’s study, in which the outcomes were at 9–12 years. Time in MVPA has a correlation with sedentary time that is not negligible (approximately −0.4 in our sample). It is possible that part of the effect modification we document is due to lower sedentary time rather than higher MVPA [54], but this is unlikely given the relatively weak (compared to MVPA) relationship of sedentary time with BMI in children [55,56,57]. A pragmatic approach was taken by using FIML to handle missing confounder data, but known patterns of non-response in the accelerometer data could have potentially biased the results [58]. The MCS was designed to be nationally representative at inception [23], but due to attrition and non-response our results might not be generalizable to the UK paediatric population or specific sub-groups (e.g. ethnic minorities).
In conclusion, we provide robust evidence that higher MVPA in childhood attenuates the positive association of infant weight gain with adolescent adiposity in boys, but not girls. Boys with rapid weight gain who meet the MVPA guideline of ≥60 min/day were found to have comparable BMI and % fat at 14 years to boys whose infant weight gain was normal. In boys known to have demonstrated rapid infant weight gain, increasing childhood MVPA levels, with the target of ≥60 min/day, might therefore go a long way to towards offsetting their increased risk for adolescent obesity.
Supplementary information is available at the International Journal of Obesity’s website.
Code availability
Available from the first author upon request.
References
NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–42.
Johnson W, Li L, Kuh D, Hardy R. How has the age-related process of overweight or obesity development changed over time? Co-ordinated analyses of individual participant data from five United Kingdom birth cohorts. PLoS Med. 2015;12:e1001828.
The Health and Social Care Information Centre. The National Child Measurement Programme: England, 2018/19 school year. London, UK: The Health and Social Care Information Centre; 2020.
Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320:967–71.
Zheng M, Lamb KE, Grimes C, Laws R, Bolton K, Ong KK, et al. Rapid weight gain during infancy and subsequent adiposity: a systematic review and meta-analysis of evidence. Obes Rev. 2018;19:321–32.
Weng SF, Redsell SA, Swift JA, Yang M, Glazebrook CP. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch Dis Child. 2012;97:1019–26.
Heslehurst N, Vieira R, Akhter Z, Bailey H, Slack E, Ngongalah L, et al. The association between maternal body mass index and child obesity: a systematic review and meta-analysis. PLoS Med. 2019;16:e1002817.
Yu ZB, Han SP, Zhu GZ, Zhu C, Wang XJ, Cao XG, et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes Rev. 2011;12:525–42.
Rotevatn TA, Melendez-Torres GJ, Overgaard C, Peven K, Hyldgaard Nilsen J, Boggild H, et al. Understanding rapid infant weight gain prevention: a systematic review of quantitative and qualitative evidence. Eur J Public Health. 2019.
Ejlerskov KT, Christensen LB, Ritz C, Jensen SM, Molgaard C, Michaelsen KF. The impact of early growth patterns and infant feeding on body composition at 3 years of age. Br J Nutr. 2015;114:316–27.
Karaolis-Danckert N, Buyken AE, Kulig M, Kroke A, Forster J, Kamin W, et al. How pre- and postnatal risk factors modify the effect of rapid weight gain in infancy and early childhood on subsequent fat mass development: results from the Multicenter Allergy Study 90. Am J Clin Nutr. 2008;87:1356–64.
Karaolis-Danckert N, Gunther AL, Kroke A, Hornberg C, Buyken AE. How early dietary factors modify the effect of rapid weight gain in infancy on subsequent body-composition development in term children whose birth weight was appropriate for gestational age. Am J Clin Nutr. 2007;86:1700–8.
Matthews EK, Wei J, Cunningham SA. Relationship between prenatal growth, postnatal growth and childhood obesity: a review. Eur J Clin Nutr. 2017;71:919–30.
Collings PJ, Brage S, Ridgway CL, Harvey NC, Godfrey KM, Inskip HM, et al. Physical activity intensity, sedentary time, and body composition in preschoolers. Am J Clin Nutr. 2013;97:1020–8.
Sardinha LB, Marques A, Minderico C, Ekelund U. Cross-sectional and prospective impact of reallocating sedentary time to physical activity on children’s body composition. Pediatr Obes. 2017;12:373–9.
Kolle E, Horta BL, Wells J, Brage S, Barros FC, Ekelund U, et al. Does objectively measured physical activity modify the association between early weight gain and fat mass in young adulthood? BMC Public Health. 2017;17:905.
Bernhardsen GP, Stensrud T, Nystad W, Dalene KE, Kolle E, Ekelund U. Early life risk factors for childhood obesity—does physical activity modify the associations? The MoBa cohort study. Scand J Med Sci Sports. 2019;29:1636–46.
Cooper AR, Goodman A, Page AS, Sherar LB, Esliger DW, van Sluijs EM, et al. Objectively measured physical activity and sedentary time in youth: the International Children’s Accelerometry Database (ICAD). Int J Behav Nutr Phys Act. 2015;12:113.
Farooq A, Martin A, Janssen X, Wilson MG, Gibson AM, Hughes A, et al. Longitudinal changes in moderate-to-vigorous-intensity physical activity in children and adolescents: a systematic review and meta-analysis. Obes Rev. 2020;21:e12953.
Steene-Johannessen J, Hansen BH, Dalene KE, Kolle E, Northstone K, Moller NC, et al. Variations in accelerometry measured physical activity and sedentary time across Europe—harmonized analyses of 47,497 children and adolescents. Int J Behav Nutr Phys Act. 2020;17:38.
Johnson W, Bann D, Hardy R. Infant weight gain and adolescent body mass index: comparison across two British cohorts born in 1946 and 2001. Arch Dis Child. 2018;103:974–80.
Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141–7.
Hansen K. Millennium Cohort Study: first, second, third, fourth, and fifth surveys: a guide to the datasets. 8th edition. London, UK: Centre for Longitudinal Studies at the UCL Institute of Education; 2014.
Tate AR, Dezateux C, Cole TJ, Davidson L. Millennium Cohort Study Child Health G. Factors affecting a mother’s recall of her baby’s birth weight. Int J Epidemiol. 2005;34:688–95.
World Health Organization. WHO Child Growth Standards. Geneva, Switzerland: World Health Organization; 2006.
Cole TJ. The development of growth references and growth charts. Ann Hum Biol. 2012;39:382–94.
Fitzsimons E. Millennium Cohort Study. Sixth survey 2015-2016. User guide. London, UK: Centre for Longitudinal Studies, UCL Institute of Education; 2017.
de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–7.
Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012;7:284–94.
Griffiths LJ, Cortina-Borja M, Sera F, Pouliou T, Geraci M, Rich C, et al. How active are our children? Findings from the Millennium Cohort Study. BMJ Open. 2013;3:e002893.
Pulsford RM, Cortina-Borja M, Rich C, Kinnafick FE, Dezateux C, Griffiths LJ. Actigraph accelerometer-defined boundaries for sedentary behaviour and physical activity intensities in 7 year old children. PLoS ONE. 2011;6:e21822.
U.S. Department of Health and Human Service. Physical activity guidelines for Americans. Washington, DC, USA: U.S. Department of Health and Human Service; 2008.
Poulsen G, Kurinczuk JJ, Wolke D, Boyle EM, Field D, Alfirevic Z, et al. Accurate reporting of expected delivery date by mothers 9 months after birth. J Clin Epidemiol. 2011;64:1444–50.
Bland R. Measuring “social class”: a discussion of the Registrar-General’s Classification. Sociology. 1979;13:283–91.
Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393.
Enders C. The performance of the full information maximum likelihood estimator in multiple regression models with missing data. Educ Psychol Meas. 2001;61:713–40.
Bross IDJ. How to use ridit analysis. Biometrics. 1958;14:18–38.
Hamer M, Stamatakis E. Relative proportion of vigorous physical activity, total volume of moderate to vigorous activity, and body mass index in youth: the Millennium Cohort Study. Int J Obes. 2018;42:1239–42.
Jago R, Salway R, Emm-Collison L, Sebire SJ, Thompson JL, Lawlor DA. Association of BMI category with change in children’s physical activity between ages 6 and 11 years: a longitudinal study. Int J Obes. 2020;44:104–13.
Admassu B, Ritz C, Wells JCK, Girma T, Andersen GS, Belachew T, et al. Accretion of fat-free mass rather than fat mass in infancy is positively associated with linear growth in childhood. J Nutr. 2018;148:607–15.
Chomtho S, Wells JC, Williams JE, Davies PS, Lucas A, Fewtrell MS. Infant growth and later body composition: evidence from the 4-component model. Am J Clin Nutr. 2008;87:1776–84.
Patro B, Liber A, Zalewski B, Poston L, Szajewska H, Koletzko B. Maternal and paternal body mass index and offspring obesity: a systematic review. Ann Nutr Metab. 2013;63:32–41.
Richmond RC, Timpson NJ, Felix JF, Palmer T, Gaillard R, McMahon G, et al. Using genetic variation to explore the causal effect of maternal pregnancy adiposity on future offspring adiposity: a Mendelian randomisation study. PLoS Med. 2017;14:e1002221.
Schnurr TM, Morgen CS, Borisevich D, Beaumont RN, Engelbrechtsen L, Angquist L, et al. The influence of transmitted and non-transmitted parental BMI-associated alleles on the risk of overweight in childhood. Sci Rep. 2020;10:4806.
den Hoed M, Brage S, Zhao JH, Westgate K, Nessa A, Ekelund U, et al. Heritability of objectively assessed daily physical activity and sedentary behavior. Am J Clin Nutr. 2013;98:1317–25.
Beardsall K, Ong KK, Murphy N, Ahmed ML, Zhao JH, Peeters MW, et al. Heritability of childhood weight gain from birth and risk markers for adult metabolic disease in prepubertal twins. J Clin Endocrinol Metab. 2009;94:3708–13.
Butte NF, Gregorich SE, Tschann JM, Penilla C, Pasch LA, De Groat CL, et al. Longitudinal effects of parental, child and neighborhood factors on moderate-vigorous physical activity and sedentary time in Latino children. Int J Behav Nutr Phys Act. 2014;11:108.
Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–95.
Johnson W. Analytical strategies in human growth research. Am J Hum Biol. 2015;27:69–83.
Keijzer-Veen MG, Euser AM, van Montfoort N, Dekker FW, Vandenbroucke JP, Van Houwelingen HC. A regression model with unexplained residuals was preferred in the analysis of the fetal origins of adult diseases hypothesis. J Clin Epidemiol. 2005;58:1320–4.
Brix N, Ernst A, Lauridsen LLB, Parner E, Stovring H, Olsen J, et al. Timing of puberty in boys and girls: a population-based study. Paediatr Perinat Epidemiol. 2019;33:70–8.
Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303.
Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23.
Arnold KF, Berrie L, Tennant PWG, Gilthorpe MS. A causal inference perspective on the analysis of compositional data. Int J Epidemiol. 2020.
Froberg A, Raustorp A. Objectively measured sedentary behaviour and cardio-metabolic risk in youth: a review of evidence. Eur J Pediatr. 2014;173:845–60.
Verswijveren S, Lamb KE, Bell LA, Timperio A, Salmon J, Ridgers ND. Associations between activity patterns and cardio-metabolic risk factors in children and adolescents: a systematic review. PLoS ONE. 2018;13:e0201947.
Wijndaele K, White T, Andersen LB, Bugge A, Kolle E, Northstone K, et al. Substituting prolonged sedentary time and cardiovascular risk in children and youth: a meta-analysis within the International Children’s Accelerometry Database (ICAD). Int J Behav Nutr Phys Act. 2019;16:96.
Rich C, Cortina-Borja M, Dezateux C, Geraci M, Sera F, Calderwood L, et al. Predictors of non-response in a UK-wide cohort study of children’s accelerometer-determined physical activity using postal methods. BMJ Open. 2013;3:e002290.
Funding
This work was funded by the UK Medical Research Council (WJ New Investigator Research Grant: MR/P023347/1). WJ acknowledges support from the National Institute for Health Research (NIHR) Leicester Biomedical Research Centre, which is a partnership between University Hospitals of Leicester NHS Trust, Loughborough University, and the University of Leicester.
Author information
Authors and Affiliations
Contributions
WJ conceptualized the study, carried out the analyses, and drafted the initial manuscript. All authors made substantial contributions to the interpretation of the data, revised the manuscript critically for important intellectual content, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Johnson, W., Norris, T., De Freitas, R. et al. Is the positive relationship of infant weight gain with adolescent adiposity attenuated by moderate-to-vigorous physical activity in childhood? Evidence from the Millennium Cohort Study. Int J Obes 45, 84–94 (2021). https://doi.org/10.1038/s41366-020-00656-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-020-00656-7
- Springer Nature Limited