Abstract
Background/objectives
Short-term breastfeeding from mothers with gestational diabetes (GDM) may programme metabolism and increase offspring diabetes risk. We examined the association of in utero GDM exposure with adiposity from infancy to adolescence, and whether any association was modified by breastfeeding during early infancy.
Methods
In the prospective Chinese birth cohort “Children of 1997” (n = 7342, 88% follow-up rate), generalised estimate equations with multiple imputation were used to assess associations of in utero GDM exposure with age- and sex-specific body mass index (BMI) z-score during infancy (3 and 9 months), childhood (2– < 8 years) and adolescence (8–16 years), adjusted for sex, parity, maternal age, birth place, preeclampisa, smoking, and family socio-economic position. We also tested whether the associations differed by mode of infant feeding (always formula-fed, mixed, always breastfed) during the first three months of life.
Results
In utero GDM exposure (7.5%) was associated with a lower BMI z-score during infancy (−0.13, 95% confidence interval (CI) −0.22, −0.05) but higher BMI z-scores during childhood (0.14, 95% CI 0.03, 0.25) and adolescence (0.25 95% CI 0.11, 0.38). Breastfeeding for the first three months did not modify the association of in utero GDM status with subsequent BMI (all p values for interaction >0.4).
Conclusions
In utero GDM exposure was associated with greater adiposity during childhood and adolescence. Breastfeeding in early infancy from mothers with GDM was not associated with greater adiposity in children and thus should still be encouraged.
Similar content being viewed by others
Introduction
Children born to mothers with gestational diabetes (GDM) are at greater risk of obesity and abnormal glucose tolerance [1, 2], emphasising the importance of infant and child care in this vulnerable group. The influence of breastfeeding by mothers with GDM on long-term metabolic health of their offspring is of concern because breast milk from these mothers, particularly those with glucose intolerance in the early postpartum period, may contain different levels of peptide hormones, such as ghrelin, that may affect energy metabolism [3]. Rodent studies suggest that milk from mothers with GDM might detrimentally affect food intake, adiposity, and metabolism [4]. Observational studies concerning the relation of breastfeeding by mothers with GDM with their offspring’s metabolic health are scarce and findings are mixed. A small study in Germany reported that the volume of breast milk ingested from diabetic mothers during the 1st week postpartum was positively associated with overweight at 2 years old [5]. The extended analysis from the same study reported that the volume of breast milk ingested from diabetic mothers during the 1st week postpartum nullified the associations of dose and duration of breastfeeding from diabetic mothers during late neonatal period (2nd to 4th week postpartum) with relative body weight at 2 years, indicating the first week of life may be more important in nutritional programming [6]. In contrast, other studies suggest benefits of breastfeeding in the offspring of mothers with GDM. A small study showed that in utero exposure to GDM was associated with childhood adiposity [7] and faster childhood growth trajectories [8] among children breastfed for <6 months but not in those breastfed for longer. The Growing Up Today Study, composing of children of women participating in the US Nurses’ Health Study II, reported that exclusive breastfeeding compared to formula feeding was associated with a lower risk of obesity among children and adolescents with in utero exposure to GDM [9]. However, in situations where higher socio-economic position is associated with both health and breastfeeding, benefits of breastfeeding may be overestimated [10], while adverse consequences of breastfeeding, regardless of mother’s GDM status, are likely to be underestimated. As such, the long-term impact of breastfeeding by mothers with GDM on their children’s long-term metabolic health is unclear.
Here, we examined the association of in utero GDM exposure with adiposity from infancy to adolescence, and whether the association differed by mode of feeding in early infancy in the large, population representative Hong Kong birth cohort, “Children of 1997” [11], which has the advantage of little association of breastfeeding with higher socio-economic position [12], minimising the socio-economic confounding in the observed health benefits of breastfeeding. We focused on the impact of a short duration of breastfeeding because breastfeeding in 1990s was commonly short and it has been suggested that being breastfed by mothers with GDM during the early postpartum period may be most detrimental to metabolic health [13]. We tested the hypothesis that in utero exposure to GDM is associated with (1) adverse metabolic health in adolescence, proxied by greater body mass index (BMI), (2) particularly among those who were breastfed in the early days of life.
Subjects and methods
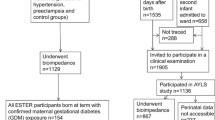
The “Children of 1997” birth cohort
Hong Kong’s “Children of 1997” birth cohort [11] is a population representative Chinese birth cohort (n = 8327) that covered 88% of all births from 1 April 1997 to 31 May 1997. The study was initially established to investigate the impact of second hand smoke exposure on infant health [14]. Families were recruited at their first postnatal visit to the 49 Maternal and Child Health Centres in Hong Kong. Baseline and feeding characteristics were obtained using a self-administered questionnaire at recruitment, 3 months, 9 months, and 18 months. Since 2005, we have carried out passive and active follow-up, by means of data linkage and surveys, to obtain health related information, including routine measurements of height and weight from the Maternal and Child Health Centres and the Student Health Service. In Hong Kong, Maternal and Child Health Centres provide free postnatal check-ups to all Hong Kong born children until 5 years. The Student Health Service provides annual health check-ups for students at all primary and secondary schools.
The study was reviewed by and received approval from the University of Hong Kong, Hospital Authority Hong Kong West Cluster Joint Institutional Review Board and the Joint Chinese University of Hong Kong—New Territories East Cluster Clinical Research Ethics Committee. Informed consent was obtained from the parents/guardians whose child participated in the birth cohort “Children of 1997”.
Exposure—in utero exposure to GDM
In utero exposure to GDM was classified as “present” or “absent” based on self-report of GDM in the relevant pregnancy by the mothers. During 1996–97, GDM was diagnosed using the 75 g oral glucose tolerance test according to the 1999 World Health Organization criteria, i.e., fasting plasma glucose of >7.0 mmol/L and 2-hour plasma glucose level of 11.1 mmol/L or above. We used the response from the earliest survey if mothers answered this question more than once. We carried out a validation study among mothers of birth cohort children delivered in the largest maternity hospital in Hong Kong (n = 820, 9.8% of all) to check the self-reported GDM status in the relevant pregnancy against clinically diagnosed GDM extracted from medical records. Although the response rate was hindered by the surge in phone spams in the city and mothers were reluctant to provide their identifier number for the validation study, we successfully obtained consent from and retrieved medical records for 94 mothers and found 97% reported their GDM status correctly and the few invalid self-reports concern over-reporting.
Outcome—Adiposity from infancy to adolescence
Adiposity were proxied by age- (in days) and sex-specific z-scores for BMI relative to the 2006 World Health Organization (WHO) growth standards [15] for 0 to <5 years and the 2007 WHO growth reference [16] for 5–16 years, calculated by interpolating the WHO references onto a daily scale using the akima package in R. We assessed the association of in utero exposure to GDM with BMI z-score in three life stages separately, infancy (3 and 9 months), childhood (2–<8 years) and adolescence (8–16 years), based on the Infancy, Childhood, and Puberty growth model [17], which divided human growth into three phases that reflect the endocrine control mechanisms of the growth process, which strongly related to BMI. Weight and length/height were retrieved from Maternal and Child Health Centres and the Student Health Service using data linkage [18,19,20].
Effect modifier—mode of infant feeding
Feeding in the first three months (0–3 months) of life was considered as always formula-fed, (i.e., never breastfed), mixed feeding (i.e, fed both breast milk and formula milk) or always breastfed (i.e., exclusively breastfed), as previously described [18,19,20] .
Statistical analyses
We used the χ2-test and analysis of variance to assess the difference in characteristics by in utero GDM exposure and mode of infant feeding. We used generalised estimating equations to assess the adjusted associations of in utero GDM exposure with BMI z-scores during infancy (3 and 9 months), childhood (2–<8 years) and adolescence (8–16 years). Our preliminary analysis suggested that similar estimates of the associations of in utero GDM with BMI z-score were obtained when using “exchangeable” (i.e., assuming correlation between measurements were consistent) or “unstructured” correlation structure of BMI z-score (Supplementary Table A1), so we used an exchangeable correlation structure, which used fewer degrees of freedom. We tested whether the associations of in utero GDM exposure with BMI varied by mode of infant feeding from the significance of interaction terms. We repeated the analysis with height z-score, relative to the 2006 WHO growth standards [15] and 2007 WHO growth reference [16], for completeness. We adjusted for sex, parity, age at measurements and potential confounders, including maternal characteristics (age and birth place) and family socio-economic position (proxied by highest parental education) (Model 1). We carried out sensitivity analyses to check if there was any change in the associations after adjusting for pregnancy conditions in the index pregnancy, which did not have clear causal relation with GDM (presence of preeclampisa and maternal smoking) (Model 2) as well as mother’s current BMI and history of type 2 diabetes as the proxy for genetic predispositions to overweight among the cohort participants (Model 3).
We used multiple imputation to predict missing confounders based on a flexible additive regression model with predictive mean matching [21] incorporating data on exposure, covariates and outcomes [22]. Presence of gestational diabetes and preeclampsia in the index pregnancy was imputed for 17%, mother’s current BMI and history of type 2 diabetes for 43%, and other covariates for <5%. We summarised the results from 10 imputed datasets into single estimates with confidence intervals (CI) adjusted for missing data uncertainty [21]. We also performed complete case analyses without imputation. Statistical analyses were performed using Stata version 13 (Stata Corp, College station, Tex) and R version 3.1.2 (R Development Core Team, Vienna, Austria).
Results
Participant characteristics
Of the original 8327 “Children of 1997” participants, as of 30th April 2012, 8298 had not withdrawn (n = 26 withdrawn). Over 80% of the participants had at least one BMI measurement during infancy, childhood or adolescence. Breastfeeding status at 0–3 months was available for 98% of participants. In total, 7297 (88%) were included in the analyses. Those included in the analyses had similar baseline characteristics, including the proportion with in utero GDM exposure, birth characteristics and socio-demographic characteristics as the whole birth cohort (Supplementary Table A2). The Cohen effect sizes were small (<0.2).
Compared with participants without in utero GDM exposure, those with in utero GDM exposure (7.5%) had similar birth weight but shorter gestational age (with more late preterm and early term and fewer post-term births) and thus slightly lower birth weight for gestational age. Their mothers were on average older and more likely to be born in Hong Kong (than the rest of China). (Table 1) Their parents were more likely to have higher education and were less likely to live in public housing. Other pregnancy/birth characteristics did not differ by in utero GDM exposure, except a slightly higher rate of preeclampsia and a lower rate of smoking during pregnancy among mothers with GDM. Mothers with GDM during the index pregnancy had greater current BMI (mean: 23.6 kg/m2) than other mothers (22.7 kg/m2) and were more likely to have a history of type 2 diabetes reported >10 years later.
Breastfeeding in the early postnatal period
In the first 3 months of life, about half (57%) of the cohort participants were only fed on infant formula, 6.3% were always breastfed and 37% had mixed feeding, including those who were partially breastfed for any length of time or exclusively breastfed for < 3 months.
Breastfeeding at 0–3 months was unrelated to in utero GDM exposure (Table 2). Those who were delivered by caesarean section or exposed to in utero maternal smoking were less likely to be always breastfed. Mothers who sustained exclusive breastfeeding at 3 months were more likely to be migrants from mainland China who had lower education, as such always being breastfed at 0–3 months was not associated with higher parental education. BMI from infancy to adolescence did not differ by mode of infant feeding, except at 3 months old when those who were always formula-fed had lower BMI (data not shown).
Associations of in utero GDM exposure with adiposity
In utero GDM exposure was negatively associated with BMI z-score during infancy (−0.13, 95% CI −0.22, −0.05), but was positively associated with BMI z-score during childhood (0.14, 95% CI 0.03, 0.25) and adolescence (0.25, 95% CI 0.11, 0.38) adjusted for sex, parity, maternal age at delivery, maternal smoking during pregnancy, highest parental education, and mother’s place of birth (Model 1, Table 3). The positive association was stronger in adolescence. Further adjustment for mother’s current BMI and history of type 2 diabetes (Model 2) or for birth weight, gestational age, and pubertal timing (Model 3) attenuated these positive associations, but the association of in utero GDM exposure with greater BMI in adolescence remained. None of the p values for interaction between in utero GDM exposure and mode of infant feeding was significant (all >0.4, Table 3). However the associations of in utero GDM exposure with greater BMI in childhood and adolescence were less marked among the small number of children who were always breastfed at 0–3 months (n = 31, Table 4).
We obtained similar results if we limited the analysis to participants who had BMI at all three stages (76%) and complete case analyses (Supplementary Table A3). In utero exposure to GDM was unrelated to height z-score from infancy to adolescence (data not shown).
Discussion
Children with in utero GDM exposure had lower BMI during infancy but higher BMI in childhood and particularly in adolescence than others, indicating that GDM related adiposity emerges in childhood, and manifests in adolescence. The stratified analysis indicated that exclusive breastfeeding for 3 months was not associated with a stronger relation of in utero GDM exposure with higher BMI, but rather might be associated with a weaker association of in utero GDM exposure with higher BMI. However, this finding was based on a small number of children who were exclusively breastfed by GDM mothers, and thus requires confirmation in larger studies.
Children born to mothers with GDM have a higher risk of becoming overweight and developing glucose intolerance and type 2 diabetes in adulthood [23, 24]. A small prospective cohort study from Hong Kong however reported a null association of in utero GDM exposure with BMI in childhood and adolescence, potentially owing to a small sample size (<200) [2, 25]. Our finding is consistent with observations in a British birth cohort [26] and other observational studies [27] that children with in utero GDM exposure are more prone to adiposity before adulthood. The associations of in utero GDM exposure with later adiposity were attenuated by further adjustment for mother’s current BMI and history of type 2 diabetes and thus may be partly due to shared genetic predisposition, shared environment or common factors resulting from both in utero exposure to GDM and mother’s history of diabetes. However the association in adolescence was strong and independent of socio-economic position, mother’s current BMI and history of type 2 diabetes. Recent studies have provided potential underlying molecular mechanisms by which maternal hyperglycaemia may exert metabolic programming effects on the developing fetus, including those involving DNA methylation of genes related to metabolism [28] and leptin [29, 30], suggesting a plausible causal role played by in utero GDM exposure on subsequent adiposity.
Exclusive breastfeeding for three months did not clearly affect the association of in utero GDM exposure with BMI from infancy to adolescence. Although the interaction testing was underpowered due to the small number of children exclusively breastfed by GDM mothers, the estimates was not consistent with any adverse consequences of exclusively breastfeeding by GDM mothers. As such, adverse effects on adiposity of being exclusively breastfed during early infancy by a mother with GDM seem unlikely, and benefits, if any, more likely. The health consequences of being breastfed from a mother with GDM have been controversial because breast milk from mothers with GDM, particularly in the early postpartum period, may have different composition, in terms of insulin, glucose, ghrelin [3], and preptin [31], which may affect metabolism of the newborn. The limited evidence of adverse impacts of being breastfed from by a mother with GDM mainly focused on infancy. A small study in Germany reported greater risks of overweight at 2 years old among infants fed with breast milk for a week from mothers with diabetes compared to those who fed with banked breast milk from mothers without diabetes [5, 6]. However, this study included mothers with type 1 diabetes and thus was not directly comparable to ours. A birth cohort from Singapore showed predominant (including exclusive) breastfeeding of 4 months or longer was associated with greater gain in weight and BMI from birth to 6 months only among infants of GDM mothers [32]. We instead observed a greater gestational age specific birth weight z-score but a lower BMI (but not shorter spine length) during infancy among children with in utero GDM exposure, implying their slower weight growth during infancy, regardless of mode of infant feeding. Our observation is consistent with the “catch-down” in weight reported in infants of mothers with GDM in Caucasians and Pima Indians [33, 34]. This Singaporean birth cohort has also reported an association between maternal glycaemia and “catch-down” in infant weight in obese mothers, however the p value for interaction by breastfeeding was not reported [35]. We cannot rule out that more severe GDM mothers may be less likely to breastfeed [36], such that the adverse impact of breastfeeding from a GDM mother may have been underestimated. Different degrees of postpartum glycaemia may contribute to varying impacts of being breastfed by GDM mothers. Further prospective studies with detailed maternal conditions after delivery are required to clarify the consequences of breastfeeding by mothers with GDM who suffer from postpartum hyperglycaemia.
It has been suggested that benefits of breastfeeding protecting against obesity [37] and type 2 diabetes [38, 39], and may attenuate the risks of obesity and glucose intolerance associated with in utero GDM exposure, as shown in a few observational studies [7, 9, 40, 41]. Our study did not find a significant difference in the association of mother’s GDM status with adiposity by mode of feeding, potentially owing to the small number of children who were exclusively breastfed by GDM mothers. However stratified analysis showed a lower BMI among children of GDM mothers who were exclusively breastfed in early infancy. We cannot rule out the different associations by strata is a chance finding. But our study was strengthened by a lack of association exclusively breastfeeding at 0–3 months with higher socio-economic position, such that socio-economic confounding by better health outcomes among breastfed children, which has been observed in long-term developed settings [42] was minimised. A lack of benefits of breastfeeding by GDM mothers is consistent with the null association of breastfeeding with adiposity previously observed in our cohort [12] and cohorts in other settings with little social patterning of breastfeeding [10] or of breastfeeding promotion in a randomised controlled trial [43]. On the other hand, observational studies reporting health benefits of breastfeeding for children of GDM mothers mainly referred to longer breastfeeding duration [7, 9, 40, 41]. Breastfeeding duration in 1990s in Hong Kong was generally short and rate of exclusively breastfeeding was low. Our setting was underpowered to test potential benefits of breastfeeding from GDM mothers if longer duration of breastfeeding is more beneficial than short-term breastfeeding among infants exposed to in utero GDM [7].
Public health implications
Children with in utero exposure to GDM had lower BMI during infancy but higher BMI in childhood and particularly in adolescence. With compensatory mechanisms between consecutive growth phases, catch-down growth in infancy may promote more rapid childhood growth [17], a growth trajectory that has been associated with a higher risk of type 2 diabetes [44]. Although shared genetic and living environments may contribute to some of the link between in utero exposure to GDM and subsequent adiposity, our findings support the importance of a life course approach in combating the obesity and type 2 diabetes epidemics [45], such as the prevention and control of GDM as well as of early obesity intervention targeting children exposed to in utero GDM. Our findings also suggest that short exclusive breastfeeding by GDM mothers did not contribute to greater adiposity in children overall and thus does not discourage breastfeeding in GDM mothers, especially those without postpartum hyperglycaemia, although confirmation from intervention studies and randomised controlled trials is warranted.
Limitations
Some limitations of the study require consideration. GDM status was self-reported. However a validation study of 94 mothers suggested good agreement between self-reported GDM status and clinically assessed GDM status. The majority (97%) reported their GDM status correctly and the very few invalid reports were over-reporting, making the observed adverse consequences of maternal GDM conservative. We did not have information on treatment of GDM and treatment may improve pregnancy glucose control. However this will only make our association of in utero exposure to GDM with later adiposity more conservative. Attending the Maternal and Child Health Centre and the Student Health Service is voluntary. However 80–90% of the whole cohort was included in the main analyses and they were not very different from the whole cohort, implicating little selection bias. BMI does not distinguish fat mass from lean mass and may not be positively linked with adiposity and metabolic risk at pre-pubertal ages [46] and high BMI may not necessary reflect great adiposity among individuals with greater percentage of muscle mass. Weight and length/height data were obtained from data linkage and therefore we were unable to assess their validity and reliability. However, a standard protocol and the same measuring tools were used in all health assessments. Any measurement error is likely to be non-differential and bias towards the null. Finally, breastfeeding duration was short in this cohort, such that our impact of breastfeeding cannot directly be extended to those with long breastfeeding duration without verification from further studies.
Conclusions
In utero GDM exposure was associated with greater adiposity in childhood and adolescence. Prevention and control of GDM and long-term follow-up of children with in utero GDM exposure might provide early intervention opportunities to combat the obesity and diabetes epidemics, but would require confirmation in further intervention studies. Further studies with larger numbers of children exclusively breastfed by GDM mothers and longer breastfeeding duration are also warranted to confirm that breastfeeding by mothers with GDM does not contribute to greater adiposity in their children, but might contribute to less adiposity.
Change history
02 May 2019
In the original version of this article, the Publisher incorrectly listed the affiliation of the author, G.M. Leung. The correct affiliation for this author should be: School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China.
References
Durnwald C, Landon M. Fetal links to chronic disease: the role of gestational diabetes mellitus. Am J Perinatol. 2012;30:343–346.
Tam WH, Ma RC, Yang X, Ko GT, Tong PC, Cockram CS, et al. Glucose intolerance and cardiometabolic risk in children exposed to maternal gestational diabetes mellitus in utero. Pediatrics. 2008;122:1229–1234.
Aydin S. The presence of the peptides apelin, ghrelin and nesfatin-1 in the human breast milk, and the lowering of their levels in patients with gestational diabetes mellitus. Peptides. 2010;31:2236–2240.
Bouret SG, Simerly RB. Developmental programming of hypothalamic feeding circuits. Clin Genet. 2006;70:295–301.
Plagemann A, Harder T, Franke K, Kohlhoff R. Long-term impact of neonatal breast-feeding on body weight and glucose tolerance in children of diabetic mothers. Diabetes Care. 2002;25:16–22.
Rodekamp E, Harder T, Kohlhoff R, Franke K, Dudenhausen JW, Plagemann A. Long-term impact of breast-feeding on body weight and glucose tolerance in children of diabetic mothers: role of the late neonatal period and early infancy. Diabetes Care. 2005;28:1457–1462.
Crume TL, Ogden L, Maligie M, Sheffield S, Bischoff KJ, McDuffie R, et al. Long-term impact of neonatal breastfeeding on childhood adiposity and fat distribution among children exposed to diabetes in utero Diabetes Care. 2011;34:641–645.
Crume TL, Ogden LG, Mayer-Davis EJ, Hamman RF, Norris JM, Bischoff KJ, et al. The impact of neonatal breast-feeding on growth trajectories of youth exposed and unexposed to diabetes in utero: the EPOCH Study. Int J Obes (Lond). 2012;36:529–534.
Mayer-Davis EJ, Rifas-Shiman SL, Zhou L, Hu FB, Colditz GA, Gillman MW. Breast-feeding and risk for childhood obesity: does maternal diabetes or obesity status matter? Diabetes Care. 2006;29:2231–2237.
Brion MJ, Lawlor DA, Matijasevich A, Horta B, Anselmi L, Araujo CL, et al. What are the causal effects of breastfeeding on IQ, obesity and blood pressure? Evidence from comparing high-income with middle-income cohorts. Int J Epidemiol. 2011;40:670–680.
Schooling CM, Hui LL, Ho LM, Lam TH, Leung GM. Cohort Profile: “Children of 1997”: a Hong Kong Chinese birth cohort. Int J Epidemiol. 2012;41:611–620.
Kwok MK, Schooling CM, Lam TH, Leung GM. Does breastfeeding protect against childhood overweight? Hong Kong’s ‘Children of 1997’ birth cohort. Int J Epidemiol. 2010;39:297–305.
Plagemann A, Harder T. Fuel-mediated teratogenesis and breastfeeding. Diabetes Care. 2011;34:779–781.
Lam TH, Leung GM, Ho LM. The effects of environmental tobacco smoke on health services utilization in the first eighteen months of life. Pediatrics. 2001;107:E91.
WHO. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85.
de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;89:660–667.
Karlberg J. A biologically-oriented mathematical model (ICP) for human growth. Acta Paediatr Scand Suppl. 1989;350:70–94.
Kwok MK, Leung GM, Lam TH, Schooling CM. Breastfeeding, childhood milk consumption, and onset of puberty. Pediatrics. 2012;130:e631–e639.
Kwok MK, Leung GM, Schooling CM. Breast feeding and early adolescent behaviour, self-esteem and depression: Hong Kong’s ‘Children of 1997’ birth cohort. Arch Dis Child. 2013;98:887–894.
Kwok MK, Leung GM, Schooling CM. Breastfeeding and adolescent blood pressure: evidence from Hong Kong’s “Children of 1997” Birth Cohort. Am J Epidemiol. 2013;178:928–936.
Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15.
Moons KG, Donders RA, Stijnen T, Harrell FE Jr. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59:1092–101.
Moore TR. Fetal exposure to gestational diabetes contributes to subsequent adult metabolic syndrome. Am J Obstet Gynecol. 2010;202:643–649.
Garcia-Vargas L, Addison SS, Nistala R, Kurukulasuriya D, Sowers JR. Gestational diabetes and the offspring: implications in the development of the cardiorenal metabolic syndrome in offspring. Cardiorenal Med. 2012;2:134–142.
Tam WH, Ma RC, Yang X, Li AM, Ko GT, Kong AP, et al. Glucose intolerance and cardiometabolic risk in adolescents exposed to maternal gestational diabetes: a 15-year follow-up study. Diabetes Care. 2010;33:1382–1384.
Lawlor DA, Fraser A, Lindsay RS, Ness A, Dabelea D, Catalano P, et al. Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia. 2010;53:89–97.
Philipps LH, Santhakumaran S, Gale C, Prior E, Logan KM, Hyde MJ, et al. The diabetic pregnancy and offspring BMI in childhood: a systematic review and meta-analysis. Diabetologia. 2011;54:1957–1966.
Houde AA, Ruchat SM, Allard C, Baillargeon JP, St-Pierre J, Perron P, et al. LRP1B, BRD2 and CACNA1D: new candidate genes in fetal metabolic programming of newborns exposed to maternal hyperglycemia. Epigenomics. 2015;7:1111–1122.
Allard C, Desgagne V, Patenaude J, Lacroix M, Guillemette L, Battista MC, et al. Mendelian randomization supports causality between maternal hyperglycemia and epigenetic regulation of leptin gene in newborns. Epigenetics. 2015;10:342–351.
Cote S, Gagne-Ouellet V, Guay SP, Allard C, Houde AA, Perron P, et al. PPARGC1alpha gene DNA methylation variations in human placenta mediate the link between maternal hyperglycemia and leptin levels in newborns. Clin Epigenetics. 2016;8:72.
Aydin S, Celik O, Gurates B, Sahin I, Ulas M, Yilmaz M, et al. Concentrations of preptin, salusins and hepcidins in plasma and milk of lactating women with or without gestational diabetes mellitus. Peptides. 2013;49:123–310.
Aris IM, Soh SE, Tint MT, Saw SM, Rajadurai VS, Godfrey KM, et al. Associations of infant milk feed type on early postnatal growth of offspring exposed and unexposed to gestational diabetes in utero. Eur J Nutr. 2015;56:55–64.
Touger L, Looker HC, Krakoff J, Lindsay RS, Cook V, Knowler WC. Early growth in offspring of diabetic mothers. Diabetes Care. 2005;28:585–589.
Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. The Northwestern University Diabetes in Pregnancy Center. Diabetes Care. 1998;21:B142–B149.
Aris IM, Soh SE, Tint MT, Saw SM, Rajadurai VS, Godfrey KM, et al. Associations of gestational glycemia and prepregnancy adiposity with offspring growth and adiposity in an Asian population. Am J Clin Nutr. 2015;102:1104–1112.
Fenger-Gron J, Fenger-Gron M, Blunck CH, Schonemann-Rigel H, Wielandt HB. Low breastfeeding rates and body mass index in Danish children of women with gestational diabetes mellitus. Int Breastfeed J. 2015;10:26.
Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115:1367–1377.
Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr. 2006;84:1043–1054.
Horta BL, Loret de MC, Victora CG. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: a systematic review and meta-analysis. Acta Paediatr Suppl. 2015;104:30–37.
Pettitt DJ, Forman MR, Hanson RL, Knowler WC, Bennett PH. Breastfeeding and incidence of non-insulin-dependent diabetes mellitus in Pima Indians. Lancet. 1997;350:166–168.
Schaefer-Graf UM, Hartmann R, Pawliczak J, Passow D, bou-Dakn M, Vetter K, et al. Association of breast-feeding and early childhood overweight in children from mothers with gestational diabetes mellitus. Diabetes Care. 2006;29:1105–1107.
Brion MJ. Commentary: assessing the impact of breastfeeding on child health: where conventional methods alone fall short for reliably establishing causal inference. Int J Epidemiol. 2010;39:306–307.
Martin RM, Patel R, Kramer MS, Guthrie L, Vilchuck K, Bogdanovich N, et al. Effects of promoting longer-term and exclusive breastfeeding on adiposity and insulin-like growth factor-I at age 11.5 years: a randomized trial. JAMA. 2013;309:1005–1013.
Eriksson JG, Forsen TJ, Osmond C, Barker DJ. Pathways of infant and childhood growth that lead to type 2 diabetes. Diabetes Care. 2003;26:3006–3010.
World Health Organisation. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. 2013. Geneva, Switzerland.
Bouhours-Nouet N, Dufresne S, de Casson FB, Mathieu E, Douay O, Gatelais F, et al. High birth weight and early postnatal weight gain protect obese children and adolescents from truncal adiposity and insulin resistance: metabolically healthy but obese subjects? Diabetes Care. 2008;31:1031–1036.
Acknowledgements
We thank colleagues at the Student Health Service and Family Health Service of the Department of Health for their assistance and collaboration. We also thank the late Dr. Connie O for coordinating the project and all the fieldwork for the initial study in 1997–1998. This work is a sub-study of the “Children of 1997” birth cohort which was initially supported by the Health Care and Promotion Fund, Health and Welfare Bureau, Government of the Hong Kong SAR [HCPF Grant # 216106] and re-established in 2005 with support from the Health and Health Services Research Fund [HHSRF Grant # 03040771], and the University Research Committee Strategic Research Theme (SRT) of Public Health, The University of Hong Kong. This sub-study builds on information added to the birth cohort by RFCID grant # 04050172 and HHSRF grant # 08090761, and was funded by the Health and Health Services Research Fund [HHSRF Grant # 12132731], Government of the Hong Kong SAR.
Author contributions
Drs Hui and Schooling developed the study conception, directed the study’s analytic strategy and wrote the manuscript. Drs Li, Nelson, Lee, and Leung contributed to the interpretation of the data and critically revised the paper. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Hui, L.L., Li, A.M., Nelson, E.A.S. et al. In utero exposure to gestational diabetes and adiposity: does breastfeeding make a difference?. Int J Obes 42, 1317–1325 (2018). https://doi.org/10.1038/s41366-018-0077-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0077-2
- Springer Nature Limited
This article is cited by
-
Breastfeeding may benefit cardiometabolic health of children exposed to increased gestational glycemia in utero
European Journal of Nutrition (2022)
-
Breastfeeding and growth trajectory from birth to 5 years among children exposed and unexposed to gestational diabetes mellitus in utero
Journal of Perinatology (2021)