Abstract
Background/objectives
Plasma steroid hormone levels vary between men and women, but their associations with BMI and adiposity are controversial. Furthermore, little is known about the role of exercise programs on the relationship between steroid hormones and adiposity. This report evaluates these relationships for plasma levels of adrenal, gonadal, and conjugated steroids with body composition and fat distribution in sedentary men and women, aged 17–65 years, and their responses to an exercise program.
Subjects/methods
In the sedentary state, 270 men (29% Blacks) and 304 women (34% Blacks) from the HERITAGE Family Study were available. Among them, 242 men and 238 women completed a 20-week fully standardized exercise program. Fourteen steroid hormones and SHBG concentrations were assayed in a fasted state and were compared for their associations with adiposity in men and women and in response to the exercise program. Covariates adjusted for in partial correlation analysis were age, ancestry, menopause status (women), and oral contraceptives/hormone replacement treatment status (women) at baseline, as well as baseline value of the trait for the training response. Differences among normal weight, overweight, and obese subjects were also considered. Statistical significance was set at P < 0.0001.
Results
Baseline levels of dihydrotesterone (DHT), 17 hydroxy progesterone (OHPROG), sex hormone-binding globulin (SHBG), and testosterone (TESTO) were negatively associated with fat mass and abdominal fat (P < 0.0001) in men and for SHBG in women (P < 0.0001). TESTO was not correlated with fat-free mass in men or women, but was significantly associated with % fat-free mass in men. No association was detected between baseline steroid hormone levels and changes in adiposity traits in response to 20 weeks of exercise.
Conclusion
In men, low DHT, OHPROG, SHBG, and TESTO were associated with higher adiposity and abdominal and visceral fat. A similar adiposity profile was observed in women with low SHBG.
Similar content being viewed by others
Introduction
There is evidence that blood levels of steroid hormones and their binding globulins influence body composition and fat distribution, but the findings are controversial, and the magnitude of the associations is generally unknown.
In men [1,2,3,4,5], there are consistently inverse associations between testosterone (TESTO) and fat mass measures starting around 30 years of age [1,2,3,4]. In women, the relationship is positive in young [6] and middle-aged females adjusted for menopausal status [2] but negative after menopause [7]. The relation between lean mass and TESTO is rather controversial: while no associations were reported in some studies of men [2, 3, 8] and women [2, 6], other studies found a positive correlation in men [9, 10] and women [7] or an inverse association in men [5, 11, 12]. Declines in TESTO with age were not associated with lean mass in either sex in late midlife [2].
Regarding body fat distribution, TESTO was shown to be negatively associated with various traits reflecting abdominal fat levels in men [1, 4, 12], whereas the relations were found to be nonsignificant [7, 13] or positive [14] in postmenopausal women. Previous findings are also equivocal for sex hormone-binding globulin (SHBG). In men, SHBG was negatively correlated with fat mass [1, 5, 11], indicators of abdominal fat accumulation [8, 10, 12], and lean mass [1, 3, 5, 11]. In women, SHBG was inversely associated with lean mass [2] and fat mass [15], whereas the association between SHBG and fat distribution was found to be nonsignificant [6, 16] or negative [15].
Some studies have reported positive associations between plasma levels of estradiol (E2) with lean mass [3], fat mass [1], body mass index (BMI), and waist circumference [17] in men, as well as with fat mass [18] and fat distribution in women [15]. Other reports have found no association with adiposity in men [19, 20]. Blood levels of DHT were negatively correlated with fat mass and abdominal fat accumulation traits in women [21], but the findings were not replicated in another study [7]. Dehydroepiandrosterone (DHEA) sulfate (DHEAS) was shown to be inversely [16, 22], positively [23], or not correlated [18, 21, 23, 24] with fat distribution in women.
One study dealt with the effects of regular exercise in older men. Baseline plasma levels of TESTO did not correlate with the beneficial effects of a 12-month, home-based, moderate-to-vigorous aerobic training program on body composition [25].
In summary, these conflicting observations may be caused by differences in age coverage (young [16], middle [2, 4], older [3, 10, 20], or mixed [1, 5, 9, 11, 12]), be specific to one sex [1, 3, 4], vary by ancestry (black [15], white, mixed [1], or undefined [26]), menopausal status (pre [2, 6, 15, 24], post [2, 7, 14, 18, 23, 27], early-post, late-post [13]), health status (health [6, 7], obesity [4, 16, 22], diseases [21]), physical activity level (activity [28], inactivity, sustained exercisers [8]), or methodology used to assess concentrations of steroid hormones, particularly androgens [29]. Furthermore, most reports are based on only one or a few hormones.
Here, we are taking advantage of the multicenter HERITAGE Family Study to examine the relationships between plasma steroid hormone levels with body composition and indicators of fat distribution in the sedentary state and after a standardized exercise training program in Blacks and Whites, men and women ranging in age from 17 to 65 years, using a panel of 14 steroid hormones plus SHBG.
Methods
Subjects
The HERITAGE Family Study cohort has been previously described [30]. Sedentary subjects aged 17–65 years took part in a standardized 20-week endurance exercise program. They were recruited at four institutions located in Austin (Texas), Phoenix (Arizona) (the site moved later to Indianapolis (Indiana)), Minneapolis (Minnesota), and Quebec City (Canada). Recruitment focused on nuclear families, and parents and their adult offspring were recruited. The study protocol had been previously approved by the institutional review board at each of the four clinical centers of HERITAGE. Informed written consent was obtained from each subject.
Anthropometric, body composition, and fat distribution measurements
Body weight, height, waist, and hip circumferences were measured following standardized procedures. Body density was measured using the hydrostatic weighing technique. The mean of the highest three (of 10) measurements was used in the calculation of percent body fat from body density using the equation of Siri for Caucasian men [31], Lohman for Caucasian women [32], Schutte et al. for Black men [33], and Ortiz et al. for Black women [34]. Fat mass was obtained by multiplying body weight by percent body fat (% body fat). These measurements have been shown to be highly reproducible with no difference among clinical centers or drift over time in the course of data collection [35]. Intraclass coefficients for repeated measurers and coefficients of variation (SD/mean × 100) were calculated for all body composition and adiposity measures. Briefly, body fat percentage and fat mass calculated from body density as described above are characterized by intraclass correlations of 0.99 with CVs extending from 0.8% for body fat percentage to 0.6 kg for fat mass (based on three repeated tests over 3 weeks in 60 adults) [35].
The computed tomography (CT) scans were used to measure abdominal total fat (ATF), abdominal subcutaneous fat (ASF), and abdominal visceral fat (AVF) [36, 37]. The CT scans were obtained between the fourth (L4) and fifth (L5) lumbar vertebrae while subjects were supine with arms extended above the head. ATF and AVF areas were calculated by delineating the areas with a graph pen and then computing the surface areas by using an attenuation range of −190 to −30 Hounsfield units. The AVF area was measured by drawing a line within the inner portion of the muscle wall surrounding the abdominal cavity. ASF area was calculated by subtracting AVF from ATF. In a recent paper, inter-raters and intra-rater intraclass coefficients for CT abdominal and visceral fat were of the order of 0.99 with CVs ranging from 0.5 to 2.2% [38]. Repeated measures based on a fatty phantom in HERITAGE generated test–retest correlations not different from 1.0 with a coefficient of variation around 1%.
Endurance exercise training program
Each subject in the HERITAGE study exercised three times per week for 20 weeks on cycle ergometers. The intensity of the training was customized for each individual on the basis of heart rate (HR) and VO2 measurements taken at two baseline maximal exercise tests. Details of the exercise training protocol can be found elsewhere [30]. Briefly, subjects trained at the HR associated with 55% of baseline VO2max for 30 min per session for the first 2 weeks. The duration and intensity were gradually increased every 2 weeks, until reaching 50 min and 75% of the HR associated with baseline VO2max. This level was maintained for the final 6 weeks of training. All training was performed on Universal Aerobicycles (Cedar Rapids, IA) and power output was controlled by direct HR monitoring using the Universal Gym Mednet (Cedar Rapids, IA) computerized system. The protocol was standardized across all four clinical centers and supervised to ensure that the equipment was working properly and that participants were compliant with the protocol. The present report is based on completers (N = 480) defined as having performed at least 57 of the required 60 exercise sessions and having all adiposity and steroid data.
Plasma steroid hormone and SHBG concentrations
The hormonal assays have been previously described [19, 39]. Fourteen steroid hormones or their derivatives and SHBG concentrations were assayed: adrenal androgen precursors: DHEA, androstenedione (DELTA4), DHEAS; active androgens: TESTO, and dihydrotesterone (DHT); androgen metabolites: androstane 3α17βdiol glucuronide (DIOLG), and androsterone glucuronide (ADTG); pregnenolone fatty acid ester (PREGE), progesterone (PROG), and E2; aldosterone (nmol/L), cortisol (nmol/L). Additionally, free androgen index (FAI) and E2/T were calculated as TESTO/SHBG × 100 and as E2/TESTO × 100, respectively. Supplementary Table 1 (Table S1) provides the full name of each hormone, the abbreviations used in the present report and a brief comment on the source of production or transformation of the hormones and their precursors.
On 2 consecutive days, two blood samples were obtained after a 12-h fast pre- and post-exercise program. For eumenorrheic women, all samples were obtained in the early follicular phase of the menstrual cycle. None of the women of reproductive age had dramatically irregular menstrual cycles as determined by questionnaire and an interview. After centrifugation, aliquots were frozen at −80oC. For nonconjugated steroids, ALDO, DHEA, and TESTO were differentially extracted with hexane-ethyl acetate. Petroleum ether was used for the extraction of DELTA4 and DHT. In-house RIAs were used to measure levels of these four steroids. PROG, OHPRG, CORT, E2, and DHEAS were assayed using commercial kits (Diagnostics Systems Lab, Webster, TX, USA). For glucuronide (ADTG and DIOLG) and ester (DHEAE and PREGE)-conjugated steroids, ethanol extraction was performed, followed by C18 column chromatography. Glucuronide conjugates were submitted to hydrolysis with β-glucuronidase (Sigma Co., St Louis, MO, USA). Fatty acid derivatives were submitted to saponification. Steroids were then separated by elution on LH-20 columns and measured by radioimmunoassay (RIA). SHBG was determined with an IRMA-Count solid phase immunoradiometric assay using 125I (Diagnostics Systems Laboratories, Inc.). All assays were performed in the laboratory of Dr. Alain Belanger, Molecular Endocrinology, Laval University Medical Center (CHUL), Québec, Canada.
Statistical analysis
The present study is based on sedentary adults from the HERITAGE Family Study who had complete hormonal and body composition and fat distribution data at baseline and who completed a standardized exercise training program. The mean of two measurements both before and after the exercise program has been used for all hormones and SHBG levels. Data of men and women were analyzed separately. Paired t-tests were used to compare variables of body composition and fat distribution at baseline and after the exercise program.
The baseline value of steroid hormones, SHBG, FAI, and E2/T in men and women are available in Table S2. Mean and median values are presented, as well as 95% confidence intervals. Testing for skewness of distributions revealed that there was no indication of major skewness problems (skewness < 3.0), with the exception of DIOLG in men (skewness = 4.7) and E2 in women (skewness = 4.4). Both were log transformed to reduce skewness.
In men, Pearson product–moment correlations were used to quantify the relationships between baseline body composition or fat distribution traits (after adjustment for age and ancestry) and their exercise training responses (after adjustment for baseline value, age, and ancestry) with each fasting plasma steroid hormone, SHBG concentration, FAI, and E2/T. One-way analysis of variance (ANOVA) was used to compare each steroid across quartiles of baseline body composition or fat distribution variables and their training responses. Quartiles were defined for each age and ancestry group and then pooled.
In women, oral contraceptives (OCs) and hormone replacement therapy (HRT) status had a significant impact on some steroids of the hormonal panel at baseline (Table S3, S4 for OC and HRT, respectively) and post-exercise training (Table S5, S6). Pre-menopause and post-menopause status also influenced the level of some hormones at baseline (Table S7) and in response to the exercise program (Table S8). As a result, correlation coefficients were used to quantify the relationships between baseline body composition and fat distribution traits (after adjustment for age, ancestry, menopause status, and OC/HRT status) and their exercise training response (after adjustment for baseline value, age, ancestry, menopause status, and OC/HRT status) with fasting plasma steroid hormone, SHBG concentration, FAI, E2/T. The general linear model (GLM) procedure was used to compare each steroid, SHBG, FAI, and E2/T across quartiles of baseline body composition or fat distribution variables and their training responses with menopause status and OC/HRT status as covariates. Quartiles were defined taking into account age and ancestry. The GLM procedure was used to compare each steroid, SHBG, FAI, and E2/T across the various BMI categories with age and ancestry as covariates: underweight (<18.5 kg/m2), normal weight (≥18.5 to <25 kg/m2), overweight (≥25 to <30 kg/m2), and obesity (≥30 kg/m2) in men and women.
Power analysis of sample size
Partial correlation analysis was used to assess the relationship between plasma steroid hormones and body composition/fat distribution at baseline and training response, with adjustment for age, ancestry, menopause status (women), OC/HRT status (women), and baseline value (training response). Based on previously published studies, we posit that the true correlations will be in the range from 0.25 to 0.50, with α = 0.0001 and two-sided tests. If the true correlations are about 0.50, a sample size of about 60 is required to achieve a statistical power of about 85%. For coefficients of the order of 0.25, only about 140 subjects are needed if the alpha threshold is set at 0.05 but about 375 subjects are required for a threshold of 0.0001. Thus, when the multiple testing stringent alpha level is used, an adequate statistical power is achieved when correlations are based on the whole sample of men or women with correlations >0.25. Lower statistical power is attained when subgroups are compared and when correlations reflect smaller effect sizes.
All analyses were performed using the SAS statistical package (SAS Institute, Inc., Cary, NC). Given the number of statistical tests performed, a Bonferroni-adjusted level of significance was calculated and set at a P-value threshold of <0.0001.
Results
Table 1 shows the distributions of the sedentary subjects before and in response to endurance training stratified by age, gender, and ancestry. At baseline, there were 270 men and 304 women with complete data (29% of males and 34% of females were Blacks). For the training response traits, there were 242 men and 238 women (25% of men and 24% of women were Blacks). At baseline, there are 261 premenopausal and 43 postmenopausal women. Among them, 83 premenopausal women were on OC, and 21 postmenopausal women reported using HRT. There were 198 premenopausal and 41 postmenopausal women who completed the exercise program. Among them, 71 were on OC and 19 were postmenopausal women on HRT.
Table 2 summarizes the associations of steroid hormone concentration levels with body composition/fat distribution in men. Shown are partial correlations (controlling for age and ancestry) between baseline plasma steroid hormone levels and baseline body composition/fat distribution. DHT, 17 hydroxy PROG (OHPROG), SHBG, and TESTO were significantly negatively correlated with nearly all body composition/fat distribution variables. SHBG and TESTO were not correlated with absolute fat-free mass but inversely associated with fat-free mass/H2 (Table 2) and positively associated with percentage fat-free mass (Table S9). Additionally, DHT, OHPROG, and SHBG were positively correlated with percentage fat-free mass (Table S9).
Table 3 depicts the partial correlations (controlling for age, ancestry, menopause status, OC/HRT status) in women. SHBG was significantly and negatively correlated with all fat mass/distribution variables, with the exception of the ASF/AVF ratio. The plasma level of SHBG was also significantly negatively correlated with fat-free mass (fat-free mass/H2, and fat-free mass) but positively associated with percentage fat-free mass (Table S10). TESTO was not correlated with fat-free mass, fat-free mass/H2 and percentage of fat-free mass. DHT was significantly and positively correlated with fat-free mass and fat-free mass/H2 (Table S10). FAI was positively associated with all body composition/fat distribution variables except ASF/AVF ratio.
Hormonal levels exhibited substantial differences across the four quartiles of body composition and fat distribution in men (Table S11-S21). Plasma levels of SHBG (Figs. 1a, b) and TESTO (Figs. 1c–f) decreased significantly from Q1 to Q4 for nearly all body composition and fat distribution variables. The hormonal levels across quartiles of adiposity variables in women are detailed in Tables S22-S32. After adjustment for age, ancestry and OC/HRT status, the plasma levels of SHBG decreased progressively from Q1 to Q4 (Figs. 2a–c), and that of FAI (Figs. 2d–f) increased significantly from Q1 to Q4 for all variables.
Hormone levels by quartiles of baseline body composition and fat distribution variables in men. a Plasma level of SHBG in Q4 of BMI was lower than Q1 and Q2. b Plasma level of SHBG in Q4 of waist girth was lower than Q1. c Plasma level of TESTO in Q4 of BMI was lower than Q1 and Q2. d Plasma level of TESTO in Q4 of percent body fat was lower than Q1. e Plasma level of TESTO in Q4 of waist girth was lower than Q1. f Plasma level of TESTO in Q4 of AVF was lower than Q1. SHBG sex hormone-binding globulin, TESTO Testosterone, AVF abdominal visceral fat. Values are shown as mean ± SD. One-way ANOVA was used to compare each plasma steroid across quartiles of baseline body composition or fat distribution variables. ***P < 0.0001
Hormone levels by quartiles of baseline body composition and fat distribution in women. a Plasma level of SHBG in Q4 of BMI was lower than Q1 and Q2. b Plasma level of SHBG in Q4 of waist girth was lower than Q1 and Q2. c Plasma level of SHBG in Q4 of AVF was lower than Q1 and Q2. d Plasma level of TESTO in Q4 of BMI was higher significantly than that of Q1, Q2 and Q3. e Plasma level of TESTO in Q4 of waist girth was higher Q1, Q2 and Q3. f Plasma level of TESTO in Q4 of AVF was higher than Q1. Free androgen index (FAI), calculated as TESTO/SHBG × 100. AVF abdominal visceral fat. Values are shown as mean ± SEM. The general linear model (GLM) procedure was used to compare each plasma steroid across quartiles of baseline body composition or fat distribution variables. ***P < 0.0001
Figure 3 depicts hormonal levels that differed significantly among normal weight, overweight, and obese subgroups in men and women, as defined by standard BMI thresholds. In men, with age and ancestry as covariance, the plasma levels of SHBG and TESTO decreased gradually from the normal weight to the obese group. In women, when age, ancestry, menopause status, and OC/HRT status were used as covariates, the levels of SHBG decreased progressively across normal weight, overweight, and obese categories, whereas FAI increased across the same weight gradient. Other hormone levels across BMI categories are detailed in Table S33.
Hormone levels among normal weight, overweight, and obese men and women. a Plasma level of SHBG was higher in normal weight group than overweight and obese. b Plasma level of TESTO was higher in normal weight group than obese. c Plasma level of SHBG was higher in normal weight group than overweight and obese. d FAI was lower in normal weight group than overweight and obese. SHBG sex hormone-binding globulin, TESTO testosterone. Values are shown as mean ± SEM. The GLM procedure was used to compare each plasma steroid across the various BMI categories with age and ancestry as covariates. ***P < 0.0001
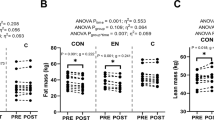
In response to the exercise program, percent body fat, waist girth, and AVF decreased significantly, but individual differences in response (△) were large (see Table 1). Table S34 summarizes the other body composition and fat distribution exercise training responses (△). We verified the degree of skewness of response distributions and did not find any strong evidence that skewness was an issue (all < 3.0). Body composition and fat distribution variables changed significantly in response to the exercise training program (P < 0.0001), except for BMI. In brief, there were no significant correlations between baseline hormonal levels and the exercise training response of body composition and fat distribution traits in men or women. Moreover, hormonal levels were not different across quartiles of body composition and fat distribution responses. These results are shown in Tables S35-S56.
Discussion
The present study is the most comprehensive report published to date on the relationships between a large panel of plasma steroid hormones and body composition or indicators of fat distribution in sedentary men and women at baseline and in response to a standardized and fully monitored 20-week exercise program.
Negative associations were found between fat mass and adipose tissue distribution variables (BMI, percent of body fat, fat mass/H2, waist circumference, hip circumference, WHR, ATF, AVF, and ASF) with plasma levels of DHT, OHPROG, SHBG, and TESTO in men. Surprisingly, the plasma concentration of TESTO was inversely correlated with fat mass/H2 but not with total fat-free mass, whereas it was positively correlated with percentage fat-free mass. In contrast, in women, SHBG was negatively correlated with all fat mass and distribution variables, whereas FAI was positively associated with all of them. Baseline plasma steroid levels, SHBG concentrations, FAI, or E2/T were not associated with the changes in body composition and fat distribution traits resulting from exposure to the 20-week endurance exercise program after adjustment for baseline values, age, ancestry, menopause status, and OC/HRT (women).
The present results reveal that body composition and fat distribution are negatively correlated with SHBG in men and women and with TESTO in men. This sex dimorphism might be partly attributed to the marked sex-related difference of SHBG binding to TESTO [40]. Non-SHBG-bound TESTO seems to be the best parameter for serum levels of bioactive T20. Androgens exert their effects on adipocyte differentiation in a depot-specific manner, via the androgen receptor, leading to modulation of adipocyte size and specific fat depot expansion [41]. Androgens also impact key adipocyte functions including insulin signaling, lipid metabolism, fatty acid uptake, and adipokine productions, with frequently observed sex-specific effects [42]. Furthermore, sex differences in body fat distribution are commonly recognized, with males usually characterized by a predominant accumulation of fat in the abdominal region, whereas women generally display a greater proportion of their body fat in the gluteal–femoral region [43].
An excessive amount of body fat, such as in obesity, can lead to impaired testicular T biosynthesis. Furthermore, depressed levels of plasma TESTO increases fat deposition, particularly AVF. As abdominal fat is a risk factor for the development of metabolic syndrome, type 2 diabetes mellitus and cardiovascular disease [44], abdominal adiposity and the ensuing hyperinsulinemia [45] can synergistically reduce SHBG production [46]. Thus, low physiological levels of TESTO and SHBG could represent components of a hormonal milieu that is favorable to fat storage with potential adverse metabolic impact.
In men, the major source of E2 is through the conversion of TESTO via aromatase, which is significantly expressed in adipose tissue, suggesting that higher levels of adiposity favor synthesis of E2 [1]. We did not find that E2 was associated with body composition and fat distribution traits in men and in women in contrast to positive associations reported in 30- to 90-year-old men from the United States [1], 69- to 80-year-old Swedish men [3], and 40- to 80-year-old Dutch men [17], as well as in premenopausal African–American women [15]. Importantly, in the present study, age, ancestry, menopause, OC, and HRT were controlled for.
In women with androgen excess, higher TESTO level is a fairly consistent predictor of abdominal adipose tissue accumulation, but it is not the case in non-hyperandrogenic women [47]. Our results confirm that androgens are not related to fat mass and visceral fat accumulation in women. Hence, in women without clinical manifestations of hyperandrogenism, there is no clear association between androgens and adiposity, particularly abdominal and visceral fat levels.
We found that baseline steroid hormonal levels were not associated with the adiposity changes observed in response to the exercise training program. This is concordant with other reports even though prior studies were based on rather small sample sizes. For instance, Hawkins et al. [25] also did not find that TESTO, DHT, and SHBG were associated with exercise-induced changes in body fat in men after 12 months of a home-based exercise intervention.
Interestingly, the World Anti-Doping Agency (WADA) introduced a ‘hyperandrogenism’ rule in 2011 that aims at excluding women with a serum TESTO > 10 nmol/L from participating in elite sport. It is based on the premise that the greater lean body mass in men is a consequence of their higher serum TESTO [48]. The Court of Arbitration for Sport suspended the ‘hyperandrogenism regulation’ in 2015. Unless sufficient scientific evidence is provided in support of the ‘hyperandrogenism’ rule within 2 years, it will be withdrawn permanently. Even though our findings are based on sedentary women, as opposed to athletes, they strongly suggest that endogenous plasma TESTO levels in the normal range are not associated with absolute or relative fat-free mass in women once age, ancestry, menopause status, OC, and HRT have been taken into account. However, in the present study, none of the women reached the 10 nmol/L threshold (the highest plasma level of TESTO was 5.905 nmol/L).
The present study is characterized by several strong points. It is based on a relatively large sample with substantial subgroups defined by age, ancestry, and sex. Two blood samples obtained on consecutive days were used to assay the steroid hormones. Of particular significance, all blood samples were drawn in the early follicular phase of the menstrual cycle for eumenorrheic women. Also of significance, information on use of OC and HRT was available and could be adjusted for in the present analyses. Furthermore, as a large number of tests of significance was performed, an alpha threshold of P < 0.0001 was used to reduce the risk of false-positive results. Additionally, body composition and fat distribution phenotypes were measured directly in the laboratory on all participants and a large panel of steroid hormones was assayed. Finally, adiposity traits changed marginally but significantly (P < 0.0001) in response to the standardized exercise training program, with considerable individual difference in the changes observed.
There are some limitations to the data reported herein. As fat-free mass was assessed by underwater weighing, we could not distinguish between truncal vs. limb lean masses, a distinction that was shown to be useful in some studies [6, 49]. As our sample of postmenopausal women was not large enough, we could not compare early and late menopausal periods [13]. Additionally, despite the fact that the study is based on a relatively large sample size, it was not sufficiently large to compare various age groups within sex and ethnic groups. Finally, the data of the HERITAGE study used herein, with the exception of the response to the exercise intervention, are observational and cross-sectional in nature, which imposes limits on the assessment of causality.
We conclude that baseline levels of DHT, OGPROG, SHBG, and TESTO in men and SHBG and FAI in women are associated with individual differences in fat mass and fat distribution phenotypes. TESTO was not correlated with absolute fat-free mass in men and women, but DHT was positively correlated with fat-free mass in women, and TESTO was positively corrected with % fat-free mass in men. Additionally, no association could be detected between baseline hormonal levels and adiposity changes following a standardized and monitored 20-week exercise program. Low physiological concentrations of DHT, OHPROG, SHBG, and TESTO are potential risk factors for high adiposity and undesirable patterns of fat distribution in men. The same appears to be true for SHBG in women.
References
Gates MA, Mekary RA, Chiu GR, Ding EL, Wittert GA, Araujo AB. Sex steroid hormone levels and body composition in men. J Clin Endocrinol Metab. 2013;98:2442–50.
Bann D, Wu FC, Keevil B, Lashen H, Adams J, Hardy R, et al. Changes in testosterone related to body composition in late midlife: findings from the 1946 British birth cohort study. Obes (Silver Spring). 2015;23:1486–92.
Vandenput L, Mellstrom D, Karlsson MK, Orwoll E, Labrie F, Ljunggren O, et al. Serum estradiol is associated with lean mass in elderly Swedish men. Eur J Endocrinol. 2010;162:737–45.
Tchernof A, Despres JP, Belanger A, Dupont A, Prud’homme D, Moorjani S, et al. Reduced testosterone and adrenal C19 steroid levels in obese men. Metabolism. 1995;44:513–9.
Lee K, Sung J, Song YM. Genetic and environmental influence on the association between testosterone, sex hormone-binding globulin and body composition in Korean men. Clin Endocrinol (Oxf). 2015;83:236–45.
Keller JL, Casson PR, Toth MJ. Relationship of androgens to body composition, energy and substrate metabolism and aerobic capacity in healthy, young women. Steroids. 2011;76:1247–51.
Casson PR, Toth MJ, Johnson JV, Stanczyk FZ, Casey CL, Dixon ME. Correlation of serum androgens with anthropometric and metabolic indices in healthy, nonobese postmenopausal women. J Clin Endocrinol Metab. 2010;95:4276–82.
Crewther BT, Heke TL, Keogh JW. The effects of a resistance-training program on strength, body composition and baseline hormones in male athletes training concurrently for rugby union 7’s. J Sports Med Phys Fit. 2013;53:34–41.
Mouser JG, Loprinzi PD, Loenneke JP. The association between physiologic testosterone levels, lean mass, and fat mass in a nationally representative sample of men in the United States. Steroids. 2016;115:62–66.
Roy TA, Blackman MR, Harman SM, Tobin JD, Schrager M, Metter EJ. Interrelationships of serum testosterone and free testosterone index with FFM and strength in aging men. Am J Physiol Endocrinol Metab. 2002;283:E284–94.
Trabert B, Graubard BI, Nyante SJ, Rifai N, Bradwin G, Platz EA, et al. Relationship of sex steroid hormones with body size and with body composition measured by dual-energy X-ray absorptiometry in US men. Cancer Causes Control. 2012;23:1881–91.
Blouin K, Despres JP, Couillard C, Tremblay A, Prud’homme D, Bouchard C, et al. Contribution of age and declining androgen levels to features of the metabolic syndrome in men. Metabolism. 2005;54:1034–40.
Cao Y, Zhang S, Zou S, Xia X. The relationship between endogenous androgens and body fat distribution in early and late postmenopausal women. PLoS ONE. 2013;8:e58448.
Liedtke S, Schmidt ME, Vrieling A, Lukanova A, Becker S, Kaaks R, et al. Postmenopausal sex hormones in relation to body fat distribution. Obes (Silver Spring). 2012;20:1088–95.
Paxton RJ, King DW, Garcia-Prieto C, Connors SK, Hernandez M, Gor BJ, et al. Associations between body size and serum estradiol and sex hormone-binding globulin levels in premenopausal African American women. J Clin Endocrinol Metab. 2013;98:E485–90.
De Simone M, Verrotti A, Iughetti L, Palumbo M, Farello G, Di Cesare E, et al. Increased visceral adipose tissue is associated with increased circulating insulin and decreased sex hormone binding globulin levels in massively obese adolescent girls. J Endocrinol Invest. 2001;24:438–44.
Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40-80 years. Eur J Endocrinol. 2003;149:583–9.
Mahabir S, Baer DJ, Johnson LL, Hartman TJ, Dorgan JF, Campbell WS, et al. Usefulness of body mass index as a sufficient adiposity measurement for sex hormone concentration associations in postmenopausal women. Cancer Epidemiol Biomark Prev. 2006;15:2502–7.
Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, Skinner JS, et al. Contribution of body fatness and adipose tissue distribution to the age variation in plasma steroid hormone concentrations in men: the HERITAGE Family Study. J Clin Endocrinol Metab. 2000;85:1026–31.
van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85:3276–82.
Cote JA, Lessard J, Mailloux J, Laberge P, Rheaume C, Tchernof A. Circulating 5alpha-dihydrotestosterone, abdominal obesity and adipocyte characteristics in women. Horm Mol Biol Clin Investig. 2012;12:391–400.
De Pergola G, Triggiani V, Giorgino F, Cospite MR, Garruti G, Cignarelli M, et al. The free testosterone to dehydroepiandrosterone sulphate molar ratio as a marker of visceral fat accumulation in premenopausal obese women. Int J Obes Relat Metab Disord. 1994;18:659–64.
Barrett-Connor E, Ferrara A. Dehydroepiandrosterone, dehydroepiandrosterone sulfate, obesity, waist-hip ratio, and noninsulin-dependent diabetes in postmenopausal women: the Rancho Bernardo Study. J Clin Endocrinol Metab. 1996;81:59–64.
Williams DP, Boyden TW, Pamenter RW, Lohman TG, Going SB. Relationship of body fat percentage and fat distribution with dehydroepiandrosterone sulfate in premenopausal females. J Clin Endocrinol Metab. 1993;77:80–5.
Hawkins VN, Foster-Schubert K, Chubak J, Sorensen B, Ulrich CM, Stancyzk FZ, et al. Effect of exercise on serum sex hormones in men: a 12-month randomized clinical trial. Med Sci Sports Exerc. 2008;40:223–33.
Webborn N, Williams A, McNamee M, Bouchard C, Pitsiladis Y, Ahmetov I, et al. Direct-to-consumer genetic testing for predicting sports performance and talent identification: consensus statement. Br J Sports Med. 2015;49:1486–91.
McTiernan A, Tworoger SS, Rajan KB, Yasui Y, Sorenson B, Ulrich CM, et al. Effect of exercise on serum androgens in postmenopausal women: a 12-month randomized clinical trial. Cancer Epidemiol Biomark Prev. 2004;13:1099–105.
Hayes LD, Grace FM, Sculthorpe N, Herbert P, Kilduff LP, Baker JS. Does chronic exercise attenuate age-related physiological decline in males? Res Sports Med. 2013;21:343–54.
Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–13.
Bouchard C, Leon AS, Rao DC, Skinner JS, Wilmore JH, Gagnon J. The HERITAGE family study. Aims, design, and measurement protocol. Med Sci Sports Exerc. 1995;27:721–9.
Siri WE. The gross composition of the body. Adv Biol Med Phys. 1956;4:239–80.
Lohman TG. Applicability of body composition techniques and constants for children and youths. Exerc Sport Sci Rev. 1986;14:325–57.
Schutte JE, Townsend EJ, Hugg J, Shoup RF, Malina RM, Blomqvist CG. Density of lean body mass is greater in blacks than in whites. J Appl Physiol Respir Environ Exerc Physiol. 1984;56:1647–9.
Ortiz O, Russell M, Daley TL, Baumgartner RN, Waki M, Lichtman S, et al. Differences in skeletal muscle and bone mineral mass between black and white females and their relevance to estimates of body composition. Am J Clin Nutr. 1992;55:8–13.
Wilmore JH, Stanforth PR, Domenick MA, Gagnon J, Daw EW, Leon AS, et al. Reproducibility of anthropometric and body composition measurements: the HERITAGE Family Study. Int J Obes Relat Metab Disord. 1997;21:297–303.
Sjostrom L, Kvist H, Cederblad A, Tylen U. Determination of total adipose tissue and body fat in women by computed tomography, 40K, and tritium. Am J Physiol. 1986;250:E736–45.
Stanforth PR, Jackson AS, Green JS, Gagnon J, Rankinen T, Despres JP, et al. Generalized abdominal visceral fat prediction models for black and white adults aged 17-65 y: the HERITAGE Family Study. Int J Obes Relat Metab Disord. 2004;28:925–32.
Lee YH, Hsiao HF, Yang HT, Huang SY, Chan WP. Reproducibility and repeatability of computer tomography-based measurement of abdominal subcutaneous and visceral adipose tissues. Sci Rep. 2017;7:40389
Ukkola O, Gagnon J, Rankinen T, Thompson PA, Hong Y, Leon AS, et al. Age, body mass index, race and other determinants of steroid hormone variability: the HERITAGE Family Study. Eur J Endocrinol. 2001;145:1–9.
Grasa MD, Gulfo J, Camps N, Alcala R, Monserrat L, Moreno-Navarrete JM, et al. Modulation of SHBG binding to testosterone and estradiol by sex and morbid obesity. Eur J Endocrinol. 2017;176:393–404.
Blouin K, Nadeau M, Perreault M, Veilleux A, Drolet R, Marceau P, et al. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clin Endocrinol (Oxf). 2010;72:176–88.
O’Reilly MW, House PJ, Tomlinson JW. Understanding androgen action in adipose tissue. J Steroid Biochem Mol Biol. 2014;143:277–84.
Blouin K, Boivin A, Tchernof A. Androgens and body fat distribution. J Steroid Biochem Mol Biol. 2008;108:272–80.
Saad F, Aversa A, Isidori AM, Gooren LJ. Testosterone as potential effective therapy in treatment of obesity in men with testosterone deficiency: a review. Curr Diabetes Rev. 2012;8:131–43.
Despres JP, Nadeau A, Tremblay A, Ferland M, Moorjani S, Lupien PJ, et al. Role of deep abdominal fat in the association between regional adipose tissue distribution and glucose tolerance in obese women. Diabetes. 1989;38:304–9.
Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab. 1988;67:460–4.
Tchernof A, Brochu D, Maltais-Payette I, Fouad Mansour M, Marchand GB, Carreau AM, Kapeluto J. Androgens and the regulation of adiposity and body fat distribution in humans. Compr Physiol 2018; In press.
Sonksen P. Determination and regulation of body composition in elite athletes. Br J Sports Med. 2018;52:219–229
LeBlanc ES, Wang PY, Lee CG, Barrett-Connor E, Cauley JA, Hoffman AR, et al. Higher testosterone levels are associated with less loss of lean body mass in older men. J Clin Endocrinol Metab. 2011;96:3855–63.
Acknowledgements
The HERITAGE Family Study has been supported over the years by multiple grants from the National Institute for Heart, Lung and Blood Diseases of the National Institutes of Health (HL45670, CB and TR; HL47323, ASL; HL47317, DC Rao; HL47327, JS Skinner; HL47321, JH Wilmore, deceased). CB is partially funded by the John W. Barton Sr. Chair in Genetics and Nutrition, and by the NIH-funded COBRE grant (NIH 8 P30GM118430-01). ZH is funded by the China Scholarship Council (file no. 201603620001) and China Institute of Sport Science (2015-01, 2016-01). We would like to express our gratitude to Dr. Alain Belanger (retired) and his staff from the Molecular Endocrinology Laboratory of the Laval University Medical Center in Quebec City, Canada, for the assays of the steroids and their dedication to the HERITAGE Family Study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AT receives research funding from Johnson & Johnson Medical Companies for studies unrelated to the present paper. The remaining authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
He, Z., Rankinen, T., Leon, A.S. et al. Plasma steroids, body composition, and fat distribution: effects of age, sex, and exercise training. Int J Obes 42, 1366–1377 (2018). https://doi.org/10.1038/s41366-018-0033-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-018-0033-1
- Springer Nature Limited
This article is cited by
-
The METS-IR is independently related to bone mineral density, FRAX score, and bone fracture among U.S. non-diabetic adults: a cross-sectional study based on NHANES
BMC Musculoskeletal Disorders (2023)
-
Sex differences in the associations between adiposity distribution and cardiometabolic risk factors in overweight or obese individuals: a cross-sectional study
BMC Public Health (2021)
-
Relationships between menstrual status and obesity phenotypes in women: a cross-sectional study in northern China
BMC Endocrine Disorders (2020)
-
Gut microbiota steroid sexual dimorphism and its impact on gonadal steroids: influences of obesity and menopausal status
Microbiome (2020)
-
Exercise type and fat mass loss regulate breast cancer-related sex hormones in obese and overweight postmenopausal women
European Journal of Applied Physiology (2020)