Abstract
Endometrial cancer is the most common malignancy of the female genital tract. Progesterone (P4) has been used for several decades in endometrial cancer treatment, especially in women who wish to retain fertility. However, it is unpredictable which patients will respond to P4 treatment and which may have a P4-resistant cancer. Therefore, identifying the mechanism of P4 resistance is essential to improve the therapies for endometrial cancer. Mitogen-inducible gene 6 (Mig-6) is a critical mediator of progesterone receptor (PGR) action in the uterus. In order to study the function of Mig-6 in P4 resistance, we generated a mouse model in which we specifically ablated Mig-6 in uterine epithelial cells using Sprr2f-cre mice (Sprr2fcre+Mig-6f/f). Female mutant mice develop endometrial hyperplasia due to aberrant phosphorylation of signal transducers and activators of transcription 3 (STAT3) and proliferation of the endometrial epithelial cells. The results from our immunoprecipitation and cell culture experiments showed that MIG-6 inhibited phosphorylation of STAT3 via protein interactions. Our previous study showed P4 resistance in mice with Mig-6 ablation in Pgr-positive cells (Pgrcre/+Mig-6f/f). However, Sprr2fcre+Mig-6f/f mice were P4-responsive. P4 treatment significantly decreased STAT3 phosphorylation and epithelial proliferation in the uterus of mutant mice. We showed that Mig-6 has an important function of tumor suppressor via inhibition of STAT3 phosphorylation in uterine epithelial cells, and the antitumor effects of P4 are mediated by the endometrial stroma. These data help to develop a new signaling pathway in the regulation of steroid hormones in the uterus, and to overcome P4 resistance in human reproductive diseases, such as endometrial cancer.
Similar content being viewed by others
Introduction
Endometrial cancer is a well-known gynecologic malignancy of the female reproductive tract. In the United States, endometrial cancer affected 60 050 women and caused 10 470 deaths in 2016.1 It comprises 7% of all cancer in women. The majority of endometrial cancer is endometrioid adenocarcinoma, which is derived from epithelial cells of the endometrium.2 The development of endometrial hyperplasia, a proliferative process in the epithelium, is a critical risk factor of endometrioid carcinoma.3 The regulation of uterine epithelial cell and stromal cell proliferation is controlled by estrogen (E2) and progesterone (P4), both of which are ovarian steroid hormones.4
P4 is a steroid hormone produced by the ovaries. Luteinizing hormone and chorionic gonadotropin regulate the synthesis and secretion of P4 during the menstrual cycle and pregnancy.5 Coordinated actions of the progesterone receptor (PGR) mediate the P4 response in the endometrium.6 Stromal–epithelial communication is important for uterine function.7 PGR inhibits E2-mediated epithelial cell proliferation via mediating epithelial–stromal crosstalk.6, 8 P4 lessens E2-stimulated uterine epithelial proliferation by modulating the gene expression in the uterine stromal cells.9 Whereas the effect of P4 on uterine function is mediated by epithelial–stromal crosstalk, the exact molecular mechanism of epithelial–stromal crosstalk remains to be elucidated.9
A steroid hormone imbalance could lead to aberrant endometrial proliferation and endometrial cancer. P4 therapy is used against endometrial hyperplasia and early endometrial cancer in patients who want to preserve fertility.10, 11 P4 and its analogs can have an effect on suppression of endometrial cancer proliferation.12 However, many studies suggest limiting the use of P4 therapy due to its low response rates in endometrial cancer.13 Despite previous studies on P4 therapy, the underlying mechanisms of P4 resistance are still poorly understood.
Mitogen-inducible gene 6 (MIG-6; also referred to as Receptor-Associated Late Transducer (RALT), ERBB receptor feedback inhibitor 1 (ERRFI1) and gene 33) is a 50-kDa cytoplasmic protein. MIG-6 is identified as an early-response gene that can be transcriptionally regulated by epidermal growth factor, transforming growth factor alpha and stress factors.14, 15 MIG-6 is also induced by mitogenic stimuli in a cell cycle-dependent manner.16 MIG-6 exhibits important tumor-suppressor capabilities by regulating migration and invasion, cell proliferation and the rate of G1–S phase progression.17, 18, 19, 20 The low level of Mig-6 is observed in human hepatocellular carcinoma,19 breast carcinomas,21 papillary thyroid cancer,18 glioblastoma,22 non-small cell lung cancer23 and endometrial cancer.24
Previously, we demonstrated that Mig-6 has a critical function in the development of endometrial hyperplasia and E2-induced endometrial cancer as a mediator of PGR functions to suppress E2 signaling in the uterus.24, 25 Mig-6 suppresses tumorigenesis of endometrial cancer that is related with Pten deficiency and ERK activation in endometrial cancer.26 MIG-6 is identified as an adaptor protein that consists of important protein–protein interaction domains, an epidermal growth factor receptor-binding domain, an src homology 3-binding motif, a 14-3-3-binding domain and a Cdc42- and Rac-interactive binding (CRIB) domain,27, 28 but it does not have a domain with enzymatic activity.15 We identified signal transducers and activators of transcription 3 (STAT3) as an MIG-6-associated protein.26 Inappropriate expression of phosphorylation of STAT3 leads to tumorigenesis.29 STAT3 is phosphorylated by receptor-associated Janus kinases in response to growth factors and cytokines, and is subsequently translocated to the cell nucleus where it acts as a transcriptional activator.30 STAT3 is a key signal transducer and regulator of gene expression that is critical to routine cellular processes including cell proliferation, development, angiogenesis, differentiation, survival and immune function.31 It is reported that STAT3 is associated with tumorigenesis and acts as an oncogene.32 Aberrant activation of STAT3 was identified in human endometrial cancer tissues as well as endometrial cancer cells.33 In addition, STAT3 has been used as a cancer therapeutic target because it has a pivotal role in oncogenic function and immunosuppression.34 The functional relationship between MIG-6 and STAT3 in endometrial cancer development, however, remains elusive.
We developed uterine epithelium-specific Mig-6 knockout mice by crossbreeding floxed Mig-6 (Mig-6f/f) mice with Sprr2fcre mice to analyze the function of epithelial Mig-6 for endometrial tumorigenesis.35 We demonstrated that Mig-6 has an important role during the development of endometrial hyperplasia. In addition, P4 treatment prevents the development of endometrial hyperplasia in mutant mice. Furthermore, Mig-6 signaling has a critical role in regulating epithelial proliferation by mediating phosphorylation of STAT3. Our results demonstrate that activation of endometrial stromal P4 signaling, including stromal Mig-6, prevents endometrial hyperplasia of mutant mice by regulating STAT3 activity.
Results
The ablation of Mig-6 in the endometrial epithelial cells of mouse
In the previous study, we found that epithelial Mig-6 is a critical tumor suppressor in the uterus of Wnt7acre+Mig-6f/f mice.25 However, Mig-6 is also expressed in the skin, and deletion of Mig-6 results in skin tumor formation over a wound.15, 36 Wnt7a-Cre activity was not only detected in uterine epithelia, but also in the ovary and skin. The ablation of Mig-6 by Wnt7a-Cre leads to tumor formation at any surgical wounds in the skin, which limits surgical applications, including ovariectomy and subcutaneous injection of steroid hormone pellets for endometrial cancer studies in mice.36 Therefore, we generated a mouse model in which we specifically ablated endometrial epithelial Mig-6 using Sprr2f-cre mice35 (Sprr2fcre+Mig-6f/f) to study the function of epithelial Mig-6 in the uterus. The epithelium-specific deletion of Mig-6 in mutant mice was proven by immunofluorescence analysis (Figure 1). MIG-6 was expressed in all compartments of the uterus in control mice. Mutant mice showed that MIG-6 levels were identified in the stromal cells but not in the epithelial cells of the uterus, whereas MIG-6 was not observed in the epithelial cells or in the stromal cells of the Pgrcre/+Mig-6f/f mouse uterus. Our immunofluorescence analysis demonstrates our successful generation of uterine epithelial-specific Mig-6-ablated mice.
Endometrial hyperplasia development by conditional epithelial Mig-6 ablation in the mouse uterus
According to our previous research, Pgrcre/+Mig-6f/f and Wnt7acre+Mig-6f/f mice display endometrial hyperplasia and cancer due to dysregulation of E2 and P4.24, 25 To examine the development and advancement of endometrial hyperplasia and cancer in the mutant mouse uterus, we investigated the uterine weight, gross appearance and histologic morphology in control and mutant mice at 9 weeks, 10 weeks and 5 months of age. The weight of the mutant mouse uterus was significantly increased compared with that of the control mice after 10 weeks of age (Figures 2a and b). Histological analysis of these uteri showed a development of endometrial hyperplasia in the uterus of mutant mice from 10 weeks of age (Figure 2c). The uteri revealed a higher number of endometrial epithelial cells and an increase in the epithelium/stroma ratio in the uterus of mutant mice. Endometrial hyperplasia is caused by excessive proliferation of endometrial gland cells.37 We next investigated whether endometrial hyperplasia in mutant mice is caused by excessive endometrial epithelial cell proliferation. The levels of Ki67, a proliferation marker, were examined in the uterus of control and mutant mice at 10 weeks of age by immunohistochemical staining. The level of Ki67 was significantly higher within the epithelium of mutant mice compared with control mice (Figures 3a and b); however, stromal proliferation was not different between the mice. These results showed that the uterus of the epithelium-specific Mig-6-ablated mice develops endometrial hyperplasia caused by an increased cell proliferation from 10 weeks of age. These microscopic anatomical changes indicate that the uterus of mutant mice exhibits endometrial hyperplasia, which can increase the chances of developing endometrial cancer in humans.
Development of endometrial hyperplasia in Sprr2fcre+Mig-6f/f mouse uterus. (a) The ratio of uterine weight to body weight of Mig-6f/f and Sprr2fcre+Mig-6f/f mice at 9 and 10 weeks and 5 months. (b) Morphology of Mig-6f/f and Sprr2fcre+Mig-6f/f mice during endometrial hyperplasia development and progression. (c) Histology of uteri from mice with epithelial Mig-6 ablation at 9 and 10 weeks and 5 months. The results represent the mean±s.e.m. *P<0.05 and **P<0.01.
Increase in epithelial cell proliferation by epithelial Mig-6 ablation in the mouse uterus. (a) Quantification of Ki67-positive cells in epithelial cells of Mig-6f/f and Sprr2fcre+Mig-6f/f mice. (b) Immunohistochemical analysis of Ki67 in Mig-6f/f and Sprr2fcre+Mig-6f/f mice. The results represent the mean±s.e.m. *P<0.05.
Inhibition of STAT3 by interaction with MIG-6
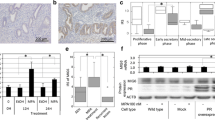
STAT3 is an MIG-6-associated protein26 and has an important part in cell proliferation.31, 38 Therefore, we examined the level of STAT3 with an immunohistochemical analysis in the uterus of female control and mutant mice at 10 weeks of age. Levels of phosphorylated STAT3 were significantly higher in the uterine epithelium of mutant mice compared with control mice (Figures 4a and b); however, phosphorylated STAT3 in stromal cells of mutant mice showed no change. In addition, total STAT3 levels were not different in uterine stromal and epithelial cells of female control and mutant mice (Figures 4c and d). In order to analyze whether MIG-6 physically interacts with STAT3 to suppress its phosphorylation, we co-transfected FLAG-tagged MIG-6 and/or V5-tagged STAT3 expression vectors to Ishikawa human endometrial adenocarcinoma cell line, and the cell lysates were immunoprecipitated with FLAG antibodies (Figure 4e). Next, we performed immunoprecipitation using protein lysates from the uteri of control and Pgrcre/+Mig-6f/f mice. Immunoprecipitation was applied with anti-STAT3, anti-MIG-6 and anti-IgG antibodies, and then examined by western blot analysis to identify an interaction between MIG-6 and STAT3. We were able to demonstrate the interaction between MIG-6 and STAT3 in the mouse uterus (Figure 4f). The results showed that MIG-6 physically interacts with STAT3 protein.
Inhibition of STAT3 phosphorylation by interacting with MIG-6. Quantification of pSTAT3- (a) and STAT3- (c) positive cells in epithelial cells of Mig-6f/f and Sprr2fcre+Mig-6f/f mice. Immunohistochemical analysis of pSTAT3 (b) and STAT3 (d) in Mig-6f/f and Sprr2fcre+Mig-6f/f mice. The protein interaction between MIG-6 and STAT3 by immunoprecipitation and western blot analysis in vitro (e) and in vivo (f). The results represent the mean±s.e.m. *P<0.05.
MIG-6 suppresses STAT3 phosphorylation
To investigate whether MIG-6 affects phosphorylation of STAT3, we co-transfected an MIG-6-expressed vector to Ishikawa cells, and treated with or without leukemia inhibitory factor, a known activator of STAT3, for 10 min.39 Our western blot analysis revealed that phosphorylation of STAT3 was increased by leukemia inhibitory factor. The MIG-6 overexpression significantly decreased STAT3 phosphorylation (Figures 5a and b). Our results indicate that MIG-6 suppresses the phosphorylation of STAT3 in endometrial epithelial cells.
Regulation of STAT3 activity by MIG-6. (a) Flag-tagged MIG-6-transfected Ishikawa cell lysates were analyzed by western blotting in the presence or absence of leukemia inhibitory factor (LIF; 100 ng/ml) treatment for 10 min. (b) Intensity of pSTAT3 was obtained using Image J software (National Institute of Health, Bethesda, MD, USA) for western blot analysis. The results represent the mean±s.e.m. ***P<0.001.
Prevention of the development of endometrial hyperplasia in epithelial Mig-6-ablated mouse uterus by P4 treatment
To determine the responsiveness of P4 on endometrial hyperplasia development in mutant mice, we treated 9-week-old female control and mutant mice with vehicle or P4 for 1 week by subcutaneous injection. Mutant mice that were treated with vehicle exhibited a significantly higher uterine weight, and an increase in gross size compared with vehicle-treated control mice. The histological analysis showed endometrial hyperplasia in mutant mice treated with vehicle. However, there was no difference in uterine weight and gross size between female control and mutant mice after P4 treatment (Figures 6a and b). Whereas mutant mice treated with vehicle developed endometrial hyperplasia in the uterus, P4-treated mice showed a normal endometrium (Figure 6c). We could not observe any difference between female control and mutant mice after the P4 treatment. These data propose that mutant mice were responsive to P4 and that this prevented the development of endometrial hyperplasia.
Prevention of endometrial hyperplasia in Sprr2fcre+Mig-6f/f mouse uterus by progesterone treatment. (a) The ratio of uterine weight to body weight of Mig-6f/f and Sprr2fcre+Mig-6f/f mice after P4 treatment. (b) Morphology of Mig-6f/f and Sprr2fcre+Mig-6f/f mice after P4 treatment. (c) Histology of uteri from Mig-6f/f and Sprr2fcre+Mig-6f/f mice after P4 treatment. The results represent the mean±s.e.m. *P<0.05.
Inhibition of active phosphorylation of STAT3 in epithelial Mig-6-ablated mouse uterus by P4 treatment
To analyze whether the observed prevention of hyperplastic phenotype was in response to recovered STAT3 signaling and proliferation, we investigated the level of epithelial cell proliferation and phosphorylation of STAT3 in the uterus of mutant mice treated for 1 week with vehicle or P4 at 9 weeks of age. Immunohistochemistry analysis results showed that levels of proliferation were significantly lowered in the P4-treated mutant mouse uterus in comparison with vehicle-treated mutant mice. In addition, phosphorylation levels of STAT3 were decreased in the uterus of mutant mice after P4 for 1 week as compared with vehicle. The level of total STAT3, however, was not affected by P4 treatment (Figure 7). These results demonstrate that P4 treatment prevents the endometrial hyperplasia development in uterine epithelial Mig-6 ablation by inhibiting STAT3 phosphorylation and endometrial epithelial cell proliferation.
Inhibition of active epithelial proliferation in Sprr2fcre+Mig-6f/f mice by progesterone treatment. (a) Quantification of Ki67-, pSTAT3- and STAT3-positive cells in epithelial cells of Mig-6f/f and Sprr2fcre+Mig-6f/f mice after P4 treatment. (b) Immunohistochemical analysis of Ki67, pSTAT3 and STAT3 in vehicle and P4-treated Sprr2fcre+Mig-6f/f mice. The results represent the mean±s.e.m. ***P<0.001.
Discussion
Mig-6 functions as a tumor suppressor through an antiproliferative role in humans.17, 18, 19, 20 We previously classified Mig-6 as a target gene of the PGR.24 Uterine-specific ablation of Mig-6 allows for the progression of endometrial hyperplasia and E2-dependent endometrial cancer due to an increase in endometrial epithelial cell proliferation by excessive E2 signaling in mice.24 To comprehend the function of epithelial Mig-6 in the uterus, we created a mouse model in which Mig-6 gene expression was ablated specifically in the Wnt7a-expressing cells (Wnt7acre+ Mig-6f/f mice).25 Wnt7acre+Mig-6f/f mice revealed a higher level of epithelial cell proliferation and an increase in the progression of endometrial hyperplasia and E2-dependent endometrial cancer.25 However, Wnt7a-Cre mice showed cre recombinase activities in skin as well as in ovarian and uterine epithelium.40 Wnt7acre+ Mig-6f/f mice have the limitation to examine the pathophysiology and tumorigenesis using steroid hormone pellets because of tumor formation at any surgical wounds in the skin. In the present study, we generated another uterine epithelium-specific Mig-6 knockout mouse model to evaluate the function of epithelial Mig-6 using a Sprr2f-cre mouse model.35 The small proline-rich protein 2F (Sprr2f) gene is specifically expressed in endometrial epithelial cells including both the luminal and glandular compartments, but not in endometrial stroma, myometrium and skin.35 Sprr2fcre+Mig-6f/f mice can overcome the limitation of the cre recombinase expression in the skin of Wnt7acre+ Mig-6f/f mice.
Sprr2fcre+Mig-6f/f mice showed development of endometrial hyperplasia from 10 weeks of age as observed in Wnt7acre+Mig-6f/f mice. Endometrioid-type endometrial adenocarcinoma and hyperplasia are associated with unopposed E2 exposure and continually increased proliferation of epithelial cells.3, 37 Levels of epithelial cell proliferation were significantly higher in the mutant mice compared with control mice at 10 weeks of age. These results suggest that increased proliferation in endometrial epithelial cells leads to the progression of endometrial hyperplasia and endometrial cancer.
Consistent activation of STAT3 leads to aberrant cell proliferation in carcinogenesis,41 indicating that STAT3 is a critical regulator of cancer cell proliferation and apoptosis. Here, we demonstrated that levels of STAT3 phosphorylation were significantly higher in the endometrial epithelial cells of mutant mice compared with control mice at the development of endometrial hyperplasia. We demonstrated that MIG-6 negatively regulates STAT3 phosphorylation through direct protein interactions in vivo and in vitro. Increased phosphorylation of STAT3 by leukemia inhibitory factor, which in turn induces further phosphorylation of STAT3, is significantly decreased by overexpressed MIG-6. These data indicate that MIG-6 inhibits uterine epithelial cell proliferation through inhibiting STAT3 phosphorylation. The progression and development of endometrial tumorigenesis is related to aberrant activation of STAT3 in endometrial epithelial cells of mutant mice.
P4 and E2, ovarian steroid hormones, are critical in the mediation of uterine events related to the establishment and maintenance of pregnancy42 as well as regulation of epithelial–stromal crosstalk through their cognate nuclear receptors.6 An imbalance of steroid hormones initiated by elevated levels of E2 and/or decreased P4 action can lead to aberrant endometrial proliferation and endometrial cancer.43 Clarifying the molecular mechanisms that regulate E2 and P4 in the uterus is paramount for understanding the pathophysiology of endometrial cancer.
There have been attempts for fertility preservation in premenopausal women with endometrial cancer through conservative treatment with high-dose P4.44 P4 can suppress the proliferation of endometrial cancer through inhibition of E2 action.45 The antagonistic effect of P4 on E2 supports the rationale for progestin-based therapy for endometrial cancer.11 To address the preventative role of P4 on endometrial hyperplasia, we treated mice with P4 for 1 week, beginning at 9 weeks of age. Female mutant mice did not exhibit an endometrial hyperplasia phenotype after P4 treatment. Mutant mice treated with P4 for 1 week showed a decrease in epithelial cell proliferation and phosphorylation level of STAT3 in the uterine epithelium. The uterus is made up of heterogeneous cell types that go through dynamic changes in order to support embryo development and implantation. These changes primarily rely on coordinated interactions mediated by P4 and E2. E2 induces epithelial proliferation in the murine uterus.46 Meanwhile, P4 inhibits E2-induced proliferation of the glandular and luminal epithelial cells. However, P4 or P4 with E2 leads to stromal cell proliferation in the uterus.46 P4 suppresses E2-stimulated epithelial proliferation via regulating stromal cell gene expressions.9 However, the mediators involved in these regulatory cell–cell interactions have not been known. We have shown that activation of stromal P4 signaling including Mig-6 has an impact on endometrial tumorigenesis. These indicate that stromal Mig-6 is a mediator for the ability of P4 to regulate E2-induced uterine proliferation.24 An understanding of the actions of hormones on the uterus requires elucidation of the mechanism of stromal and epithelial communication with each other and, further, how this epithelial–stromal crosstalk is transformed by hormonal binding to stromal versus epithelial mediators. These results provide evidence that activated stromal P4 signaling along with Mig-6 may have a role in the prevention of endometrial hyperplasia of mutant mice by inhibition of STAT3 activity. Furthermore, these data suggest that treatment with a STAT3 inhibitor could be an alternative way to overcome epithelial proliferation in endometrial hyperplasia.
Overall, these findings show that loss of Mig-6 in the endometrial epithelial cells results in endometrial hyperplasia in response to an increase in epithelial cell proliferation. MIG-6 negatively regulates the phosphorylation of STAT3 via direct protein interaction with STAT3. P4 treatment prevents the development of endometrial hyperplasia in mutant mouse uteri through inhibition of epithelial cell proliferation and excessive activation of STAT3 by P4-induced stromal Mig-6. Therefore, our studies provide a framework for understanding endometrial cancer development, and a useful animal model for studying new therapies in the treatment and prevention of endometrial cancer.
Materials and methods
Mouse tissue samples
All mouse experiments were cared for according to the protocol approved by the Institutional Animal Care and Use Committee of Michigan State University. The mice with epithelial cell-specific Mig-6 knockout in the uterus were generated using the Sprr2f-cre mouse model.35 To determine the endometrial hyperplasia development and P4 effects, vehicle (beeswax) or P4 (40 mg/pellet) pellet was injected subcutaneously into female control and mutant mice, respectively, beginning at 9 weeks of age for 1 week before killing.
Immunohistochemistry and immunofluorescence analyses
Immunostaining analyses were performed as previously described.47 Briefly, uterine sections were incubated with appropriate primary antibodies, anti-MIG-6 (Customized antibody by Dr Jeong Lab), anti-pSTAT3 (CS-9131; Cell Signaling, Danvers, MA, USA), anti-STAT3 (CS-4904; Cell Signaling) and anti-Ki67 (ab15580; Abcam, Cambridge, MA, USA), in 10% normal goat serum in phosphate-buffered saline overnight at 4 °C. For immunohistochemistry, sections were incubated with secondary antibody (Vector Laboratories, Burlingame, CA, USA) and detected using the Vectastain Elite DAB Kit (Vector Laboratories). For immunofluorescence, the sections were incubated with secondary antibody conjugated to Alexa Fluor 488-conjugated anti-mouse IgG (Invitrogen Corp., Carlsbad, CA, USA) for 2 h at room temperature. Then, the sections were mounted with 4,6-diamidino-2-phenylindole (Vector Laboratories) to enable nuclear visualization. The immunohistochemical staining intensities were graded by H-score. The H-score was calculated as previously reported.48
Cell culture and transient transfection
Ishikawa cells were cultured in Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12 (Gibco BRL, Gaithersburg, MD, USA) with 10% (v/v) fetal bovine serum (Gibco BRL) and 1% (v/v) penicillin–streptomycin (Gibco BRL) at 37 °C under 5% CO2. FLAG-tagging MIG-6 and V5-tagging STAT3 expression vectors were transfected using Lipofectamine 2000 reagent (Invitrogen Corp.) in accordance with the manufacturer’s instructions.
Immunoprecipitation
Immunoprecipitation was performed as described previously.49 Briefly, 0.5 μg of lysates were immunoprecipitated with 1 μg of antibodies to FLAG (F1804; Sigma-Aldrich, St Louis, MO, USA), STAT3 (CS-4904; Cell Signaling) or MIG-6 (Customized antibody by Dr Jeong Lab) with 30 μl of resuspended protein A-agarose (Pierce Biotechnology, Rockford, IL, USA) and incubated overnight at 4 °C. Immunocomplexes were applied to sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membrane (Millipore Corp., Bedford, MA, USA). The membrane was exposed to anti-V5 (A190-220A; Bethyl Laboratories, Montgomery, TX, USA), anti-FLAG and anti-STAT3 antibodies.
Statistical analysis
For all animal experiments, the samples were not predetermined using any statistical method. On the basis of our previous studies, five mice per group were used for all experiments to attain proper statistical power. A balance in sample size across groups was ensured by block randomization. To evaluate the result variations in group, the investigators were blinded to the group. There are no excluded samples and animals. In vitro experiments were conducted three times, and results are presented as the mean±s.e.m. of three biological replicates. Student’s t-test was used for two groups. An analysis of variance test was used for more than two groups, followed by Tukey or Bonferroni test for pairwise t-test. All statistical tests were analyzed by the GraphPad Prism 5 (San Diego, CA, USA). *P<0.05, **P<0.01 and ***P<0.001.
References
Siegel RL, Miller KD, Jemal A . Cancer statistics, 2016. CA Cancer J Clin 2016; 66: 7–30.
Di Cristofano A, Ellenson LH . Endometrial carcinoma. Annu Rev Pathol 2007; 2: 57–85.
Kurman RJ, Kaminski PF, Norris HJ . The behavior of endometrial hyperplasia. A long-term study of "untreated" hyperplasia in 170 patients. Cancer 1985; 56: 403–412.
Critchley HO, Saunders PT . Hormone receptor dynamics in a receptive human endometrium. Reprod Sci 2009; 16: 191–199.
Graham JD, Clarke CL . Physiological action of progesterone in target tissues. Endocr Rev 1997; 18: 502–519.
Rubel CA, Jeong JW, Tsai SY, Lydon JP, Demayo FJ . Epithelial-stromal interaction and progesterone receptors in the mouse uterus. Semin Reprod Med 2010; 28: 27–35.
Lee JH, Kim TH, Oh SJ, Yoo JY, Akira S, Ku BJ et al. Signal transducer and activator of transcription-3 (Stat3) plays a critical role in implantation via progesterone receptor in uterus. FASEB J 2013; 27: 2553–2563.
Franco HL, Rubel CA, Large MJ, Wetendorf M, Fernandez-Valdivia R, Jeong JW et al. Epithelial progesterone receptor exhibits pleiotropic roles in uterine development and function. FASEB J 2012; 26: 1218–1227.
Kurita T, Lee KJ, Cooke PS, Lydon JP, Cunha GR . Paracrine regulation of epithelial progesterone receptor and lactoferrin by progesterone in the mouse uterus. Biol Reprod 2000; 62: 831–838.
Kim JJ, Kurita T, Bulun SE . Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev 2013; 34: 130–162.
Hahn HS, Yoon SG, Hong JS, Hong SR, Park SJ, Lim JY et al. Conservative treatment with progestin and pregnancy outcomes in endometrial cancer. Int J Gynecol Cancer 2009; 19: 1068–1073.
Yang S, Thiel KW, Leslie KK . Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab 2011; 22: 145–152.
Decruze SB, Green JA . Hormone therapy in advanced and recurrent endometrial cancer: a systematic review. Int J Gynecol Cancer 2007; 17: 964–978.
van Laar T, Schouten T, van der Eb AJ, Terleth C . Induction of the SAPK activator MIG-6 by the alkylating agent methyl methanesulfonate. Mol Carcinog 2001; 31: 63–67.
Zhang YW, Vande Woude GF . Mig-6, signal transduction, stress response and cancer. Cell Cycle 2007; 6: 507–513.
Wick M, Burger C, Funk M, Muller R . Identification of a novel mitogen-inducible gene (mig-6): regulation during G1 progression and differentiation. Exp Cell Res 1995; 219: 527–535.
Fiorini M, Ballaro C, Sala G, Falcone G, Alema S, Segatto O . Expression of RALT, a feedback inhibitor of ErbB receptors, is subjected to an integrated transcriptional and post-translational control. Oncogene 2002; 21: 6530–6539.
Lin CI, Du J, Shen WT, Whang EE, Donner DB, Griff N et al. Mitogen-inducible gene-6 is a multifunctional adaptor protein with tumor suppressor-like activity in papillary thyroid cancer. J Clin Endocrinol Metab 2011; 96: E554–E565.
Reschke M, Ferby I, Stepniak E, Seitzer N, Horst D, Wagner EF et al. Mitogen-inducible gene-6 is a negative regulator of epidermal growth factor receptor signaling in hepatocytes and human hepatocellular carcinoma. Hepatology 2010; 51: 1383–1390.
Ying H, Zheng H, Scott K, Wiedemeyer R, Yan H, Lim C et al. Mig-6 controls EGFR trafficking and suppresses gliomagenesis. Proc Natl Acad Sci USA 2010; 107: 6912–6917.
Anastasi S, Sala G, Huiping C, Caprini E, Russo G, Iacovelli S et al. Loss of RALT/MIG-6 expression in ERBB2-amplified breast carcinomas enhances ErbB-2 oncogenic potency and favors resistance to Herceptin. Oncogene 2005; 24: 4540–4548.
Duncan CG, Killela PJ, Payne CA, Lampson B, Chen WC, Liu J et al. Integrated genomic analyses identify ERRFI1 and TACC3 as glioblastoma-targeted genes. Oncotarget 2010; 1: 265–277.
Zhang YW, Staal B, Su Y, Swiatek P, Zhao P, Cao B et al. Evidence that MIG-6 is a tumor-suppressor gene. Oncogene 2007; 26: 269–276.
Jeong JW, Lee HS, Lee KY, White LD, Broaddus RR, Zhang YW et al. Mig-6 modulates uterine steroid hormone responsiveness and exhibits altered expression in endometrial disease. Proc Natl Acad Sci USA 2009; 106: 8677–8682.
Kim TH, Lee DK, Cho SN, Orvis GD, Behringer RR, Lydon JP et al. Critical tumor suppressor function mediated by epithelial Mig-6 in endometrial cancer. Cancer Res 2013; 73: 5090–5099.
Kim TH, Franco HL, Jung SY, Qin J, Broaddus RR, Lydon JP et al. The synergistic effect of Mig-6 and Pten ablation on endometrial cancer development and progression. Oncogene 2010; 29: 3770–3780.
Pirone DM, Carter DE, Burbelo PD . Evolutionary expansion of CRIB-containing Cdc42 effector proteins. Trends Genet 2001; 17: 370–373.
Makkinje A, Quinn DA, Chen A, Cadilla CL, Force T, Bonventre JV et al. Gene 33/Mig-6, a transcriptionally inducible adapter protein that binds GTP-Cdc42 and activates SAPK/JNK. A potential marker transcript for chronic pathologic conditions, such as diabetic nephropathy. Possible role in the response to persistent stress. J Biol Chem 2000; 275: 17838–17847.
Furtek SL, Backos DS, Matheson CJ, Reigan P . Strategies and approaches of targeting STAT3 for cancer treatment. ACS Chem Biol 2016; 11: 308–318.
Xiong A, Yang Z, Shen Y, Zhou J, Shen Q . Transcription factor STAT3 as a novel molecular target for cancer prevention. Cancers 2014; 6: 926–957.
Yu H, Kortylewski M, Pardoll D . Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol 2007; 7: 41–51.
Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C et al. Stat3 as an oncogene. Cell 1999; 98: 295–303.
Chen CL, Hsieh FC, Lieblein JC, Brown J, Chan C, Wallace JA et al. Stat3 activation in human endometrial and cervical cancers. Br J Cancer 2007; 96: 591–599.
Wang X, Crowe PJ, Goldstein D, Yang JL . STAT3 inhibition, a novel approach to enhancing targeted therapy in human cancers (review). Int J Oncol 2012; 41: 1181–1191.
Contreras CM, Akbay EA, Gallardo TD, Haynie JM, Sharma S, Tagao O et al. Lkb1 inactivation is sufficient to drive endometrial cancers that are aggressive yet highly responsive to mTOR inhibitor monotherapy. Dis Models Mech 2010; 3: 181–193.
Ferby I, Reschke M, Kudlacek O, Knyazev P, Pante G, Amann K et al. Mig6 is a negative regulator of EGF receptor-mediated skin morphogenesis and tumor formation. Nat Med 2006; 12: 568–573.
Ambros RA . Simple hyperplasia of the endometrium: an evaluation of proliferative activity by Ki-67 immunostaining. Int J Gynecol Pathol 2000; 19: 206–211.
Li L, Shaw PE . Autocrine-mediated activation of STAT3 correlates with cell proliferation in breast carcinoma lines. J Biol Chem 2002; 277: 17397–17405.
Cheng JG, Chen JR, Hernandez L, Alvord WG, Stewart CL . Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proc Natl Acad Sci USA 2001; 98: 8680–8685.
Daikoku T, Ogawa Y, Terakawa J, Ogawa A, DeFalco T, Dey SK . Lactoferrin-iCre: a new mouse line to study uterine epithelial gene function. Endocrinology 2014; 155: 2718–2724.
Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C et al. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res 2011; 71: 7226–7237.
Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA Jr. et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev 1995; 9: 2266–2278.
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C et al. Cancer statistics, 2006. CA Cancer J Clin 2006; 56: 106–130.
Bovicelli A, D'Andrilli G, Giordano A, De Iaco P . Conservative treatment of early endometrial cancer. J Cell Physiol 2013; 228: 1154–1158.
Kaunitz AM . Injectable depot medroxyprogesterone acetate contraception: an update for U.S. clinicians. Int J Fertil Womens Med 1998; 43: 73–83.
Huet-Hudson YM, Andrews GK, Dey SK . Cell type-specific localization of c-myc protein in the mouse uterus: modulation by steroid hormones and analysis of the periimplantation period. Endocrinology 1989; 125: 1683–1690.
Kim BG, Yoo JY, Kim TH, Shin JH, Langenheim JF, Ferguson SD et al. Aberrant activation of signal transducer and activator of transcription-3 (STAT3) signaling in endometriosis. Hum Reprod 2015; 30: 1069–1078.
Ishibashi H, Suzuki T, Suzuki S, Moriya T, Kaneko C, Takizawa T et al. Sex steroid hormone receptors in human thymoma. J Clin Endocrinol Metab 2003; 88: 2309–2317.
Lee JH, Kim TH, Oh SJ, Yoo JY, Akira S, Ku BJ et al. Signal transducer and activator of transcription-3 (Stat3) plays a critical role in implantation via progesterone receptor in uterus. FASEB J 2013; 27: 2553–2563.
Acknowledgements
We would like to thank Dr Diego H Castrillon (University of Texas Southwestern Medical Center, Dallas, TX, USA) for Sprr2f-cre mice. We would also like to thank Hanna E Teasley and Paige Wells for manuscript preparation. This work was supported by America Cancer Society, RSG-12-084-01-TBG and National Institutes of Health (NIH, HD084478) to JWJ and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2016R1D1A1B03934346) to JYY.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Yoo, JY., Yang, W., Lee, J. et al. MIG-6 negatively regulates STAT3 phosphorylation in uterine epithelial cells. Oncogene 37, 255–262 (2018). https://doi.org/10.1038/onc.2017.335
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.335
- Springer Nature Limited
This article is cited by
-
Selumetinib overcomes gefitinib primary and acquired resistance by regulating MIG6/STAT3 in NSCLC
Archives of Pharmacal Research (2023)
-
Loss of MIG-6 results in endometrial progesterone resistance via ERBB2
Nature Communications (2022)
-
MIG-6 suppresses endometrial epithelial cell proliferation by inhibiting phospho-AKT
BMC Cancer (2018)