Abstract
Many procedures in modern clinical medicine rely on the use of electronic implants in treating conditions that range from acute coronary events to traumatic injury1,2. However, standard permanent electronic hardware acts as a nidus for infection: bacteria form biofilms along percutaneous wires, or seed haematogenously, with the potential to migrate within the body and to provoke immune-mediated pathological tissue reactions3,4. The associated surgical retrieval procedures, meanwhile, subject patients to the distress associated with re-operation and expose them to additional complications5,6,7,8. Here, we report materials, device architectures, integration strategies, and in vivo demonstrations in rats of implantable, multifunctional silicon sensors for the brain, for which all of the constituent materials naturally resorb via hydrolysis and/or metabolic action9,10,11,12, eliminating the need for extraction. Continuous monitoring of intracranial pressure and temperature illustrates functionality essential to the treatment of traumatic brain injury2,13; the measurement performance of our resorbable devices compares favourably with that of non-resorbable clinical standards. In our experiments, insulated percutaneous wires connect to an externally mounted, miniaturized wireless potentiostat for data transmission. In a separate set-up, we connect a sensor to an implanted (but only partially resorbable) data-communication system, proving the principle that there is no need for any percutaneous wiring. The devices can be adapted to sense fluid flow, motion, pH or thermal characteristics, in formats that are compatible with the body’s abdomen and extremities, as well as the deep brain, suggesting that the sensors might meet many needs in clinical medicine.
Similar content being viewed by others
Main
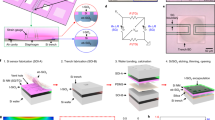
Figure 1a and Supplementary Fig. 1 show a bioresorbable pressure sensor with a magnified illustration of the active region and its cross-sectional side view. The construction involves a membrane of poly(lactic-co-glycolic acid) (PLGA, with a thickness of 30 μm), sealed against a supporting substrate of nanoporous silicon (60–80 μm thick; 71% porosity) or magnesium foil (60–80 μm thick; see Supplementary Figs 2, 3). The substrate has a square structure of relief (with a depth of 30–40 μm) etched onto its surface. The associated air cavity allows the membrane to deflect in response to pressure in the fluid surroundings. A silicon nanomembrane in a serpentine geometry serves as a piezoresistive element that rests on the surface of the membrane near one of the edges of the cavity, where deflection-induced strains are largest (Fig. 1b). The resistance of this sensing element increases monotonically in a linear fashion across the full range of pressures that are relevant to intracranial monitoring (that is, 0–70 mm Hg). An overcoat of silicon oxide (SiO2, about 100 nm thick) provides electrical passivation and a barrier against biofluids. Figure 1c and Supplementary Fig. 4 show photographs of two representative devices of different dimensions to illustrate the scalability of fabrication; the total sizes and weights are 1 mm × 2 mm × 0.08 mm (trench size: 0.67 mm × 0.8 mm × 0.03 mm) and about 0.4 mg; and 3 mm × 6 mm × 0.11 mm (trench size: 2 mm × 2.4 mm × 0.04 mm) and roughly 1 mg, respectively.
a, Schematic illustration of a biodegradable pressure sensor. The inset shows the location of the silicon-nanomembrane (Si-NM) strain gauge. b, Optical micrograph of the strain-gauge region. ‘Trench BD’, boundary of the trench. c, Image of a complete device. The outer diameter of the hypodermic needle is 1 mm. d, Left, distribution of principal strains across the PLGA layer, including the Si-NM strain gauge at the left edge, determined from finite element analysis (FEA) for an external pressure of 50 mm Hg. Right, corresponding displacement profile evaluated along the red dotted line in the left panel. εmax and dz are the principal strain and vertical displacement, respectively. e, Responses of a commercial pressure sensor (blue) and a calibrated biodegradable device (red) to time-varying pressure over a range relevant to intracranial monitoring. f, Response of a similar biodegradable device (red), but configured as an accelerometer, with comparison to a commercial sensor (blue). g, Comparison of the calibrated response of such a bioresorbable temperature sensor (red) to a commercial device (blue). h, The difference in temperature measured by two separate Si-NM temperature sensors placed near a Si-NM element for Joule heating allows assessment of flow rate. i, A single serpentine Si-NM used as both a temperature sensor and a heating element allows measurements of thermal conductivity and heat capacity. The graph shows time-dependent changes in temperature upon actuation of Joule heating in devices immersed in different liquids. The coefficients of thermal conductivity (ĸ, measured through the rate of resistance change) of hexane, toluene, ethylene glycol, and water are 0.12, 0.13, 0.26, and 0.60 W m−1 K−1, respectively. j, When the Si-NM is exposed to aqueous surroundings, its conductance depends on pH. The graph shows measurements for immersion in solutions with pH values between 2 and 10. k, Images collected at several stages of accelerated dissolution of a bioresorbable pressure sensor upon insertion into an aqueous buffer solution (pH 12) in a transparent PDMS enclosure at room temperature.
The mechanics of the system can be captured quantitatively by three-dimensional finite element analysis (FEA). Distributions of principal strains and vertical displacements evaluated at an external pressure of 50 mm Hg appear in Fig. 1d. The maximum strain for any applied pressure over the range of interest occurs at the midpoint of the left (and right) edge of the trench, thus motivating this choice of location for the silicon-nanomembrane piezoresistive element (see Supplementary Methods and Supplementary Figs 5 and 6 for details)14. The calibration between pressure and resistance is linear, with a slope of 83 Ω(mm Hg)−1, consistent with modelling results and a gauge factor of about 30, which lies within a range of expected values for monocrystalline silicon (Supplementary Fig. 7)15.
Evaluations in set-ups that resemble the intracranial cavity reveal measured pressure responses that agree quantitatively with those of clinical-standard, non-bioresorbable sensors (Fig. 1e and Supplementary Figs 8–10). With various simple modifications, this same platform can be used for precision measurement of other parameters of interest in biomedicine and clinical care. Examples include: motion sensors built with a cantilevered test mass of PLGA (that is, a single-axis accelerometer, Fig. 1f); temperature sensors that exploit the temperature-dependent resistance of silicon-nanomembrane elements set apart from the cavity structure (Fig. 1g); flow sensors in which the silicon nanomembranes serve simultaneously as heating elements and temperature sensors (Fig. 1h); thermal conductivity/diffusivity sensors that exploit related concepts (Fig. 1i); and pH sensors that rely on electrostatic gating of transport through the silicon nanomembrane (Fig. 1j). In addition, chemically functionalizing the surface of the silicon of this last device provides a route to biomolecular sensing, using schemes similar to those in conventional silicon biosensors16,17,18. The fabrication methods and operating principles for each of the modalities in Fig. 1f–j appear in Supplementary Methods and Supplementary Figs 11–15.
The uniqueness of these devices is their ability to dissolve completely into biocompatible end products when immersed in aqueous solutions, including biofluids such as cerebrospinal fluid (CSF). Hydrolysis of the silicon nanomembranes, the layers of SiO2, the thin wafers of nanoporous silicon and the magnesium foils causes loss of material at rates of 23 nm day−1, 8 nm day−1, 9 μm day−1 and 4 μm day−1, respectively, in artificial CSF (ACSF) at physiological temperature (37 °C) (Supplementary Fig. 16). Separate studies indicate that PLGA (75:25 (lactide:glycolide) composition) dissolves in biofluids within four to five weeks19. To illustrate the various stages of dissolution of a completed system, Fig. 1k shows a sequence of images of a bioresorbable pressure sensor inserted into a transparent chamber designed for accelerated testing (polydimethylsiloxane (PDMS) enclosure filled with buffer solution at pH 12 and room temperature), in which fluid exchange can occur through an array of openings around the perimeter (Supplementary Fig. 17). Supplementary Fig. 18 presents images of nanoporous silicon and silicon nanomembranes observed by scanning electron microscopy at different times during hydrolysis. The silicon nanomembrane dissolves uniformly, without fracture. By comparison, nanoporous silicon dissolves less uniformly, with a tendency to form fragments. Here, the silicon-nanomembrane and SiO2 components dissolve first, within 15 hours, followed by the nanoporous silicon, which disappears within 30 hours. In all cases, the dissolution kinetics depends strongly on the materials and the composition of the surrounding solution20,21,22. Supplementary Table 1 summarizes the hydrolysis mechanisms and dissolution rates of these materials in a representative solution. As described below, the encapsulation material and its thickness define the operational lifetimes.
Figure 2 illustrates a strategy for using these types of bioresorbable systems for wireless pressure and temperature monitoring in the intracranial space of rats. Figure 2a shows a photograph of a device like the one in Fig. 1c, but configured to allow simultaneous sensing of both pressure and temperature. The measured temperature can also be used to calibrate against parasitic effects of this parameter on the pressure determination (see Supplementary Methods and Supplementary Fig. 19). Biodegradable molybdenum wires (10 μm thick) serve as an interface to wireless communication systems. Pressing the interconnect wires (molybdenum, 10 μm thick, or magnesium, 50 μm thick) against the PLGA at elevated temperatures (65 °C) embeds them near the surface but leaves the top regions exposed, thereby allowing for deposition of biodegradable metals (molybdenum, 2 μm thick) to form electrical contact pads through stencil masks (made from the polyimide Kapton, 12.5 μm thick; Supplementary Fig. 20). The deposited molybdenum forms stable interconnects between metal wires and silicon nanomembranes that are fully embedded on PLGA. Encapsulation with a bioresorbable polymer (polyanhydride, discussed in more detail below) enhances system robustness by reducing the stress concentrations at the interconnections. Narrow strips of PLGA laminated onto the front and back sides of the wires along their entire lengths act as electrical insulation. These insulated wires connect to an externally mounted, miniaturized wireless potentiostat for transmission of data thorough percutaneous wiring. Figure 2b provides a diagram of such a system in the intracranial space of a rat model. The sensor subsystem connects via molybdenum wires to the wireless module, which is mounted on the top of the skull. Figure 2c–e summarizes the surgical process. A PLGA sheet (about 80 μm thick) and a dissolvable surgical glue (Fig. 2c) seal the craniectomy defect to close the intracranial cavity. Conventional sutures hold the surgical site closed, in a standard process23 that retains points at which the dissolvable wires emerge from the skin to allow electrical connection (Fig. 2d). These wires have dimensions comparable to those of the surgical threads, and therefore pose little additional risk. Figure 2e shows a healthy, freely moving rat with a complete system. Supplementary Fig. 21 presents images of the connections.
a, Image of bioresorbable pressure and temperature sensors integrated with dissolvable metal interconnects (sputtered molybdenum, Mo, 2 μm thick) and wires (Mo, 10 μm thick). The inset shows an optical micrograph of the serpentine Si-NM structures that form the sensing regions. The Si-NM that is not above the air cavity (left) responds only to temperature; the one at the edge of the air cavity (right) responds primarily to pressure. b, Diagram of a bioresorbable sensor system in the intracranial space of a rat, with electrical interconnects that provide an interface to an external wireless data-transmission unit for long-range operation. c, d, Demonstrations of c, an implanted bioresorbable sensor in a rat, and d, a sutured individual. A thin film of PLGA (~80 μm) and a degradable surgical glue (TISSEAL) seal the craniectomy defect to close the intracranial cavity. e, Healthy, freely moving rat equipped with a complete, biodegradable wireless intracranial sensor system.
Figure 3 summarizes the results of a comprehensive set of wireless measurements of intracranial pressure (ICP) and intracranial temperature (ICT), recorded in rats with percutaneous wired systems. The ICP traces reveal features that correspond to periodic manual abdominal compression activating the Valsalva manoeuvre, which yields rapid increases or decreases in ICP (Fig. 3a)24. Gentle changes in the rat’s position—that is, Trendelenburg (30° head-down position) and reverse Trendelenburg (30° head-up position)—produce gradual increases and decreases in ICP, respectively (Fig. 3b), as would be expected because of the corresponding accumulation and depletion of blood in the brain25. The pressure values compare well with those determined using a clinical-standard, wired ICP sensor implanted in the same region of the same animal. The wireless, bioresorbable ICT sensors perform to levels of accuracy similar to those of commercial sensors: Fig. 3c and d show comparative data collected by modulating the cranial temperature with a heating or cooling blanket placed beneath the animal.
a–e, Red, data from a transient, bioresorbable sensor; blue, data from a commercial sensor. a, Real-time wireless measurements of ICP, showing transient increases induced by the Valsalva manoeuvre. b, In vivo observation of changes in ICP as a function of time in the Trendelenburg and reverse Trendelenburg positions. ICP increases in the 30° head-down position (Trendelenburg) as compared with the supine position, and decreases in the 30° head-up position (reverse Trendelenburg). c, Gradual increase and d, decrease in ICT due to application of a heating/cooling blanket. e, Measurements of ICP over three days reveal consistent responses from devices encapsulated with biodegradable polyanhydride. f, Confocal fluorescence images of the cortical surface beneath the dissolved device at 2, 4 and 8 weeks, showing the absence of inflammatory responses. The images are double-immunostained for GFAP (glial fibrillary acidic protein) to detect astrocytes (red), and Iba1 (ionized calcium-binding adaptor molecule 1) to identify microglia/macrophages (green). The dashed line indicates the site of the implant.
The operational lifetimes of the devices are defined by dissolution of the encapsulation layers and the permeation of fluids through them. In vitro experiments using a bioresorbable pressure sensor encapsulated with a film of a specially synthesized polyanhydride (about 120 μm thick; Supplementary Fig. 22) show expected performance and accurate readings with an appropriately modified calibration factor (50 Ω(mm Hg)−1). The slow dissolution rate of the polyanhydride (about 1.3 μm day−1)—together with the modest change in sensitivity that occurs depending on the thickness of this material (about 0.34 Ω(mm Hg)−1 μm−1)—leads to a loss of accuracy of only a few per cent when operated over several days. This error falls within standards defined by the Association for the Advancement of Medical Instrumentation (AAMI) for pressure monitoring, that is, ±2 mm Hg (from 0 to 20 mm Hg) and ±10% (from 20 to 100 mm Hg)2. (Supplementary Methods and Supplementary Figs 23–25 present information on the synthesis/hydrolysis chemistry, dissolution kinetics, water permeability, and biocompatibility of the polyanhydride.)
Stable, continuous operation is possible for up to three days (Supplementary Fig. 26). Beyond this period, water tends to pass through the polyanhydride and PLGA into the electrically active regions of the device and the air cavity. The resistance remains relatively constant for seven days, and then begins to increase markedly, mainly because of dissolution of the molybdenum wires and interconnection metal (Supplementary Figs 27 and 28). Figure 3e illustrates in vivo operation for three days without notable degradation in absolute accuracy or sensitivity, as benchmarked against a standard, non-resorbable wired sensor. Supplementary Fig. 29 shows similar data from the temperature sensor, where the absence of an air cavity affords enhanced stability, and accurate measurements for six days of operation. These timeframes are relevant for clinical use: ICP and ICT are typically monitored continuously for several days after traumatic brain injury. The chemistry, thickness and composition of the encapsulating layers can be selected to extend the functional lifetimes26.
Biocompatibility of the devices through all stages of their life cycle is essential. Comprehensive studies of the immunohistochemistry of brain tissues at several times after implantation (two, four and eight weeks) demonstrate that the sensors and the by-products of their dissolution in the intracranial space are biocompatible. Representative confocal fluorescence images (see Fig. 3f for nanoporous silicon and Supplementary Fig. 30 for magnesium foil) indicate no overt reaction of brain glial cells to the sensor, and no focal aggregation of glial cells at the implantation site for all time ranges. Astrocytosis (an increase in the number of astrocyte cells) and microglial activity at the cortical surface are within normal limits, indicating no overt immune reaction to the device and its by-products. Although the percutaneous wiring does not noticeably affect animal behaviour (see Supplementary Methods and Supplementary Fig. 31), a miniaturized, fully implantable wireless communication system might offer advantages, by removing the possibility of secondary infection at the wires. A wireless system constructed mostly, but not entirely, of resorbable materials (~85% by mass and ~86% by volume)—using an advanced near-field communication-technology approach, with fully bioresorbable metal coils, substrates and encapsulation layers—appears in Extended Data Fig. 1, Methods, and Supplementary Figs 32–37.
Given that these devices function successfully in the intracranial space, they could also be used in other organs and body compartments. As an example, Fig. 4a and b illustrate ICP monitoring using the same bioresorbable device in modes with relevance to acute abdominal compartment syndrome and acute compartment syndrome of the extremity27,28. Furthermore, modifying the devices to allow them to be injected deep into tissues could address other needs in clinical treatment. For example, monitoring physiological parameters of the deep brain with intraparenchymal sensors could yield data that are unavailable from the surface or the intracranial space. In addition, because electrophysiological and metabolic abnormalities often emanate from infarcts, contusions and haematomas that damage adjacent intact tissue, sensors of pressure, temperature, pH and other physical/chemical parameters that are placed into the parenchyma within the blood-deprived (ischaemic) penumbra could advance our knowledge of secondary brain injury29,30,31. Such considerations apply not only to injured brain tissue, but also to acute or chronic ischaemia that threatens the heart, limbs, intra-abdominal organs or grafts.
Red, data from a transient biodegradable sensor; blue, data from a commercial sensor. a, b, Pressures measured in a, intra-abdominal and b, leg cavities. c, Image showing in vivo injection of a needle-shaped biodegradable pressure sensor (using a magnesium foil support, ~80 μm thick) into the brain parenchyma with a stereotactic frame and arm. The inset shows a biodegradable pressure sensor inserted into hydrogel, as evidence of the sensor’s robust mechanical construction. d, In vivo measurements of pressure in the deep brain. e, In vivo measurements of temperature in the deep brain during anaesthesia. The temperature drops during anaesthesia owing to reduced blood circulation, and returns to normal after awakening.
Modifying the geometry of the supporting structures introduced in Fig. 1 enables delivery of bioresorbable sensors into the depths of brain tissue, for direct measurements of injury or status. Figure 4c shows an example that integrates a bioresorbable ICP sensor onto a magnesium foil, formed with a tip region that allows injection into tissues of interest (Supplementary Fig. 38). Mounting the device on a stereotactic frame and fixture allows accurate positioning and controlled penetration (Supplementary Fig. 39). Figure 4d and e summarize pressure and temperature data collected at a site about 5 mm beneath the surface of the rat brain. The Valsalva manoeuvre yields data that quantitatively agree with those obtained using conventional sensors at a similar location. The device detected changes in temperature during anaesthesia (the temperature decreased, owing to reduced blood circulation) and waking up (the temperature returned to normal), as expected of intraparenchymal tissue.
The biomedical sensors reported here enable wireless data collection in body cavities and in deep tissues, with platforms that are fully bioresorbable, thereby allowing patients to be monitored until homeostasis has been achieved, and avoiding the risks associated with chronically implanted devices or their removal32,33. In vivo and in vitro experiments demonstrate precision measurements of pressure, temperature, motion, flow, thermal properties and pH, with possible extensions to biomolecular binding events. These features will be useful in diagnosing and treating a diverse range of medical conditions, from acute traumatic injuries such as extremity compartment syndrome, to chronic medical diseases such as diabetes. The materials, manufacturing methods and design layouts should be relevant to many other sensor modalities, with the potential for co-integration of advanced silicon-based integrated circuits, radio communication technologies, power supply and energy harvesters—each adapted from advances in transient electronics. Thus, it is realistic to expect that these devices could be used in sensing, recording, stimulating, and electrical control for medical monitoring and treatment, not only for the body regions explored here but also for areas such as the cardiac space and spinal system. Translating these technologies into clinical settings should provide patients and medical professionals with a vital set of tools for combating human disease.
Methods
Fabrication of bioresorbable silicon pressure sensors
Fabrication involved integration of silicon-based, piezoresistive sensing elements onto substrates of PLGA, bonded over cavities etched into the surfaces of nanoporous Si (np-Si) substrates or magnesium foils. Solid-state diffusion of boron yielded highly doped p-type monocrystalline silicon nanomembranes (Si-NMs) on silicon-on-insulator (SOI) wafers (top silicon ~300 nm thick, p-type; SOITEC, France). Eliminating the buried oxide with hydrofluoric acid allowed transfer of the Si-NMs onto a bilayer of D-PI (diluted polyimide (poly(pyromellitic dianhydride-co-4,4′-oxydianiline)), ~200 nm)/PMMA (poly(methyl methacrylate), ~300 nm) on temporary silicon carrier substrates. Photolithography and etching patterned the Si-NMs into structures with serpentine designs. Electron-beam evaporation and spin-casting defined uniform layers of SiO2 (~100 nm) and D-PI, respectively, to serve the purpose of passivation. Selective dry etching through all of the layers (D-PI/SiO2/D-PI/PMMA) formed a mesh structure that enabled release in acetone, for transfer to a film of PLGA (~30 μm). Heating these films to temperatures near the glass transition of the PLGA (65 °C) and laminating them onto np-Si substrates (or magnesium foils, ~60–80 μm) with square regions of etched relief (~30–40 μm) formed sealed air cavities upon cooling to room temperature. Additional details appear in Supplementary Information.
Calibration of the pressure response
Responses of commercial sensors under environments similar to those in the intracranial cavity allowed absolute pressure calibration for the bioresorbable devices. The experiments involved placing a bioresorbable pressure sensor inside the barrel of a syringe partially filled with ACSF (Ecocyte BioScience, USA) and with a commercial sensor (NeuLog, USA) located at its open end (orifice). Moving the plunger component of the syringe allowed reversible access to well controlled pressures throughout a range relevant to intracranial monitoring (Supplementary Fig. 9). Comparison of the electrical resistance of the bioresorbable sensor (via data acquisition (DAQ) system USB-4065, National Instruments, USA) with pressures from the commercial sensor yielded calibration curves. Additional data appear in Supplementary Information.
Connections to wireless data-transmission systems
Laser cutting of foils of molybdenum (~10 μm thick) or magnesium (~50 μm thick) yielded dissolvable narrow metal strips (that is, interconnection wires, 80 μm × 30 mm). Pressing these wires against PLGA substrates using a PDMS stamp at 65 °C embedded them into the surface of the PLGA. Sputter deposition of molybdenum (~2 μm) through high-resolution stencil masks (12.5 μm, Kapton; Dupont, USA) yielded electrical connections between the wires and contact pads on the PLGA (Supplementary Fig. 20). The opposite ends of the wires connected to externally mounted wireless communication systems (Pinnacle Technology, USA) (Supplementary Fig. 21).
Evaluation of the kinetics of device dissolution
Measurements of time-dependent changes in the thicknesses of square (100 μm × 100 μm) Si-NMs (~200 nm thick), electron-beam evaporated layers of SiO2 (~100 nm), free-standing nanoporous silicon substrates (np-Si, ~80 μm) and magnesium foils (~80 μm) due to immersion in ACSF at body temperature (37 °C) established the dissolution kinetics of the key materials. Removing samples from the ACSF every other day, rinsing them with deionized water, and measuring the thicknesses by profilometry (Dektak, USA) yielded the dissolution rate, as in Supplementary Fig. 16. Sealed reservoirs of PDMS with viewing windows allowed for observation of dissolution behaviour at the level of the completed devices. These engineered structures included access channels around the periphery to allow passive fluid exchange and diffusion with a surrounding bath (Supplementary Fig. 17).
Evaluation in animal models
Studies were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Washington University in St Louis (protocol number 20140207). Male Lewis rats weighing 250–350 g (Charles River, Wilmington, MA) received subcutaneous injections of buprenorphine hydrochloride (0.05 mg kg−1; Reckitt Benckiser Healthcare Ltd, USA) for pain management, and of ampicillin (50 mg kg−1; Sage Pharmaceuticals, USA) to prevent infection at the implantation site before the surgical process. Animals were anaesthetized with isoflurane gas and held in a stereotaxic frame for the duration of the surgical procedure and measurements. Opening a craniectomy and dural, implanting bioresorbable sensors on the cortical surface, sealing the craniectomy with a PLGA sheet (~80 μm thick) and/or biodegradable surgical glue, and suturing the skin implanted the fully resorbable biosensing system in intracranial space. Comparison testing with a clinical intracranial pressure sensor (Integra LifeSciences, USA) and commercial thermistor (DigiKey Electronics, USA) implanted in parallel to bioresorbable sensors demonstrated the functionality of the bioresorbable sensors. To implant the injectable device, the same procedure of opening a craniectomy and dural was performed. Injecting needle-shaped biosensors into the brain parenchyma (~5 mm deep) with a stereotactic frame and arm enabled monitoring of pressure and temperature in the deep-brain parenchyma. Additional details on the manual operation of pressure/temperature changes, the immunohistochemistry tests, and the surgical process and measurement at the intra-abdominal cavity and lower extremities appear in Supplementary Information. The immunohistochemistry tests used five individual rats per stage (2, 4 and 8 weeks) and device type (np-Si and magnesium-foil substrates). In vivo functionality tests of pressure and temperature sensors involved three trials using different batches of devices and animals, to establish reproducibility.
Implantable near-field-communication wireless system
The sensor introduced in Fig. 2a can be integrated with sub-dermal wireless data-transmission systems, constructed largely of bioresorbable materials, via thin, bioresorbable wires that pass through the skull. Extended Data Fig. 1a and b show an illustration of a chip-scale, near-field-communication (NFC) technology that includes bioresorbable coils, polymer substrates, encapsulation layers and resistors, a partially bioresorbable NFC chip, and non-resorbable capacitors, and a picture of this system integrated with a bioresorbable pressure sensor via biodegradable wiring. Here, micro-patterned magnesium coils (50 μm thick, outer diameter 15 mm) allow inductive coupling to an external data reader for power transfer and data transmission. A silicon-based logic chip (RF430FRL152H, Texas Instruments, USA; 4 mm × 4 mm × ~300 μm) captures the measured data at a high acquisition rate, then digitizes and processes the information for transmission to the external reader. Passive components include Si-NM resistors and capacitors. PLGA serves as the substrate and electrical passivation layer. Extended Data Fig. 1c summarizes the operating principles. The external reader wirelessly delivers power for operating the logic chip and provides the small currents needed to assess the response of the piezoresistive and thermoresistive sensors. In particular, changes in resistance associated with changes in pressure and temperature register as voltages that can be recorded and transmitted to the external reader by the NFC chip through the associated coil antenna. This NFC system is far more sophisticated than a conventional radio-frequency-identification (RFID) tag. Here, a single chip platform provides all of the computing functionality needed for high-speed data recording, real-time software filtering, and wireless transmission of sensor outputs as captured with an on-board 14-bit analogue-to-digital converter.
This system communicates through biofluids and tissue with little loss, owing to the use of magnetic coupling in a relatively low-frequency band (13.56 MHz; Supplementary Fig. 32), consistent with negligible heating associated with system operation (Supplementary Fig. 33). These characteristics enable communication distance of up to 25 mm through biological tissue. The high-speed, programmable operation of the NFC chip is critical to overall operation. Supplementary Fig. 34a presents examples of data-acquisition rates of up to 250 Hz, via recordings of oscillating voltages (sine wave) with frequencies from 1 Hz to 50 Hz. Spectrograms and other related data appear in Supplementary Fig. 34b–d. These high sampling rates allow efficient operation of digital filtering algorithms, and they also foreshadow the ability to measure biosignals such as EEG, ECoG and ECG. Supplementary Fig. 35 demonstrates the response of real-time high/low-pass filter function achieved by software programming for on-board computation with the NFC chip. Supplementary Fig. 36 shows two-channel operation of the system with/without this type of filtering during sensing. This integrated system provides wireless operation that compares quantitatively with that of a commercial wired sensor (Supplementary Fig. 37).
The wireless module is largely bioresorbable, as illustrated in Extended Data Fig. 1d through images at various stages of dissolution in ACSF at 60 °C. The magnesium coils, electrodes, interconnects and silicon resistors (240 mg; ~85% of the total mass of the NFC system) dissolve fully after 14 days. Here, the NFC chip is not bioresorbable; but fully bioresorbable complementary metal-oxide semiconductor (CMOS) circuit technologies offer the potential for constructing bioresorbable chips. In particular, recently reported schemes demonstrate that modest modifications to otherwise conventional semiconductor-manufacturing techniques allow the use of foundry fabrication facilities for construction of bioresorbable CMOS34. Even with the examples presented here, where the NFC chip is not fully bioresorbable, the associated implantation strategy minimizes risk by locating the hardware subdermally on the skull, outside the intracranial space, thereby allowing rapid, facile extraction.
In this overall architecture, the fully bioresorbable sensors reside in the intracranial space, while the NFC system resides extracranially within the subgaleal layer of the scalp. Fine, dissolvable wiring provides electrical interconnections through a burr hole in the skull, sealed with a bioresorbable surgical glue. After completing the subgaleal closure, the wireless system and sensor are fully implanted. Extended Data Fig. 1e–h show a diagram of the implantation strategy, surgical process, and wireless in vivo intracranial pressure and temperature results measured in a rat model. Here, all of the components in the intracranial region are fully bioresorbable. The non-bioresorbable components of the system remain extra-axial within the scalp, thereby minimizing the risk of provoking pathological neuroinflammation in the intracranial space. In addition, the relative material safety (as judged by the US Food and Drug Administration (FDA) class) of, for example, a subdermally implanted encapsulated non-resorbable device (such as an RFID chip) is similar to that of a titanium fixation screw. Removal of an extra-axial component involves a much lower risk than intracranial surgery. Intracranial pressure and temperature values measured in the rat model using the NFC system are comparable to those captured using commercial wired sensors.
Fabrication of a fully implantable NFC wireless system
The magnesium foil was patterned on the PDMS by using photolithography and etching with dilute hydrochloric acid (deionized water:HCl = 15:1). Transfer printing of the patterned magnesium foil onto a film of PLGA (~150 μm) formed the inductive coil and electrode. The Si-NM resistor was formed on the SOI wafer by doping with phosphorus at 950 °C and patterning the top silicon (~300 nm thick) into the trace. Undercutting the buried oxide with hydrofluoric acid and transfer printing Si-NM on PLGA formed the resistor of NFC system. Laminating the top PLGA (~150 μm) and heating it at 65 °C yielded the passivation layer. Biodegradable conductive W paste served to interconnect the NFC wireless system to the metal wire (molybdenum or magnesium)35.
References
Poole, J. E. Present guidelines for device implantation: clinical considerations and clinical challenges from pacing, implantable cardiac defibrillator, and cardiac resynchronization therapy. Circulation 129, 383–394 (2014)
Brain, T. F. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J. Neurotrauma 24, S37–S44 (2007)
Chamis, A. L. et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation 104, 1029–1033 (2001)
Hall-Stoodley, L., Costerton, J. W. & Stoodley, P. Bacterial biofilms: from the natural environment to infectious diseases. Nature Rev. Microbiol. 2, 95–108 (2004)
Maytin, M. & Epstein, L. M. Lead extraction is preferred for lead revisions and system upgrades. Circ. Arrhythm. Electrophysiol. 3, 413–424 (2010)
Boutry, C. M. et al. Towards biodegradable wireless implants. Phil. Trans. R. Soc. A 370, 2418–2432 (2012)
Ott, K. et al. Retained intracranial metallic foreign bodies: report of two cases. J. Neurosurg. 44, 80–83 (1976)
Vajramani, G. V. et al. Persistent and intractable ventriculitis due to retained ventricular catheters. Br. J. Neurosurg. 19, 496–501 (2005)
Hwang, S.-W. et al. A physically transient form of silicon electronics. Science 337, 1640–1644 (2012)
Irimia-Vladu, M. “Green” electronics: biodegradable and biocompatible materials and devices for sustainable future. Chem. Soc. Rev. 43, 588–610 (2014)
Bettinger, C. J. & Bao, Z. Organic thin film transistors fabricated on resorbable biomaterial substrates. Adv. Mater. 22, 651–655 (2010)
Luo, M., Martinez, A. W., Song, C., Herrault, F. & Allen, M. G. A microfabricated wireless RF pressure sensor made completely of biodegradable materials. J. Microelectromech. Syst. 23, 4–13 (2014)
Brogan, M. E. & Manno, E. M. Treatment of malignant brain edema and increased intracranial pressure after stroke. Curr. Treat. Options Neurol. 17, 327 (2015)
Liu, C. Foundations of MEMS Ch. 6 (Prentice Hall, 2011)
Moseley, P. T. & Crocker, J. Sensor Materials Ch. 4 (Institute of Physics Publishing, 1994)
Chang, H. et al. DNA-mediated fluctuations in ionic current through silicon oxide nanopore channels. Nano Lett. 4, 1551–1556 (2004)
Zheng, G., Patolsky, F., Cui, Y., Wang, W. U. & Lieber, C. M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nature Biotechnol. 23, 1294–1301 (2005)
Stern, E. et al. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature 445, 519–522 (2007)
Gentile, P., Chiono, V., Carmagnola, I. & Hatton, P. V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 15, 3640–3659 (2014)
Hwang, S.-W. et al. Dissolution chemistry and biocompatibility of single-crystalline silicon nanomembranes and associated materials for transient electronics. ACS Nano 8, 5843–5851 (2014)
Kang, S.-K. et al. Dissolution behaviors and applications of silicon oxides and nitrides in transient electronics. Adv. Funct. Mater. 24, 4427–4434 (2014)
Yin, L. et al. Dissolvable metals for transient electronics. Adv. Funct. Mater. 24, 645–658 (2014)
Uslaner, J. M. et al. T-type calcium channel antagonism produces antipsychotic-like effects and reduces stimulant-induced glutamate release in the nucleus accumbens of rats. Neuropharmacol. 62, 1413–1421 (2012)
Barth, K. N. M., Onesti, S. T., Krauss, W. E. & Solomon, R. A. A simple and reliable technique to monitor intracranial pressure in the rat: technical note. Neurosurgery 30, 138–140 (1992)
Haure, P., Cold, G. E., Hansen, T. M. & Larsen, J. R. The ICP-lowering effect of 10° reverse Trendelenburg position during craniotomy is stable during a 10-minute period. J. Neurosurg. Anesthesiol. 15, 297–301 (2003)
Morgan, P. W. Structure and moisture permeability of film-forming polymers. Ind. Eng. Chem. 45, 2296–2306 (1953)
Meldrum, D. R. et al. Prospective characterization and selective management of the abdominal compartment syndrome. Am. J. Surg. 174, 667–673 (1997)
Olson, S. A. & Glasgow, R. R. Acute compartment syndrome in lower extremity musculoskeletal trauma. J. Am. Acad. Orthop. Surg. 13, 436–444 (2005)
Stiefel, M. F. et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J. Neurosurg. 103, 805–811 (2005)
Timofeev, I. et al. Extracellular brain pH with or without hypoxia is a marker of profound metabolic derangement and increased mortality after traumatic brain injury. J. Cereb. Blood Flow Metab. 33, 422–427 (2013)
Suehiro, E. et al. Diverse effects of hypothermia therapy in patients with severe traumatic brain injury based on the computed tomography classification of the traumatic coma data bank. J. Neurotrauma 32, 353–358 (2015)
Mittal, R. et al. Use of bio-resorbable implants for stabilization of distal radius fractures: the United Kingdom patients’ perspective. Injury 36, 333–338 (2005)
Ye, T. et al. Management of grade III open dislocated ankle fractures: combined internal fixation with bioabsorbable screws/rods and external fixation. J. Am. Podiatr. Med. Assoc. 101, 307–315 (2011)
Yin, L., Bozler, C., Harburg, D. V., Omenetto, F. & Rogers, J. A. Materials and fabrication sequences for water soluble silicon integrated circuits at the 90 nm node. Appl. Phys. Lett. 106, 014105 (2015)
Huang, X. et al. Biodegradable materials for multilayer transient printed circuit boards. Adv. Mater. 26, 7371–7377 (2014)
Acknowledgements
S.-K.K. and co-workers are funded by the Defense Advanced Research Projects Agency. J.G.M. is supported by the National Institute of Mental Health, grant F31MH101956. The authors thank M. R. Bruchas at Washington University School of Medicine for providing immunohistochemistry facilities; M. R. MacEwan at Washington University School of Medicine for discussions on animal protocols; A. Manocchi at Transient Electronics Inc. for performing the dissolution test of polyanhydride; and H. Ning at Xerion Advanced Battery Corporation for assistance with running the BET measurements. H.C. was a Howard Hughes Medical Institute International Student Research Fellow. S.-W.H. was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant NRF-2015R1C1A1A02037560). G.P. and K.M.L. were supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (grants NRF-2007-00107 and NRF-2013M3A9D3045719).
Author information
Authors and Affiliations
Contributions
S.-K.K., S.-W.H., D.V.H., N.A.K., S.Y., J.S., H.Y., R.C.W., C.H.L., S.C., D.S.W., J.C., P.V.B. and J.A.R. designed and fabricated the sensors and interfaces. S.-K.K., S.M.L., J.S., J.K. S.H.L. and J.A.R. designed, fabricated and analysed the near-field communication system with the sensor. S.-K.K., R.K.J.M., S.M.L., D.V.H., H.C., P.G., S.Y., J.S., M.S., R.C.W., C.H.L., B.V., Z.L., Y.H., W.Z.R. and J.A.R. conceived the idea and performed the experiments and analysis. R.K.J.M., P.G., J.G.M., M.S., G.P., A.D.G., A.H.K., K.-M.L. and W.Z.R. analysed the immunohistochemistry. S.-K.K., R.K.J.M., S.-W.H., S.M.L., D.V.H., H.C., W.Z.R. and J.A.R. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Fully implantable near-field communication (NFC) system with bioresorbable interface and intracranial sensors.
a, Diagram of a fully implantable NFC system. This device uses a magnesium foil (~50 μm) for the inductive coil, interconnects and electrodes; patterned silicon nanomembranes (Si-NMs, ~300 nm) for resistors; conventional capacitors; and an advanced NFC microchip for data acquisition, processing and transmission. PLGA serves as the substrate and for encapsulation. The diameter of the entire device is about 15 mm. b, Image of this type of NFC system integrated with a bioresorbable pressure sensor. c, Diagram of the operational principles. d, Series of images showing accelerated dissolution of the NFC system inserted into an ACSF at 60 °C. e, Diagram of the implantation process. The bioresorbable sensors reside in the intracranial space, while the NFC system is located extracranially, on the outside surface of the skull, beneath the skin. Bioresorbable, thin metal wires interconnect the NFC system and the sensors. f, Real-time wireless measurements of ICP, showing transient increases induced by the Valsalva manoeuvre (red, data obtained from a transient ICP sensor; blue, data obtained from a commercial ICP sensor). g, Increase in ICT owing to application of a heating blanket around the head, as determined by bioresorbable (red) and commercial (blue) sensors. h, Demonstrations of implantation and suturing in a rat model. A biodegradable surgical glue (TISSEAL) seals the intracranial space.
Supplementary information
Supplementary Information
This file contains Supplementary Methods and Discussion, Supplementary References, Supplementary Table 1 and Supplementary Figures 1-39. (PDF 7154 kb)
Rights and permissions
About this article
Cite this article
Kang, SK., Murphy, R., Hwang, SW. et al. Bioresorbable silicon electronic sensors for the brain. Nature 530, 71–76 (2016). https://doi.org/10.1038/nature16492
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature16492
- Springer Nature Limited
This article is cited by
-
Fully bioresorbable hybrid opto-electronic neural implant system for simultaneous electrophysiological recording and optogenetic stimulation
Nature Communications (2024)
-
Stretchable liquid metal based biomedical devices
npj Flexible Electronics (2024)
-
A biodegradable and flexible neural interface for transdermal optoelectronic modulation and regeneration of peripheral nerves
Nature Communications (2024)
-
Injectable ultrasonic sensor for wireless monitoring of intracranial signals
Nature (2024)
-
Advances in Wireless, Batteryless, Implantable Electronics for Real-Time, Continuous Physiological Monitoring
Nano-Micro Letters (2024)