Abstract
Anxiety disorders constitute a major disease and social burden worldwide; however, many questions concerning the underlying molecular mechanisms still remain open. Besides the involvement of the major excitatory (glutamate) and inhibitory (gamma aminobutyric acid (GABA)) neurotransmitter circuits in anxiety disorders, the stress system has been directly implicated in the pathophysiology of these complex mental illnesses. The glucocorticoid receptor (GR) is the major receptor for the stress hormone cortisol (corticosterone in rodents) and is widely expressed in excitatory and inhibitory neurons, as well as in glial cells. However, currently it is unknown which of these cell populations mediate GR actions that eventually regulate fear- and anxiety-related behaviors. In order to address this question, we generated mice lacking the receptor specifically in forebrain glutamatergic or GABAergic neurons by breeding GRflox/flox mice to Nex-Cre or Dlx5/6-Cre mice, respectively. GR deletion specifically in glutamatergic, but not in GABAergic, neurons induced hypothalamic–pituitary–adrenal axis hyperactivity and reduced fear- and anxiety-related behavior. This was paralleled by reduced GR-dependent electrophysiological responses in the basolateral amygdala (BLA). Importantly, viral-mediated GR deletion additionally showed that fear expression, but not anxiety, is regulated by GRs in glutamatergic neurons of the BLA. This suggests that pathological anxiety likely results from altered GR signaling in glutamatergic circuits of several forebrain regions, while modulation of fear-related behavior can largely be ascribed to GR signaling in glutamatergic neurons of the BLA. Collectively, our results reveal a major contribution of GRs in the brain’s key excitatory, but not inhibitory, neurotransmitter system in the regulation of fear and anxiety behaviors, which is crucial to our understanding of the molecular mechanisms underlying anxiety disorders.
Similar content being viewed by others
Introduction
Anxiety disorders, such as generalized anxiety disorder, panic disorder, obsessive-compulsive disorder and posttraumatic stress disorder, represent the most common psychiatric illnesses, with a lifetime prevalence of approximately 30% in the United States.1, 2, 3
Dysfunctions of the major excitatory (glutamate) and inhibitory (gamma aminobutyric acid (GABA)) neurotransmitter circuits have been implicated in anxiety disorders. In particular, an imbalance between these neurotransmitter systems can lead to abnormal excitability of the anxiety-related neuronal network, thereby causing aberrant behavioral responses.4, 5, 6, 7, 8
The major environmental risk factor for anxiety disorders is exposure to traumatic and stressful life events, such as threats and social stress.9, 10 These situations activate the hypothalamic–pituitary–adrenal (HPA) axis, which ultimately leads to the enhanced secretion of glucocorticoids (GCs). GCs alter neuronal activity in various brain regions, including the hippocampus and the basolateral amygdala (BLA), which are implicated in attention, vigilance and the selection of appropriate behavioral strategies.11 This mechanism allows the body to optimally face stress challenges and adapt to environmental stimuli.
GCs act via glucocorticoid receptors (GRs) and mineralocorticoid receptors (MRs), and proper GR signaling is critical for a healthy stress response. Thus dysfunction of the HPA axis and altered GR signaling are believed to contribute to the development of anxiety disorders.11, 12, 13, 14 Earlier pharmacological and genetic approaches have clearly implicated the GR in the modulation of stress-related behaviors.15, 16, 17, 18, 19, 20, 21 Both central nervous system (CNS)- and forebrain-specific GR ablation result in decreased anxiety.16, 21 However, the underlying brain regions and specific cell types that modulate GR action on fear and anxiety still remain largely unknown.
Given the reports of previous studies,16, 20, 21, 22 and the importance of the glutamate–GABA balance for the etiology of anxiety disorders, we argued that particularly GR expression in excitatory (that is, glutamatergic) neurons would impact fear- and anxiety-related behavior. To test this, we generated conditional mouse mutants lacking the receptor in glutamatergic neurons (GRGlu-CKO mice) and contrasted this with mice lacking GR in the majority of GABAergic neurons (GRGABA-CKO mice). Subsequently, we assessed whether fear- and anxiety-related behavior are regulated by GRs in glutamatergic neurons of the BLA (GR-BLAGlu-CKO mice). Thus our models have the unique potential to specifically dissect the role of the GR in the major excitatory and inhibitory neurotransmitter system within distinct brain regions of the CNS.
Materials and methods
Detailed information on experimental procedures is provided in Supplementary Materials and Methods.
Generation of neurotransmitter-specific conditional GR KO lines
The generation of GR-floxed mice was previously described.16 Conditional GR mutant mice in glutamatergic or GABAergic cells were obtained by breeding GRflox/flox mice to Nex-Cre mice or Dlx5/6-Cre mice, respectively, using a three-generation breeding scheme (detailed information in Supplementary Materials and Methods).
Generation of Cre-driver-specific LacZ reporter lines
Mice reporting the expression of Camk2α-, Nex- and Dlx5/6-Cre drivers were generated by breeding hemizygous or heterozygous Camk2α-, Nex- and Dlx5/6-Cre mice to Rosa26+/flopCrh-IRES-LacZ reporter mice (referred to as R26LacZ), flop: floxed stop.23, 24
AAV-mediated deletion of GR in the BLA
Conditional deletion of the GR in the BLA was induced with adeno-associated virus (AAV) vectors, expressing an enhanced green fluorescent protein (EGFP) reporter and Cre recombinase under the control of the Camk2α promoter (AAV-Camk2α::EGFP-Cre, #PV1917, Penn Vector Core, University of Pennsylvania, Philadelphia, PA, USA). For the control group, AAV vectors lacking the Cre recombinase were used (AAV-Camk2α::EGFP, #PV2521, Penn Vector Core, University of Pennsylvania). Virus production, amplification and purification were performed by GeneDetect (Auckland, New Zealand). GRflox/flox mice were anesthetized with isoflurane, and 0.5 μl (BLA) of either AAV-Camk2α::EGFP-Cre or AAV-Camk2α::EGFP were bilaterally injected in the BLA at 0.06 μl min−1 by glass capillaries with tip resistance of 2–4 MΩ in a stereotactic apparatus. The following coordinates were used: BLA: 1.0 mm posterior to bregma, 3.5 mm lateral from midline, and 3.8 mm below the surface of the skull. After surgery, mice were treated for 5 days with Metacam. Behavioral testing started 4 weeks after virus injection. Successful knockout (KO) of the GR in Camk2α neurons of the BLA was verified by immunofluorescence. Animals that were not infected bilaterally in the BLA were excluded from the analysis.
Single in situ hybridization
Frozen brains were sectioned at −20 °C in a cryostat microtome at 18 μm, thaw mounted on Super Frost Plus slides, dried and stored at −80 °C. In situ hybridization using 35S UTP-labeled ribonucleotide probes (GR and corticotropin-releasing hormone (CRH)) was performed as described previously.25
Double in situ hybridization
Frozen brains were sectioned at −20 °C in a cryostat microtome at 20 μm, thaw mounted on Super Frost Plus slides, dried and stored at −80 °C. Double in situ hybridization (DISH) enabling the simultaneous detection of two different mRNA markers was performed as previously described.26
Immunohistochemistry
Immunofluorescence was performed on free-floating sections as described previously27 (detailed information in Supplementary Materials and Methods).
Neuroendocrine parameters
To determine basal corticosterone and adrenocorticotropic hormone levels, blood sampling was performed in the early morning (0830–0930 hours) and afternoon (0430–0530 hours, only corticosterone) by collecting trunk blood from animals rapidly decapitated under isoflurane anesthesia, with the time from first handling of the animal to completion of bleeding not exceeding 45 s. For evaluation of the corticosterone response to stress, we collected blood samples 30 min (response levels) after an acute stressor (a forced swim test (FST)) by tail cut.28
Behavioral testing
All behavioral tests were recorded using a videotracking system (Anymaze 4.20; Stoelting, Dublin, Ireland), unless otherwise stated. The following behavioral tests were performed in the morning between 0830 and 1230 h in the same room in which the mice were housed: open field (OF), elevated plus maze (EPM), dark–light (DaLi) box, FST, and fear conditioning paradigms. Home cage activity was assessed during the dark and light cycle. The testing procedures were performed as described in Supplementary Materials and Methods.
mEPSC recordings
Neurons in the BLA were selected for recording if they displayed a pyramidal-shaped cell body. All miniature excitatory postsynaptic current (mEPSC) were recorded with a holding potential of −70 mV. If the neuron under study displayed stable mEPSC properties during baseline recording (at least 10 min), corticosteroids were applied for ≈20 min via the perfusion medium. All data were acquired, stored and analyzed on a PC using pClamp 9.0 and Clampfit 9.2 (Axon Instruments, Berkshire, UK). Minimal cutoff for mEPSC analysis was 6 pA (experimental details in Supplementary Materials and Methods).
Statistical analysis
The data presented are shown as means+s.e.m. and were analyzed by the commercially available software SPSS 17.0 (SPSS, Chicago, IL, USA) and Sigma Plot 11.0 (Systat, Erkrath, Germany). The sample size was chosen such that with a type 1 error of 0.05 and a type 2 error of 0.2 the effect size should be at least 1.2-fold of the pooled s.d. When two groups were compared, the unpaired Student’s t-test was applied. If data were not normally distributed, the non-parametric Mann–Whitney test was used. For four-group comparisons (chronic social defeat stress), two-way analysis of variance was performed. Comparisons between mEPSC properties determined during the final 5 min of baseline recording and the final 5 min of recording in the presence of corticosterone in the same cells were analyzed by one-tailed paired t-test. The courses of locomotor activity in the OF and the freezing responses during the fear conditioning paradigms were analyzed by repeated-measure analysis of variance. P-values of <0.05 were considered significant. All data were tested for outliers using the Grubbs' test. Homogeneity of variances was tested using the Bartlett's test. Animals were allocated to experimental groups in a semirandomized manner, and data analysis was performed blinded to the group allocation.
Results
Neurotransmitter identity of GR-expressing neurons
In order to assess the distribution of GR expression within the major limbic excitatory and inhibitory brain circuits, we performed DISH. Simultaneous detection of 35S-labeled GR and digoxigenin-labeled vesicular glutamate transporter 1 (Vglut1) riboprobes revealed predominant expression of GR in glutamatergic neurons of the hippocampus, BLA and throughout the cortical layers. Minimal-to-no co-expression was observed for the central amygdala (CeA), confirming earlier findings, which demonstrate a prominent expression of GABAergic markers in this region29, 30, 31 (Figure 1a). In support, DISHs performed against GR and the GABAergic markers glutamic acid decarboxylase 65 and 67 (Gad65/67) revealed a strong co-localization in the CeA. In addition, GR mRNA was also present in GABAergic interneurons of the hippocampus and cortex and few Gad65/67 cells of the BLA (Figure 1b). In summary, GR expression within the cortex, hippocampus and BLA is predominantly confined to Vglut1-positve glutamatergic neurons, whereas expression in the CeA is largely restricted to Gad65/67-positive GABAergic neurons.
Glucocorticoid receptor (GR) is expressed in neurons of the major excitatory and inhibitory neurotransmitter systems within the brain. GR was co-localized with neurotransmitter-specific markers by double in situ hybridization using wild-type mice. (a) There is a predominant expression of GR in glutamatergic (Vglut1) neurons of the hippocampus, the basolateral amygdala (BLA) and throughout the cortex. Minimal-to-no co-expression was observed for the central amygdala (CeA). (b) GR strongly co-localized with GABAergic (Gad65/67) neurons in the CeA. In addition, GR mRNA was also present in GABAergic neurons of the hippocampus and cortex, and few Gad65/67 cells of the BLA. Black arrowheads indicate examples of cells expressing only GR (silver grains). Gray arrowheads indicate cells co-expressing GR and the respective markers (red staining). Scale bar, 50 μm. DG, dentate gyrus.
Next we used conditional mutagenesis to genetically dissect the specific involvement of GRs in glutamatergic and GABAergic neuronal subpopulations. We crossed GRflox/flox mice with Nex-Cre or Dlx5/6-Cre mice to generate the following lines: GRGlu-CKO mice, where GR is deleted in forebrain glutamatergic neurons, and GRGABA-CKO mice, carrying a GR deletion in forebrain GABAergic neurons. In situ hybridization demonstrated that lack of GR mRNA in GRGlu-CKO mice was most prominent in the cortex and limbic regions, including the BLA and dorsal and ventral hippocampus. We did not observe a significant loss of GRs in the dentate gyrus, which might be caused by the repopulation of GR-expressing newborn neurons during adulthood, considering that the NEX promoter is only transiently active in the dentate gyrus granule cells.32 In addition, the absence of GR was also detected in a number of paraventricular nucleus (PVN) neurons (Figure 2a). Notably, mice (non-specifically) lacking the GR in principal forebrain neurons have previously been generated by breeding GRflox/flox mice with Camk2α-Cre mice. Although Camk2α is predominately expressed in excitatory projection neurons, it is also found in GABAergic medium spiny neurons of the striatum.33, 34, 35, 36 In addition, GABAergic Camk2α-positive neurons have recently been identified in the bed nucleus of the stria terminalis and shown to modulate anxiety-related behavior.37 Consequently, Nex-Cre-mediated inactivation represents a much more selective approach to assess GR deletion specifically in glutamatergic neurons. In fact, analyses of LacZ mRNA expression in Camk2α-Cre and Nex-Cre reporter mice showed that, in contrast to Nex-mediated Cre activity, Camk2α-Cre is additionally expressed in a subset of neurons of the caudate putamen, CeA, septum and bed nucleus of the stria terminalis (Supplementary Figure S1). Moreover, the LacZ expression pattern in Nex-Cre reporter mice (R26LacZNex) strongly resembles endogenous Vglut1 expression, which is largely absent from the striatum, as well as from the thalamic and hypothalamic nuclei.30, 38, 39 However, lack of GR in a subset of PVN neurons in GRGlu-CKO mice also suggests recombination in Vglut2-containing neurosecretory PVN neurons.39, 40, 41
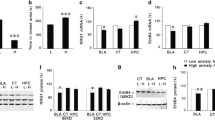
Neurotransmitter-specific GR-CKO lines lack GR expression in a cell type-specific manner. (a) Expression of GR mRNA was assessed by in situ hybridization in wild-type and neurotransmitter-specific GR-CKO lines. Autoradiographs of glucocorticoid receptor (GR) mRNA expression pattern in brain sections of wild-type, GRGlu-CKO and GRGABA-CKO mice. Areas of interest are highlighted with arrowheads and dashed lines. CPu, caudate putamen; Pir, piriform cortex; Ctx, cortex; PVN, paraventricular nucleus; dHc, dorsal hippocampus; CeA, central amygdala; BLA, basolateral amygdala; vHc, ventral hippocampus. (b) Coronal sections of control and mutant mice were stained for GR protein and DAPI (4,6-diamidino-2-phenylindole). DG, dentate gyrus. Scale bar, 250 μm. GR deletion in GRGlu-CKO mice is most prominent in limbic structures, such as the BLA, PVN and hippocampus, whereas lack of GR in GRGABA-CKO mice was mainly observed in the CeA.
Deletion of GR mRNA in GRGABA-CKO mice was most obvious not only in GABAergic neurons of the caudate putamen and CeA but also detected in hippocampal and cortical interneurons (Figure 2a and Supplementary Figure S2). The latter only constitute a small fraction of cortical and hippocampal neurons, and hence of GR-expressing cells, which is reflected in the apparent lack of the GR mRNA deletion pattern in GRGABA-CKO mice. However, scattered expression of LacZ mRNA in the cortex and hippocampus of Dlx-Cre reporter mice (R26LacZDlx5/6) clearly shows Dlx5/6-Cre-mediated recombination in these regions (Supplementary Figure S1). In addition, previous work has confirmed the exclusive GABAergic identity of Cre-expressing neurons in Dlx5/6-Cre mice.26, 42, 43 Lack of GR expression in GRGlu-CKO and GRGABA-CKO mice was also evident at the protein level (Figure 2b and Supplementary Figure S2). Overall, the pattern of GR deletion in both conditional KO lines nicely mirrored the co-expression patterns observed with DISH, highlighting the selective neurotransmitter type-specific deletion properties of the generated mouse mutants.
Absence of GRs in forebrain glutamatergic neurons results in HPA axis hyperactivity
GRGlu-CKO mice exhibit absence of GR expression not only in glutamatergic neurons of the PVN (an important feedback site for GCs) but also in the hippocampus and the BLA, which represent additional key players and regulators of the HPA axis. We therefore investigated whether neurotransmitter-specific deletion of GR in forebrain glutamatergic or GABAergic circuits would have an effect on HPA axis regulation.
We found that basal am and pm corticosterone levels were significantly increased in GRGlu-CKO compared with GRGlu-Ctrl mice (Figure 3a). Moreover, response levels taken 30 min after an acute stressor (FST) showed significantly increased corticosterone levels in GRGlu-CKO (Figure 3b), in line with impaired negative feedback via GRs. Remarkably, we did not observe any changes in corticosterone levels in the GRGABA-CKO mice (Figures 3c and d). Consequently, we analyzed adrenal and thymus gland weight, as alterations in these organs are often associated with changes in HPA axis activity. Indeed, adrenal gland weight was significantly increased, and thymus weight was significantly decreased in GRGlu-CKO mice compared with controls (Figures 3e and f), while there were no changes at all in GRGABA-CKO mice (Supplementary Figures S3A and B). Similarly, basal adrenocorticotropic hormone levels were increased in GRGlu-CKO, but not in GRGABA-CKO mice, supporting a predominant central HPA-hyperdrive in GRGlu-CKO animals (Figure 3g, Supplementary Figure S3C). Moreover, we detected significantly increased CRH mRNA levels in the PVN of GRGlu-CKO mice (Figure 3h). Although there were no differences in body weight of GRGlu-CKO mice, we found significantly reduced body weight in GRGABA-CKO mice (Supplementary Figures S3D and E). Because of the robust endocrine phenotype of GRGlu-CKO mice, we continued to check for potential differences in circadian behavior. To investigate baseline activity in a familiar environment that is not compromised by novelty, home cage activity was monitored during the dark and light phase by an automated infrared tracking system. However, we observed no differences in locomotor activity in GRGlu-CKO mice compared with littermate controls (Supplementary Figure S3F).
Glucocorticoid receptor (GR) signaling in glutamatergic, but not GABAergic, neurons is necessary for appropriate hypothalamic–pituitary–adrenal axis activity. (a) Basal am and pm corticosterone levels were increased in GRGlu-CKO mice (am: Mann–Whitney test (MW-test), T=153.00, P<0.001, ctrl n=12, GRGlu-CKO n=9; pm: t-test, T21=−2.697, P<0.05, ctrl n=12, GRGlu-CKO n=11). (b) Corticosterone response levels, assessed 30 min after an acute stressor, were increased in GRGlu-CKO mice (t-test, T19=−3.309, P<0.05, ctrl n=12, GRGlu-CKO n=9). (c and d) Basal am and pm, as well as response, corticosterone levels did not differ between GRGABA-CKO and control littermates (ctrl n=12, GRGABA-CKO n=10). (e) Thymus weight was decreased in GRGlu-CKO mice (t-test, T19=3.190, P<0.01, ctrl n=12, GRGlu-CKO n=9). (f) Adrenal gland weight was increased in GRGlu-CKO mice (t-test, T19=−9.484, P<0.01, ctrl n=12, GRGlu-CKO n=9). (g) Basal adrenocorticotropic hormone (ACTH) levels were increased in GRGlu-CKO mice (MW-test, T=171.00, P<0.001, ctrl n=12, GRGlu-CKO n=9). (h) (left) Corticotropin-releasing hormone (CRH) mRNA levels in the paraventricular nucleus (PVN) were increased in GRGlu-CKO mice (t-test, T17=−2.226, P<0.05, ctrl n=11, GRGlu-CKO n=8); (right) Representative in situ hybridization images of CRH mRNA expression in the PVN. *P<0.05, data are expressed as mean+s.e.m.
GR activity in forebrain glutamatergic neurons controls fear and anxiety
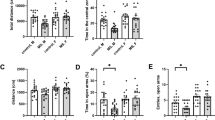
Next we assessed the behavioral consequences of GR deletion in forebrain GABAergic or glutamatergic neurons on anxiety and fear. In the EPM test, GRGlu-CKO mice showed reduced anxiety-like behavior as compared with control littermates, which is evident from increased open arm time and entries (Figure 4a). The low-anxiety phenotype of GRGlu-CKO mice was further confirmed in the DaLi box test, as GRGlu-CKO mice spent more time in the lit compartment and showed an increased distance travelled in the lit compartment compared with GRGlu-Ctrl mice (Figure 4b). The anxiolytic phenotype was independent of alterations in general locomotion, as total distance traveled in the OF test (Supplementary Figures S4A and B) and home cage activity (Supplementary Figure S3F) did not differ between the two genotypes. Moreover, stress-coping behavior in the FST was not altered in GRGlu-CKO mice (Supplementary Figures S4C and D). No changes in anxiety-related and stress-coping behavior were observed in GRGABA-CKO mice (Figures 4c and d; Supplementary Figures S4G and H); however, general locomotion was increased in GRGABA-CKO mice compared with control littermates in the OF test (Supplementary Figures S4E and F). As the contribution of forebrain GABAergic GRs to neuroendocrine and behavioral alterations may only be apparent under severe stress conditions, we subjected GRGABA-CKO mice to 3 weeks of chronic social defeat stress. However, we still observed no genotype-dependent differences with regard to corticosterone levels, anxiety, social behavior or stress coping (Supplementary Figure S5).
Glucocorticoid receptor (GR) in glutamatergic, but not in GABAergic, neurons selectively drives anxiety-related behavior and fear. (a) GRGlu-CKO mice spent more time in (t-test, T17=−3.823, P<0.001) and showed more entries into the open arms (t-test, T17=−2.602, P<0.05) of the elevated plus maze (EPM) (ctrl n=11, GRGlu-CKO n=8). (b) GRGlu-CKO mice spent more time in (Mann–Whitney test (MW-test), T=131.00, P<0.05) and showed an increased distance traveled (MW-test, T=128.00, P<0.05) in the lit compartment of the dark–light (DaLi) box (ctrl n=12, GRGlu-CKO n=9). (c and d) Anxiety-related behavior was not altered in GRGABA-CKO mice in the EPM and DaLi (ctrl n=12, GRGABA-CKO n=11. (e) GRGlu-CKO mice showed a reduced freezing response to the conditioned stimulus (tone) in a neutral environment during the course of all extinction trials (repeated-measure analysis of variance (ANOVA), F1, 22=4.976, P<0.05) after conditioning with a single tone–footshock pairing (ctrl n=13, GRGlu-CKO n=12). (f) No differences in freezing were found between GRGlu-CKO mice and control mice in the acquisition and consolidation (day 1) of another aversive fear conditioning paradigm with five tone–footshock pairings in a different batch of mice (n=12 per group). During the extinction sessions (day 2 and 7), GRGlu-CKO mice showed a significantly reduced freezing response to the conditioned stimulus (repeated-measure ANOVA, F1, 22=4.670, P<0.05 (n=12 per group). (g and h) Freezing behavior did not differ in GRGABA-CKO mice in any of the two fear conditioning paradigms (ctrl n=12, GRGABA-CKO n=11); *P<0.05, data are expressed as mean+s.e.m.
A key feature of clinical anxiety disorders is a failure to appropriately inhibit, or extinguish, fear.44 Pavlovian fear conditioning represents one of the best rodent models to assess cognitive processes related to fear. It consists of the pairing of a conditioned stimulus (CS) with an aversive unconditioned stimulus (US; electric footshock), which mainly induces increased freezing as a conditioned fear response.10 Thus we subjected both GRGlu-CKO and GRGABA-CKO mice to a fear conditioning paradigm with a single CS–US pairing. In order to subsequently assess the freezing response to the tone (cued fear) without confounding influences of contextual memory, conditioned mice were only tested in a neutral environment. GRGlu-CKO mice did not show any significant differences during fear retrieval (first three tone bins of session 1) compared with controls. However, GRGlu-CKO mice demonstrated enhanced fear extinction compared with GRGlu-Ctrl mice, as depicted in the time freezing over the course of the three extinction sessions (Figure 4e). To resolve whether the enhanced fear extinction in GRGlu-CKO mice may be the result of altered fear learning, we subjected another batch of mice to a paradigm with five CS–US pairings,45 allowing the analysis of fear acquisition, consolidation and extinction in more detail. GRGlu-CKO mice did not show any alterations in fear learning compared with GRGlu-Ctrl mice (Figure 4f, left). However, while there were also no alteration during the fear consolidation session (tone bins 1–3), GRGlu-CKO mice demonstrated significantly enhanced fear extinction compared with GRGlu-Ctrl mice during the extinction phase (tone bins 4–13) of days 2 and 7 after conditioning, reflected in significantly reduced freezing responses (Figure 4f, right). Thus the initial findings of an enhanced fear extinction phenotype in GRGlu-CKO mice is independent of fear learning during the acquisition session, which was confirmed using a different paradigm in a separate batch of mice. Interestingly, GRGABA-CKO mice did not exhibit any alterations in fear learning, consolidation and fear extinction (Figures 4g and h) in any of the two fear conditioning paradigms.
GR deletion in forebrain glutamatergic neurons prevents metaplasticity of BLA responses to corticosterone
Persistence of fear involves the BLA.46 Corticosterone strengthens (BLA-dependent) cue-related fear in C57Bl/6 mice,47 and rapid GR-dependent effects in the BLA are important for stable emotional memory.48 We reasoned that impaired GR-dependent signaling in the BLA may contribute to the less stable fear phenotype of GRGlu-CKO mice. To probe the underlying neurobiological substrate, we focused on GR-sensitive signaling specifically in BLA glutamatergic cells, that is, the frequency of mEPSCs.49 Thus BLA cells rapidly respond to a 20-min application (pulse) of 100 nm corticosterone with increased mEPSC frequency via MRs49; yet, a second pulse (20 min, 100 nm, 1 h after the first pulse) causes decreased mEPSC frequency—a phenomenon called ‘metaplasticity’—via a GR-dependent mechanism.49 This GR-dependent decrease does not occur in the hippocampus, where the second pulse causes mEPSC frequency enhancement, similar to the first.49
Basal mEPSC frequency was significantly increased in the principal BLA neurons of GRGlu-CKO compared with GRGlu-Ctrl mice (Figure 5a), comparable to mice with GR ablation in all BLA cells.49 As expected, BLA cells in GRGlu-Ctrl mice responded to a first pulse of corticosterone with increased mEPSC frequency (Figure 5b; Supplementary Table S2), whereas exposure to a second pulse caused a decrease in mEPSC frequency, earlier shown to be GR dependent (Figure 5c). In GRGlu-CKO mice, this GR-dependent reduction in mEPSC frequency in response to the second corticosterone pulse did not occur (Figure 5e). Notably, the (MR-dependent) response to the first pulse was also attenuated (Figure 5d), which was somewhat unexpected as we did not observe alterations in MR mRNA levels in the BLA of GRGlu-CKO mice (Supplementary Figure S6). Hippocampal cells (not liable to metaplasticity) of GRGlu-CKO mice showed a clearly enhanced mEPSC frequency to the second pulse (prior to corticosterone: 0.36+0.05 Hz, during corticosterone: 0.51+0.08 Hz, n=9; P=0.004).
Glucocorticoid receptor (GR) signaling in glutamatergic neurons of the basolateral amygdala (BLA) is essential for responses to corticosterone, and cued fear conditioning. (a) GRGlu-CKO compared with control mice showed an increased basal miniature excitatory postsynaptic current (mEPSC) frequency (t-test, T14=−2.192, P<0.05; ctrl n=8, GRGlu-CKO n=8). (b and c) BLA neurons of controls responded to the first pulse (20 min, 100 nm) of corticosterone with increased mEPSC frequency (P<0.05, n=8) and with a decreased mEPSC frequency to a second pulse (20 min, 100 nm) applied 1 h later (P<0.05, n=6). (d and e) BLA responses to a first corticosterone pulse were not present in GRGlu-CKO mice (n=8). BLA responses to a second pulse of corticosterone were not present in GRGlu-CKO mice (n=8). (f) Representative images of GRfloxflox mice injected with AAV-Camk2α::GFP-Cre (GR-BLAGlu-CKO) or AAV-Camk2α::GFP (GR-BLAGlu-Ctrl) in the BLA. Green fluorescent protein (GFP) in green, GR immunostaining in red. (g–j) Anxiety-related behavior was not affected in GR-BLAGlu-CKO mice (GR-BLAGlu-Ctrl n=10, GR-BLAGlu-CKO n=12). (k) GR-BLAGlu-CKO mice showed significantly attenuated fear learning during conditioning (repeated-measure analysis of variance (ANOVA), F1, 20=6.620, P<0.05) as well as decreased fear expression (day 1) and enhanced extinction (days 2 and 7) (repeated-measure ANOVA, F1, 20=6.199, P<0.05) following fear conditioning (GR-BLAGlu-Ctrl n=10, GR-BLAGlu-CKO n=12). *P<0.05, data are expressed as mean±s.e.m. DAPI, 4,6-diamidino-2-phenylindole; EPM, elevated plus maze.
GR deletion in glutamatergic neurons of the BLA alters fear but not anxiety behavior
Based on the electrophysiology results, we elaborated to which extent the fear-suppressing and anxiolytic phenotype of GRGlu-CKO mice is mediated by GRs in the glutamatergic neurons of BLA. For this, we injected AAV vectors expressing either Camk2α::GFP-Cre (GR-BLAGlu-CKO) or Camk2α::GFP (GR-BLAGlu-Ctrl) constructs into the BLA of GRflox/flox mice (Figure 5f and Supplementary Figure S7A). Thus Cre-expression is driven by the Camk2α promoter and therefore largely restricted to excitatory neurons.23, 24, 26 Moreover, in contrast to the CeA, the BLA primarily contains excitatory glutamatergic neurons (Figure 1).50, 51, 52 Consequently, Cre-mediated GR deletion in the BLA of GRflox/flox mice should almost entirely be restricted to glutamatergic neurons. Following viral injections and recovery for 4 weeks, GR-BLAGlu-Ctrl and GR-BLAGlu-CKO mice were subjected to the OF, EPM, DaLi box and the auditory fear conditioning paradigm with five CS–US pairings. Interestingly, we did not observe any differences in anxiety between GR-BLAGlu-Ctrl and GR-BLAGlu-CKO mice in the EPM or DaLi box test (Figures 5g and j) and no changes in general locomotion in the OF (Supplementary Figure S7B). In contrast, disruption of GR expression in glutamatergic neurons of the BLA resulted in significantly reduced fear learning during acquisition (Figure 5k, left). Along these lines, GR-BLAGlu-CKO mice also showed significantly reduced fear expression and enhanced fear extinction compared with control mice in the subsequent test sessions on days 1, 2 and 7 after conditioning (Figure 5k, right).
Discussion
In this study, we employed a unique set of transgenic mice to delineate whether the GR modulates neuroendocrine regulation as well as fear- and anxiety-related behavior primarily via excitatory (glutamatergic) or inhibitory (GABAergic) forebrain circuits. Our results provide substantial evidence that GR signaling in the forebrain glutamatergic, but not in the GABAergic, neurotransmitter system is crucially involved in regulating stress system activity, fear and anxiety.
Several conditional GR KO mouse studies previously contributed to our understanding of GR-mediated control of HPA axis activity. In particular, conditional deletion of GR in the CNS (GRNesCre mice) resulted in HPA axis hyperactivity, possibly owing to GR deletion in the PVN.16 Moreover, disruption of GR limited to adult forebrain neurons (forebrain-specific GR KO (FBGRKO) mice (Camk2α-Cre), which primarily, but not exclusively, lack GR in forebrain excitatory neurons) led to a mild form of HPA axis hyperactivity.53 More recently, hypercorticosteroidism was observed in PVN-specific GR KO mice (Sim1Cre-GRe3Δ mice).54 Our observations of HPA axis hyperactivity in GRGlu-CKO mice, with GR deletion in limbic structures and the PVN, are in line with these results. We detected an upregulation of Crh mRNA in the PVN of GRGlu-CKO mice, which might result from disrupted negative-feedback control in glutamatergic, CRH-expressing GR neurons, and thus further potentiate HPA axis activity. Along these lines, a recent study demonstrated that the great majority of PVN CRH neurons co-express VGLUT2.55 Thus it is likely that Nex-Cre-mediated recombination, and hence GR deletion, occurred in VGLUT2-expressing CRH neurons of the PVN. However, the precise percentage of glutamatergic CRH/GR-positive neurons in the PVN remains to be determined. Notably, glutamatergic and GABAergic forebrain neurons in GRGlu-CKO and GRGABA-CKO mice, respectively, are ‘protected’ against the raised corticosterone levels owing to GR deletion and, if anything, will show enhanced MR activation.56 From our study, we can conclude that especially the GR in forebrain glutamatergic neurons has a prominent role in mediating the negative feedback on the HPA axis; however, this does not exclude a GR-dependent role of GABAergic neurons in HPA axis regulation, for instance, as an interface between GR-carrying excitatory extrahypothalamic neurons and CRH-expressing neurons in the PVN.57
Genetic mouse models of altered GR expression have also highlighted the crucial involvement of brain GR in emotional behavior.15 Specifically, conditional overexpression of the GR in the forebrain and the limbic system (GRov mice) led to increased anxiety-like behavior.18 Fittingly, CNS-specific GR KO mice (GRNesCre mice) and FBGRKO, as well as GRGlu-CKO mice in this study, demonstrated reduced anxiety-related behavior.16, 20, 21 However, the interpretation of the anxiolytic phenotype in FBGRKO is confounded by the observed increase in locomotion during the DaLi box and EPM test. In contrast to our results, despair-related behavior was enhanced in FBGRKO mice.20, 53 Interestingly, PVN-specific GR KO mice (Sim1Cre-GRe3Δ mice) and mice lacking GR in the CeA demonstrated no alterations in anxiety-related or (in the case of Sim1Cre-GRe3Δ mice) despair behavior.54, 58 Along these lines, we observed no significant changes in anxiety upon specific deletion of the GR in glutamatergic neurons of the BLA. This potentially suggests that GR action in glutamatergic neurons of other limbic structures, such as the hippocampus or prefrontal cortex, or in glutamatergic circuits of several brain regions is primarily involved in modulating anxiety-related behavior.
Although many of the previous studies examined the effects of GR deletion on anxiety in relatively non-homogeneous populations of neurons, we were able to isolate the specific contribution of the GR in forebrain glutamatergic neurons. Not only are these behavioral effects not observed when GR is lacking in GABAergic neurons but also mice lacking the GR in dopamine-releasing neurons (GRDat-Cre mice) or in dopaminoceptive neurons (GRD1-Cre mice) did not show an overt anxiety-related phenotype.17 Also an additional challenge of the system by chronic stress did not reveal a role of GABAergic GRs in stress system regulation and anxiety. Nonetheless, we cannot rule out a potential contribution of non-Dlx5/6 expressing GABAergic interneurons in anxiety-related phenotypes. In addition, GRs in forebrain GABAergic neurons could be of relevance for other behavioral domains. In fact, the slight hyperlocomotion in GRGABA-CKO mice might be a consequence of the pronounced receptor deletion throughout the striatum/CPu, a structure which is highly relevant for the coordination of movement.
An inability to properly extinguish fear is often observed in patients suffering from anxiety disorders, such as posttraumatic stress disorder and phobias.44 Rodent studies have shown that corticosterone can facilitate and is necessary for fear extinction.47, 58, 59, 60 However, while most of these studies applied acute pharmacological approaches or targeted GR deletion in a non-cell type-specific manner, we were able to specifically dissect the contribution of GRs over a prolonged period of time (similar to the timeframe in disease development) in glutamatergic and GABAergic neurons. Lack of GR in forebrain glutamatergic, but not GABAergic, neurons led to enhanced tone-fear extinction in two different fear conditioning paradigms, while fear learning remained unaffected. Interestingly, no overt fear conditioning phenotype was reported in FBGRKO mice.58 The discrepancies in fear conditioning between FBGRKO and GRGlu-CKO mice are most likely related to differences in deletion patterns and deletion time points and the subsequently triggered compensatory mechanism. Nex-Cre-induced deletion is exclusively restricted to glutamatergic neurons (mostly Vglut1-positive), most prominently of the cortex, hippocampus and BLA, and initiated during early development (E11.5).32 On the other hand, Camk2α-induced deletion is initiated postnatally (P16–20)24 and primarily, but not exclusively, not only observed in excitatory neurons of the cortex, hippocampus and BLA but also detected in neurons of the CeA, striatum and thalamus. Importantly, our results suggest that GRs in glutamatergic neurons of the BLA mediate conditioned fear but not anxiety. Notably, whereas GRGlu-CKO mice only displayed enhanced extinction, AAV-mediated deletion of the GR in glutamatergic neurons of the BLA (GR-BLAGlu-CKO mice) additionally resulted in decreased fear learning and fear expression. The more drastic effect on conditioned fear in GR-BLAGlu-CKO mice might be explained by the more instant (viral-mediated) deletion process of the GR during adulthood specifically in glutamatergic neurons of the BLA, as opposed to the gradual GR deletion process, which occurs in GRGlu-CKO mice throughout development within the entire excitatory circuit, and might thus be more prone to induce compensatory changes. Our results are in line with previous studies, which have repeatedly implicated GR signaling in the BLA with the formation and consolidation of fear memories.22, 61, 62 Along these lines, GR antagonist application into the BLA attenuates fear-related behavior and disrupts traumatic memories.63, 64, 65 Interestingly, viral-induced deletion of GR in the CeA (which is predominantly GABAergic) was shown to reduce contextual as well as auditory cued freezing following fear conditioning.58 Collectively, these and our results support a role for the GR in the BLA and CeA in the regulation of fear-related behavior during adulthood. The fact that we observed no alterations in fear conditioning in GRGABA-CKO mice (in which GR is also deleted in the CeA) might be the result of compensatory mechanisms owing to developmental GR deletion and/or absence of GR in GABAergic neurons throughout the brain. Of course, it cannot be entirely excluded that viral spread outside the CeA in the previous study58 or the BLA in our study might have partially influenced the behavioral outcomes.
In search of a possible mechanism whereby the lack of GR expression in glutamatergic BLA neurons could lead to less stable fear memory, we assessed mEPSCs—which reflect the spontaneous release of a glutamate-containing vesicle—in principal neurons of the BLA in GRGlu-CKO and control mice. High levels of corticosterone quickly and long-lastingly enhance glutamatergic transmission in BLA neurons, via nongenomic actions requiring MRs, possibly allowing an extended timeframe for encoding of emotional aspects during stressful events.49 A second pulse of corticosterone applied 1 h later, though, leads to a quick suppression of glutamatergic transmission, a GR-dependent phenomenon. This GR-dependent reduction by a second pulse was completely abolished in GRGlu-CKO mice. Interestingly, even the response to the first pulse, which is MR-dependent, was absent in GRGlu-CKO mice, although MR mRNA levels were not altered in GRGlu-CKO mice. Possibly, protein level and localization of MRs (for example, availability in the plasma membrane) or systems downstream of the MR were changed, owing to prolonged GR knockdown and/or the associated hypercorticosteroidism. Regardless of the mechanism, the results clearly show attenuated GC signaling related to BLA glutamatergic transmission in GRGlu-CKO mice.
Taken together, our study supports that GR signaling in forebrain glutamatergic, but not GABAergic, neurons mediates fear and anxiety behavior and has a critical role in the regulation of HPA axis activity. Moreover, we were able to further disentangle GR-mediated anxiety- and fear-related behaviors. Our results suggest that pathological anxiety might result from alterations in GR signaling in glutamatergic circuits of several forebrain regions, while modulation of fear-related behavior can largely be ascribed to GR signaling in glutamatergic neurons of the BLA. Our study provides a clear dissection of GR action in phenotypically distinct neuronal populations, which adds significant clarity to its role in stress-related emotional behavior. These findings further underline the importance of GR-dependent glutamatergic pathways in the development of psychopathologies related to fear and anxiety, which is of relevance to future pharmacological approaches.
References
Norrholm SD, Ressler KJ . Genetics of anxiety and trauma-related disorders. Neuroscience 2009; 164: 272–287.
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE . Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62: 593–602.
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2163–2196.
Kalueff AV, Nutt DJ . Role of GABA in anxiety and depression. Depress Anxiety 2007; 24: 495–517.
Zorumski CF, Paul SM, Izumi Y, Covey DF, Mennerick S . Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci Biobehav Rev 2013; 37: 109–122.
Wu LJ, Kim SS, Zhuo M . Molecular targets of anxiety: from membrane to nucleus. Neurochem Res 2008; 33: 1925–1932.
Sanacora G, Treccani G, Popoli M . Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 2012; 62: 63–77.
Gross C, Hen R . The developmental origins of anxiety. Nat Rev Neurosci 2004; 5: 545–552.
Caspi A, Moffitt TE . Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci 2006; 7: 583–590.
Shin LM, Liberzon I . The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 2010; 35: 169–191.
de Kloet ER, Joëls M, Holsboer F . Stress and the brain: from adaptation to disease. Nat Rev Neurosci 2005; 6: 463–475.
Joëls M, Baram TZ . The neuro-symphony of stress. Nat Rev Neurosci 2009; 10: 459–466.
Joëls M . Impact of glucocorticoids on brain function: relevance for mood disorders. Psychoneuroendocrinology 2011; 36: 406–414.
Yehuda R . Status of glucocorticoid alterations in post-traumatic stress disorder. Ann NY Acad Sci 2009; 1179: 56–69.
Müller MB, Holsboer F . Mice with mutations in the HPA-system as models for symptoms of depression. Biol Psychiatry 2006; 59: 1104–1115.
Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 1999; 23: 99–103.
Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G et al. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science 2013; 339: 332–335.
Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S et al. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci USA 2004; 101: 11851–11856.
Wagner KV, Wang XD, Liebl C, Scharf SH, Müller MB, Schmidt MV . Pituitary glucocorticoid receptor deletion reduces vulnerability to chronic stress. Psychoneuroendocrinology 2011; 36: 579–587.
Arnett MG, Kolber BJ, Boyle MP, Muglia LJ . Behavioral insights from mouse models of forebrain—and amygdala-specific glucocorticoid receptor genetic disruption. Mol Cell Endocrinol 2011; 336: 2–5.
Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ . Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci 2006; 26: 1971–1978.
Sandi C . Glucocorticoids act on glutamatergic pathways to affect memory processes. Trends Neurosci 2011; 34: 165–176.
Lu A, Steiner MA, Whittle N, Vogl AM, Walser SM, Ableitner M et al. Conditional CRH overexpressing mice: an animal model for stress-elicited pathologies and treatments that target the central CRH system. Mol Psychiatry 2008; 13: 989.
Minichiello L, Korte M, Wolfer D, Kühn R, Unsicker K, Cestari V et al. Essential role for TrkB receptors in hippocampus-mediated learning. Neuron 1999; 24: 401–414.
Schmidt MV, Sterlemann V, Ganea K, Liebl C, Alam S, Harbich D et al. Persistent neuroendocrine and behavioral effects of a novel, etiologically relevant mouse paradigm for chronic social stress during adolescence. Psychoneuroendocrinology 2007; 32: 417–429.
Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM et al. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science 2011; 333: 1903–1907.
Wang XD, Chen Y, Wolf M, Wagner KV, Liebl C, Scharf SH et al. Forebrain CRHR1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling. Neurobiol Dis 2011; 42: 300–310.
Fluttert M, Dalm S, Oitzl MS . A refined method for sequential blood sampling by tail incision in rats. Lab Anim 2000; 34: 372–378.
Herman JP, Mueller NK, Figueiredo H . Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann NY Acad Sci 2004; 1018: 35–45.
Fremeau RT, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ et al. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron 2001; 31: 247–260.
Day HE, Curran EJ, Watson SJ, Akil H . Distinct neurochemical populations in the rat central nucleus of the amygdala and bed nucleus of the stria terminalis: evidence for their selective activation by interleukin-1beta. J Comp Neurol 1999; 413: 113–128.
Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave KA . Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis 2006; 44: 611–621.
Klug JR, Mathur BN, Kash TL, Wang HD, Matthews RT, Robison AJ et al. Genetic inhibition of CaMKII in dorsal striatal medium spiny neurons reduces functional excitatory synapses and enhances intrinsic excitability. PLoS One 2012; 7: e45323.
Robison AJ, Vialou V, Sun HS, Labonte B, Golden SA, Dias C et al. Fluoxetine epigenetically alters the CaMKIIa promoter in nucleus accumbens to regulate DeltaFosB binding and antidepressant effects. Neuropsychopharmacology 2014; 39: 1178–1186.
Robison AJ, Vialou V, Mazei-Robison M, Feng J, Kourrich S, Collins M et al. Behavioral and structural responses to chronic cocaine require a feedforward loop involving DeltaFosB and calcium/calmodulin-dependent protein kinase II in the nucleus accumbens shell. J Neurosci 2013; 33: 4295–4307.
Erondu NE, Kennedy MB . Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci 1985; 5: 3270–3277.
Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL et al. Distinct extended amygdala circuits for divergent motivational states. Nature 2013; 496: 224–228.
Varoqui H, Schäfer MKH, Zhu H, Weihe E, Erickson JD . Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci 2002; 22: 142–155.
Hisano S . Vesicular glutamate transporters in the brain. Anat Sci Int 2003; 78: 191–204.
Hrabovszky E, Wittmann G, Turi GF, Liposits Z, Fekete C . Hypophysiotropic thyrotropin-releasing hormone and corticotropin-releasing hormone neurons of the rat contain vesicular glutamate transporter-2. Endocrinology 2005; 146: 341–347.
Singh-Taylor A, Korosi A, Molet J, Gunn BG, Baram TZ . Synaptic rewiring of stress-sensitive neurons by early-life experience: a mechanism for resilience? Neurobiol Stress 2015; 1: 109–115.
Monory K, Massa F, Egertová M, Eder M, Blaudzun H, Westenbroek R et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 2006; 51: 455–466.
Dine J, Kühne C, Deussing JM, Eder M . Optogenetic evocation of field inhibitory postsynaptic potentials in hippocampal slices: a simple and reliable approach for studying pharmacological effects on GABAA and GABAB receptor-mediated neurotransmission. Front Cell Neurosci 2014; 8: 2.
Graham BM, Milad MR . The study of fear extinction: implications for anxiety disorders. Am J Psychiatry 2011; 168: 1255–1265.
Dias BG, Ressler KJ . Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci 2014; 17: 89–96.
Bhagat SM, Butler SS, Taylor JR, McEwen BS, Strittmatter SM . Erasure of fear memories is prevented by Nogo Receptor 1 in adulthood. Mol Psychiatry; doi: 10.1038/mp.2015.179 (e-pub ahead of print 1 December 2015).
Brinks V, De Kloet ER, Oitzl MS . Corticosterone facilitates extinction of fear memory in BALB/c mice but strengthens cue related fear in C57BL/6 mice. Exp Neurol 2009; 216: 375–382.
Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL et al. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci USA 2009; 106: 4888–4893.
Karst H, Berger S, Erdmann G, Schütz G, Joels M . Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci USA 2010; 107: 14449–14454.
McDonald AJ. Cell types and intrinsic connections of the amygdala. In: Aggleton JP (ed). The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Wiley-Liss: New York, USA, 1992, pp 69–76.
Spampanato J, Polepalli J, Sah P . Interneurons in the basolateral amygdala. Neuropharmacology 2011; 60: 765–773.
Sah P, Faber ESL, Lopez De Armentia M, Power J . The amygdaloid complex: anatomy and physiology. Physiol Rev 2003; 83: 803–834.
Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y et al. Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. PNAS 2005; 102: 473–478.
Laryea G, Schütz G, Muglia LJ . Disrupting hypothalamic glucocorticoid receptors causes HPA axis hyperactivity and excess adiposity. Mol Endocrinol 2013; 27: 1655–1665.
Dabrowska J, Hazra R, Guo JD, Dewitt S, Rainnie DG . Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Front Neurosci 2013; 7: 156.
Joels M, Karst H, DeRijk R, de Kloet ER . The coming out of the brain mineralocorticoid receptor. Trends Neurosci 2008; 31: 1–7.
Ulrich-Lai YM, Herman JP . Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 2009; 10: 397–409.
Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ . Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc Natl Acad Sci USA 2008; 105: 12004–12009.
Blundell J, Blaiss CA, Lagace DC, Eisch AJ, Powell CM . Block of glucocorticoid synthesis during re-activation inhibits extinction of an established fear memory. Neurobiol Learn Mem 2011; 95: 453–460.
Yang YL, Chao PK, Lu KT . Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology 2006; 31: 912–924.
Myers KM, Davis M . Mechanisms of fear extinction. Mol Psychiatry 2007; 12: 120–150.
McGaugh JL, Roozendaal B . Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol 2002; 12: 205–210.
Donley MP, Schulkin J, Rosen JB . Glucocorticoid receptor antagonism in the basolateral amygdala and ventral hippocampus interferes with long-term memory of contextual fear. Behav Brain Res 2005; 164: 197–205.
Conrad CD, MacMillan DD, Tsekhanov S, Wright RL, Baran SE, Fuchs RA . Influence of chronic corticosterone and glucocorticoid receptor antagonism in the amygdala on fear conditioning. Neurobiol Learn Mem 2004; 81: 185–199.
Tronel S, Alberini CM . Persistent disruption of a traumatic memory by postretrieval inactivation of glucocorticoid receptors in the amygdala. Biol Psychiatry 2007; 62: 33–39.
Acknowledgements
This work was supported by the Max Planck Society. We thank Daniela Harbich, Bianca Schmid and Ania Mederer for their excellent technical assistance; Carine Dournes for sharing unpublished data, Günther Schütz (German Cancer Research Center, Heidelberg, Germany) for originally sharing GRflox/flox mice; and Klaus-Armin Nave (Max Planck Institute of Experimental Medicine, Göttingen, Germany) for originally sharing Nex-Cre mice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Hartmann, J., Dedic, N., Pöhlmann, M. et al. Forebrain glutamatergic, but not GABAergic, neurons mediate anxiogenic effects of the glucocorticoid receptor. Mol Psychiatry 22, 466–475 (2017). https://doi.org/10.1038/mp.2016.87
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.87
- Springer Nature Limited
This article is cited by
-
The cortisol switch between vulnerability and resilience
Molecular Psychiatry (2024)
-
Restoring Wnt signaling in a hormone-simulated postpartum depression model remediated imbalanced neurotransmission and depressive-like behaviors
Molecular Medicine (2023)
-
Pathogenesis of Post-Traumatic Stress Disorder and Therapeutic Targets
Neuroscience and Behavioral Physiology (2023)
-
Activation of the anterior cingulate cortex ameliorates anxiety in a preclinical model of fetal alcohol spectrum disorders
Translational Psychiatry (2022)
-
Blood levels of T-Cell Receptor Excision Circles (TRECs) provide an index of exposure to traumatic stress in mice and humans
Translational Psychiatry (2022)