Abstract
Antidepressant drugs are commonly prescribed treatments for anxiety disorders, and there is growing interest in understanding how these drugs impact fear extinction because extinction learning is pivotal to successful exposure-based therapy (EBT). A key objective within this domain is understanding how antidepressants alter the activation of specific elements of the limbic-based network that governs such fear processing. Chronic treatment with the antidepressant tianeptine has been shown to reduce the acquisition of extinction learning in rats, yet the drug’s acute influence on activation in prefrontal and amygdalar regions, and on extinction learning are not well understood. To assess its influence on cellular activation, rats were injected with tianeptine and Fos immunoreactivity was measured in these regions. Acute tianeptine treatment selectively altered Fos expression within subdivisions of the central nucleus of the amygdala (CEA) in a bidirectional manner that varied in relation to ongoing activation within the capsular subdivision and its prefrontal and intra-amygdalar inputs. This pattern of results suggests that the drug can conditionally modulate the activation of CEA subdivisions, which contain microcircuits strongly implicated in fear processing. The effect of acute tianeptine was also examined with respect to the acquisition, consolidation and expression of fear extinction in rats. Acute tianeptine attenuated extinction learning as well as the recall of extinction memory, which underscores that acute dosing with the drug could alter learning during EBT. Together these findings provide a new perspective for understanding the mechanism supporting tianeptine’s clinical efficacy, as well as its potential influence on CEA-based learning mechanisms.
Similar content being viewed by others
Introduction
Exposure-based therapy (EBT) and antidepressant drugs are two widely prescribed treatments for anxiety disorders.1, 2 In EBT, a patient is exposed to a feared stimulus in the absence of danger in order to overcome anxiety. Because patients can undergo both treatments simultaneously, the impact of such drug treatments on the effectiveness of EBT is an important topic.3, 4, 5
Tianeptine is an antidepressant drug that also shows benefits for anxiety symptoms in patients,6, 7, 8, 9 which does not have an acute anxiogenic effect that can complicate treatment.10, 11, 12 Unlike many other antidepressants, tianeptine displays no affinity for known neurotransmitter receptors, nor does it inhibit the reuptake of catecholamines in the brain,13 but it does exhibit potent effects on glutamatergic transmission.14, 15, 16, 17, 18
In rodent studies, chronic tianeptine treatment produces robust effects on both stress and fear processes. Chronic tianeptine administration reverses stress-induced alterations in structural plasticity in the hippocampus,13 and also impairs the acquisition of both auditory fear conditioning10 and auditory fear extinction,19 which both involve limbic-dependent learning relevant to EBT.20, 21 In fear conditioning, an originally neutral conditional stimulus (such as a tone or context) comes to elicit fear responses after it has been associated with an aversive unconditional stimulus, such as a footshock. Later during fear-extinction training the feared conditional stimulus is repeatedly presented without the unconditional stimulus. This training leads to decreased fear responding to the conditional stimulus that mimics the fear reduction observed after EBT.
Although the amygdala and medial prefrontal cortex contribute to the regulation of both the stress response22 and fear behavior,23, 24 the acute influence of tianeptine on cellular activation in these regions, and on fear extinction, has been poorly understood. During an elevated platform stress experiment, we injected rats with tianeptine, and measured the immunoreactivity of the immediate early gene Fos in the infralimbic cortex (IL), prelimbic cortex (PL), lateral amygdala (LA), basolateral amygdala (BA), medial amygdala (MEA) and subdivisions of the central nucleus of the amygdala (CEA). We observed that tianeptine produced bidirectional alterations on the activation of the CEA subdivisions. Given the important role of these subdivisions in fear processing, we also tested its influence on fear extinction and found that acute tianeptine attenuated both the acquisition and expression of fear extinction. Our results support the idea that tianeptine can selectively modulate the activation of CEA subdivisions, which might be crucial for understanding its capacity for altering fear processing.
Materials and methods
Animals
Adult male rats of the Sprague Dawley (Experiments 1 and 2), Long Evans (Experiments 3 and 4) and Wistar (Experiments 5 and 6) (see Supplementary Table 1 for explanation of the strain choices). Animals were housed two or four per cage with free access to food and water in a temperature-controlled facility (20–24 ºC) with a 12 h/12 h light/dark cycle. All procedures were conducted during the light phase and conformed to national (JO 887-848) and European (2010/63/EU) animal experimentation regulations.
Drugs and injections
Tianeptine (Servier, Suresnes, France) was freshly dissolved in saline and administered at a 10 mg kg−1 dose. In all cases, these intraperitoneal injections were administered either 30 min before the start of a behavioral procedure, or immediately after. Sodium pentobarbital (Cenravet, Taden, France) was administered in the Fos experiments at a dose of 60 mg kg−1 for anesthesia and 300 mg kg−1 for terminal anesthesia.
Immediate early gene expression
To characterize the impact of tianeptine on cellular activation, two experiments measured tianeptine-induced Fos expression in the amygdala and prefrontal cortex:
Experiment 1: Rats (n=42) were either transferred individually to a brightly lit unfamiliar room where they were placed on an elevated platform (20 × 21 cm surface, 100 cm above the ground) for 30 min (stress rats), or they remained in their home cages (control rats). Afterwards, all rats received an injection of pentobarbital (60 mg kg−1) followed by an injection of saline or tianeptine (10 mg kg−1; 1 ml kg−1) before being returned to their home cage. Pentobarbital was included to mimic conditions used previously during in vivo electrophysiological experiments.18, 25
Experiment 2: For 3 days, rats (n=22) were weighed, handled and they received one injection of saline to habituate the effects of the injection procedure on Fos expression. The following day, rats received two injections: either saline or pentobarbital (60 mg kg−1) followed by either saline or tianeptine (10 mg kg−1).
Ninety minutes after drug injections, all rats were terminally anesthetized with pentobarbital (300 mg kg−1) and perfused transcardially with saline followed by 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4). Brains were collected, post-fixed and transferred into a cryoprotectant solution (30% sucrose in phosphate buffer). Serial coronal sections (50 μm) were cut with a microtome and stored at 4 °C in phosphate buffer containing 0.02% sodium azide.
Fos protein, immunocytochemistry was conducted as described previously.26 Sections were incubated overnight at room temperature with a primary rabbit polyclonal antibody directed at the N terminus of the Fos protein (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA, USA). A biotinylated goat anti-rabbit antibody (1:2000, Jackson ImmunoResearch Laboratories, West Grove, PA, USA) was used as the secondary (2-hour incubation at room temperature). Staining was revealed using the avidin-biotin peroxidase method (ABC kit, Vector Laboratories, Burlingame, CA, USA) coupled to diaminobenzidine. Sections were mounted, dehydrated and coverslipped using Eukitt mounting medium. To control for immunostaining specificity, some sections were processed as described above, but without the primary or secondary antibody (Supplementary Figure S1).
Quantitative analyses of positively labeled nuclei were performed using a computerized image-processing system (Explora Nova, Inc., La Rochelle, France) coupled to a DM6000 Leica microscope. Structures were anatomically defined according to the Paxinos and Watson atlas and the previous work of Jolkkonen and colleagues.27, 28 The borders of the area were determined with the use of the Nissl-stained adjacent sections. Immunoreactive neurons were counted bilaterally (minimum of three sections per hemisphere, and six per rat). A constant density threshold operation background (Mercator software) and target acceptance criterion were used to record the number of Fos-positive nuclei for each of the sections. These numbers were then averaged for each animal in each group to give the mean values used for statistics. All measurements were carried out by an experimenter blinded to the experimental conditions.
Behavioral procedures
General procedures
Rats were handled for ~2 min prior to the conditioning day, and all experimental sessions occurred at 24 h intervals. Scrambled footshocks and tones were delivered via computer-controlled systems (Imetronic, Pessac, France or Med Associates, St. Albans, VT, USA; see Supplementary Table 2 for description of the conditioning contexts). Freezing behavior was estimated from video according to an instantaneous time-sampling procedure.29 Freezing was defined as the absence of any visible movement except that required for breathing. The observers were blinded to drug treatments when they rated freezing behavior. The inter-observer correlation was >0.95. Animals were matched to the drug or saline groups based on freezing observed during conditioning and/or extinction.
Auditory fear procedures
Rats were conditioned in a rectangular chamber with a stainless-steel grid floor (Context A) that was scented with 1% ammonia hydroxide and illuminated by the house lights. After 180 s, rats received a sequence of three tone-shock parings (60 s inter-trial interval; tone: 20 s, 2000 Hz, 78 dB; footshock: 0.6 mA, 2 s; 60 s post-shock interval). Extinction training occurred in Context B (dark chamber with a curved wall insert scented by 1% acetic acid) and began with a 180 s pre-tone interval followed by the delivery of 32 tones (30 s inter-trial interval; 60 s post-tone interval). The test consisted of a 180 s pre-tone interval followed by the presentation of a single tone. Experiment 3 included one extinction session and the test occurred in Context B. Experiment 4 included two extinction sessions and half the animals were tested in either Context A or Context B.
Context fear procedures
Rats were placed in a rectangular chamber with a grid floor (Context A or C) for 480 s. After 120 s, the rats received a series of three unsignaled footshocks (0.5 mA, 1 s; 120 s inter-trial interval). Extinction training consisted of a 480 s shock-free reexposure to the conditioning chamber. The extinction retention test consisted of a 240 s shock-free exposure to the chamber.
Statistics
Data were analyzed with Student’s t-tests (unpaired except where indicated), repeated-measures ANOVAs and factorial ANOVAs. P<0.05 was used as a criterion for statistical significance. All analyses were performed with Statistica software (StatSoft, Tulsa, OK, USA).
Results
Acute tianeptine treatment produced bidirectional effects on Fos+ levels in the CEA depending on the ongoing activational profile of the prefrontal-amygdala network
Our initial goal for the current investigation was to follow up prior findings that demonstrated tianeptine can reverse the stress-induced disruption of long-term potentiation in the medial prefrontal cortex.25 Therefore, we characterized its impact on cellular activation measured by Fos expression in the medial prefrontal cortex and amygdala. The previous long-term potentiation experiments were conducted in vivo in pentobarbital-anesthetized rats. Accordingly, we mimicked these conditions and rats either underwent platform stress for 30 min or remained in homecages, and then were given injections of pentobarbital (60 mg kg−1) followed by an injection of saline or tianeptine (10 mg kg−1) before being killed for tissue processing. This experiment was conducted as two replications. Similar stress and drug effects were observed in each, yet there was some variability in the group means and s.d. across the replications (Supplementary Figure S2). Therefore, a z-score standardization procedure was used to pool the data sets, whereby we calculated a standard score (z-score=(x−μ)/σ) for each data value (x) using the corresponding mean (μ) and s.d. (σ) from each brain region tabulated during each replication.30 These pooled data were analyzed with factorial ANOVAs with factors for Stress and Drug.
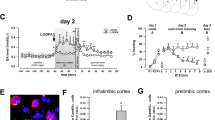
As shown in Figure 1, stress exposure elevated FOS+ levels in the IL, PL, LA, BA and MEA (all F’s(1,38) >43.96, all P’s<0.000001), but there were no significant effects of tianeptine treatment in these regions (Drug and Stress × Drug: all F’s(1,38) <1.24). In contrast, stress did not alter Fos+ levels in the CEA (F(1,38)=2.34, P=0.13), and tianeptine treatment was associated with a significant decrease in the number of Fos+ cells in the CEA, independent of stress (Drug: F(1,38)=51.08, P<0.000001; Stress × Drug: F(1,38)<1). Post hoc contrasts confirmed that each tianeptine-treated group was decreased compared with the corresponding saline controls. Likewise, a double-labeling assay from a subset of experimental animals demonstrated a similar drug effect, and confirmed that the majority of Fos+ cells in the CEA were likely GABAergic (Supplementary Figure S3).
Acute tianeptine treatment selectively decreased the number of Fos+ neurons in the central nucleus of the amygdala. (a) Fos+ counts expressed as z-scores by brain area in rats given saline or drug injections immediately after the control or stress procedure. Error bars: standard error of the means (±s.e.m.). (b) Z-scores of the CEA Fos+ data from individual rats. Horizontal bars represent the group means. #: significant contrast between the corresponding control and stress group. *: significant contrast between the corresponding saline and tianeptine group.
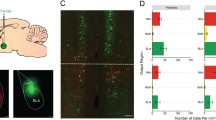
Given the growing interest in amygdala microcircuits that involve distinct subdivisions within the amygdala31 following the global Fos analysis, we recounted Fos+ cells in the capsular (CEAc), medial (CEAm) and lateral (CEAl) subdivisions of the CEA. Visual inspection of the data indicated that the CEAc displayed a relatively higher level of Fos background expression compared with the other subdivisions (Figure 2a). Statistical analysis of either the raw or z-score values gave similar results, and there were no significant stress effects. Tianeptine treatment decreased Fos levels in each of the subdivisions (F’s(1,38) CEAc=51.59, P<0.000001; CEAm=6.64, P=0.014; CEAl=18.35, P=0.00012). Thus, in conditions involving relatively high background expression in the CEAc, tianeptine treatment decreased Fos expression throughout the CEA.
Acute tianeptine treatment produced bidirectional effects on the activation of the central amygdala subdivisions that varied with respect to ongoing activation in the region. Average number of Fos+ cells counted in the central amygdala subdivisions from Experiments 1 and 2 (a and b). Representative photo micrographs showing examples of Fos immunoreactivity from each experiment (left). Summary of behavioral treatments (above). Scale bars 100-μm. Error bars: standard error of the means (±s.e.m.). d: significant main effect of drug. *: significant contrast between the corresponding saline and tianeptine group.
The rats in Experiment 1 underwent minimal handling prior to the test day and they were anesthetized with pentobarbital during the 90 min period before being killed (Table 1). Experiment 2 was conducted to address whether these treatments might have influenced the drug effect we observed in the CEA. Thus, rats were habituated to saline injections for 3 days. On the test day, they all underwent the control treatment followed by a sequence of two injections: saline or pentobarbital followed by saline or tianeptine (Table 1). Data were analyzed with a factorial ANOVA with the factors Drug and Anesthesia.
As shown in Figure 2b, saline-treated rats displayed substantially lower levels of Fos+ cells in the CEAc compared with Experiment 1. This difference could not be attributed to pentobarbital treatment, however, because the anesthetic did not alter Fos+ counts in any of the subdivisions (all F’s (1,18)<1.60), nor did it interact with tianeptine treatment (Drug × Anesthesia: all F’s(1,18)< 1). Together, these results suggest that the handling procedures utilized in each experiment contributed to different Fos+ backgrounds observed in the CEA. Remarkably, tianeptine had a contrasting influence on the activation of the CEA subdivisions compared with Experiment 1 (where the CEAc displayed high background). Here, in conditions of relatively low Fos+ background in the CEAc, tianeptine significantly increased the number of Fos+ neurons in the CEAl and CEAm (F’s(1,18): CEAl=11.56, P=0.0031; CEAm=4.85, P=0.041), but not in the CEAc (F(1,18)<1).
To further characterize these bidirectional drug effects, we performed an analysis of the raw Fos+ data from only those groups from Experiments 1 and 2 that had equivalent treatments on the test day (Experiment 1: control-saline, control-tianeptine; Experiment 2: pentobarbital-saline, pentobarbital-tianeptine). These groups underwent the control treatment and they were given pentobarbital on the test day, but they had different handling regimens prior to testing, and were analyzed with a factorial ANOVA with factors for Experiment and Drug. Consistent with the above analyses, tianeptine treatment rendered opposite effects on Fos levels in the CEAm and CEAl across the two studies (Experiment × Drug: CEAm F(1,24)=6.18, P=0.020; CEAl F(1,24)=10.52, P=0.0035) as well as an significant interaction in the CEAc (F(1,24)=10.52, P=0.0035). Interestingly, there was also substantially lower levels of overall Fos activation in the IL, PL, LA and BA regions during Experiment 1 compared with Experiment 2 (IL: 239±49 vs 759±44; PL: 219±45 vs 867±44; LA: 7.93±1.77 vs 61.53±3.56; BA: 59±12 vs 246±24; all F’s(1,24)>52.48, all P’s <0.000001). These additional analyses support the idea that the handling regimens used in each study produced differential activational profiles in the prefrontal-amygdala network at the time of testing, and tianeptine had a bidirectional influence on the Fos+ levels within CEA subdivisions in a manner that varies with respect to ongoing activity of the network.
Acute tianeptine attenuates the acquisition and expression of fear extinction
Given the relevance of the CEA to fear processing, the above results motivated a series of experiments concerning the impact of acute tianeptine on the acquisition and expression of both auditory (Experiments 3 and 4) and contextual fear (Experiments 5 and 6) extinction.
Auditory fear extinction
Experiment 3: Twenty-four hours after auditory fear conditioning, rats given saline or tianeptine before auditory extinction training showed similar freezing during the pre-tone interval (t(20)<1) and during the first two tones (t(20)<1), but tianeptine-treated rats displayed more freezing during the tones overall (F(1,20)=5.89, P=0.025) (Figure 3a). The next day the tianeptine rats also displayed significantly more freezing during the drug-free extinction retention test (F(1,20)=5.36, P=0.031). Thus, tianeptine attenuated the acquisition of fear extinction for auditory cues.
Acute tianeptine treatment attenuated the acquisition and expression of auditory fear extinction. (a) Experiment 3: Tianeptine pre-treatment attenuated the extinction of freezing to a tone cue that had previously been paired with footshock. (b and c) Experiment 4: Tianeptine pre-treatment selectivity increased freezing to a previously extinguished tone cue when different groups of rats were tested in either the extinction or fear-conditioning contexts, respectively. (d) Freezing data from the expression of extinction test collapsed across test contexts. Error bars: standard error of the means (±s.e.m.). *: statistically significant group difference. +: statistically significant increase in freezing to the tone in relation to the pre-tone interval (paired t-test). × : statistically significant Drug × Test interval interaction.
Experiment 4: Rats underwent auditory fear conditioning and two extinction training sessions before being matched to four groups that were given saline or tianeptine 30 min before being placed in either the extinction or fear-conditioning contexts during the extinction retention test. During the pre-tone interval, rats tested in the fear-conditioning context displayed more freezing compared with those tested in the extinction context (F(1,31)=8.34, P=0.007), but tianeptine treatment did not influence these levels (F(1,31)=1.58, P=0.22; Drug × Test context: F(1,31)<1) (Figures 3b and c). Interestingly, animals given tianeptine showed a robust increase in freezing upon tone onset, whereas saline-treated animals did not (Drug × Test interval F(1,31)=6.28, P=0.018) (Figure 3d). Together, these results indicate that tianeptine treatment did not produce a general increase in freezing behavior. Instead the drug selectively elevated freezing to the previously extinguished tone, suggesting that the drug impaired the recall of auditory extinction memory.
Contextual fear extinction
Experiment 5: Twenty-four hours after context fear conditioning with unsignaled footshocks, rats were injected with saline or tianeptine before contextual extinction training in the conditioning chamber. Saline-treated rats showed decreased freezing across the extinction session, whereas tianeptine-treated rats displayed sustained freezing at the end (Figure 4a) (Drug × Time: F(3,84)=2.76, P=0.047). The next day, the tianeptine rats also displayed significantly more freezing during the drug-free extinction retention test (F(1,28)=9.44, P=0.0047). Thus, similar to the case of auditory fear extinction, tianeptine attenuated the acquisition of extinction of fear conjured by contextual cues.
Acute tianeptine treatment attenuated the acquisition and expression of contextual fear extinction. (a) Experiment 5: Tianeptine pre-treatment attenuated the extinction of context freezing in a chamber that had previously been paired with footshock. (b) Experiment 6: Tianeptine pre-treatment increased freezing to a previously extinguished shock-paired chamber. Error bars represent standard error of the means (±s.e.m.). *: statistically significant group difference. × : statistically significant Drug × Time interaction.
Experiment 6: Rats were fear conditioned with unsignaled footshocks and were reexposed to the conditioning chamber on the following day. They displayed increased freezing during fear conditioning and decreased freezing during extinction training (Figure 4b). On the following day, rats were administered saline or tianeptine prior to a reexposure to the chamber. Tianeptine-treated rats displayed elevated freezing compared with the saline controls (F(1,16)=13.60, P=0.002). Thus, tianeptine increased freezing in a shock-associated context that had been previously extinguished, which is consistent with interpretation that tianeptine impaired the recall of extinction memory.
An additional experiment (Supplementary Figure S4) indicated that when administered before context conditioning, tianeptine increased freezing at the end of the session, but this effect did not carry over to a drug-free fear-retention test. Also, tianeptine given immediately after context conditioning, or immediately after context extinction training, did not alter the consolidation of the fear acquisition or extinction memories measured the next day (Supplementary Figures S5). Furthermore, acute tianeptine slightly increased locomotion and decreased rearing as rats explored a dark-illuminated field, but the drug neither altered exploration evoked by the novel bright light gradient, nor dark preference behavior (Supplementary Figure S6). Together these results are consistent with the hypothesis that tianeptine has fairly selective effects on fear-extinction processes without generally altering fear or motor processing.
Discussion
In the present study, we demonstrated that tianeptine can exert bidirectional effects on Fos+ levels in the subdivisions of the CEA. We also showed that tianeptine attenuates both fear-extinction learning and the recall of fear-extinction memory. These results suggest that tianeptine can act as a conditional modulator of CEA activation, which is interesting in light of the increasing evidence that neuronal microcircuits found within amygdala nuclei are specifically recruited during distinct aspects of fear processing.31, 32, 33, 34, 35, 36, 37, 38 Moreover, the correlation between our Fos and behavioral results supports the hypothesis that tianeptine’s modulation of the CEA contributes to its attenuation of fear extinction.
In the current study, we counted Fos+ neurons in several elements of the prefrontal-amygdala network that has been strongly implicated in fear acquisition and fear-extinction processes. Because tianeptine is thought to influence neurons primarily by modulating glutamatergic signaling,13, 15, 16, 18 it might be anticipated that the drug would render widespread effects on the activation of glutamatergic cells and their targets within in the network. Instead, our analysis detected drug effects exclusively within the CEA, which implies that the CEA may be especially sensitive to the drug. An interesting feature of the present findings was also that the drug induced changes in Fos+ levels that were bidirectional when comparing the results from the two studies, and the bidirectionality was correlated with the relative activation observed in the CEAc and its inputs. This pattern suggests that the effect of tianeptine on CEA activation is conditional, inasmuch as the drug induced distinct effects depending on the current state of ongoing activation in the network.
The CEA contains a high percentage of GABAergic neurons39 and receives glutamatergic inputs that synapse onto the nucleus’s complex intrinsic circuitry.27, 40 The CEA’s capsular and lateral divisions are composed mainly of GABAergic medium spiny neurons (MSNs),41, 42, 43 which contribute to the inhibitory control of output neurons in the CEAm,32 and they contain subpopulations of neurons that form mutually inhibitory and functionally distinct neurocircuits.34, 35 The CEA also receives direct input from numerous brain regions, including the IL, PL, BA, LA, intercalated cell clusters and the parabrachial nucleus.27, 44, 45 Projections arising in other amygdala nuclei terminate mainly in the CEAc or CEAm,44 whereas the majority of inputs from the medial prefrontal cortex and parabrachial nucleus arrive in the CEAc.27, 46
How might tianeptine cause these bidirectional effects? One possibility is that the relative activation in the network favors distinct modes of synaptic transmission (such as tonic vs phasic inhibition34), or synaptic plasticity47 that are differentially affected by the drug. Indirect support for this view comes from the precedent that tianeptine selectively favors the induction of some forms of synaptic plasticity,48 and it can cause contrasting effects on glutamatergic signaling depending on the cellular context.49 The drug might also be operating on distinct subpopulations of neurons or synapses in the CEA that are differentially recruited depending on ongoing activation.32, 37, 50 Tianeptine’s conditional influence is also interesting with respect to the MSNs that are present in the CEAc. In other brain regions, MSNs are known to be involved with the gating of information flow from afferents,51, 52 so it’s possible that MSNs in the CEAc (which receive the bulk of input to the CEA) engage in a similar function,42, 53 and that tianeptine could alter such gating. Clarifying the drug’s mode of action in the CEA will be an important step in understanding how this antidepressant drug alters amygdala microcircuits and emotional processing.
Chronic tianeptine treatment (23 doses) impairs the acquisition of auditory fear extinction, whereas subchronic treatment (11 doses) produces no discernible effect.19 Taken together with our results, it is likely that acute and chronic tianeptine treatment impair the acquisition of extinction via different mechanisms. Namely, chronic treatments likely produce adaptive changes in the brain that develop over weeks and that involve the downregulation of NMDA receptors,19 or the increased localization of the SNAP-25 protein in vesicles.14 In contrast, the immediate effects of a single dose of the drug likely involve rapid changes in synaptic transmission or cell excitability.49 This idea is supported by previous observations showing that tianeptine potently impacts AMPA receptor trafficking,16 which is a cellular process that has been implicated in the formation of extinction memory.54 It is also noteworthy that the CEAc and CEAl contain a high percentage of NR2B subunit-containing NMDA receptors,55 because these receptors also have been strongly implicated in extinction processes.56
Our results present two related findings: tianeptine modulated activation within subdivisions of the CEA and the drug attenuated behavioral extinction. An important question is whether the Fos effects are pertinent to explaining the drug’s influence on extinction. Although we did not test this question directly, there are reasons to consider the hypothesized link plausible: first, our results strongly support the view that tianeptine potently impacts the activation of the CEA, without apparent effects in many other elements of the prefrontal-amygdala network that are considered important for fear extinction. Second, the CEA (especially the CEAc) is directly connected with the IL, BA and intercalated cell cluster, which are strongly implicated in fear-extinction processes.31, 44, 57, 58 Third, previous reports have demonstrated that fear-extinction training is associated with increased Fos expression in the CEA.59, 60 Fourth, in Experiment 1 we observed tianeptine decreased Fos levels in the CEA of both control and explicitly stressed animals, which implies that drug can influence CEA activation over a range of circumstances, including others that give rise to high Fos levels in the nucleus (such as fear extinction). Together these results support the hypothesis that tianeptine’s modulation of the CEA is critical for understanding is behavioral effects.
A previous study reported that acute tianeptine treatment did not alter within-session freezing during a procedure equivalent to an extinction training session,11 which contrasts with our results. This discrepancy might be explained by a methodological difference whereby our conditioning procedure rendered substantially a higher degree of conditioned freezing at the start of the test, which was likely more amenable for detecting an impairment of fear extinction. It is also notable that consistent with previous findings10 we observed that acute tianeptine treatment did not influence the long-term retention of fear behavior when given prior to context conditioning. Thus, although the drug attenuates extinction, it does not occlude all fear processing.
The CEA has been considered integral in fear-response production,35, 61 including behavioral freezing. Our results show that tianeptine increased freezing in the presence of a previously extinguished cue or context. Importantly, tianeptine and saline rats displayed similar levels of freezing to background cues during the pre-tone period of the auditory extinction retention test, which argues against the possibility that the drug causes a general increase in freezing, or that it is intrinsically anxiogenic. Rather, the pattern is consistent with the interpretation that tianeptine increases freezing because it interferes with the recall of the extinction memory that normally suppresses the conditioned freezing behavior. Also, the pattern of the drug effects observed during several control experiments indicate that it is unlikely that the alterations in freezing observed in this report result from drug-elicited motor impairment.
Amygdala processing is fundamental to stress and fear regulation.22, 23, 31 Our results suggest that tianeptine can selectively modulate CEA subdivisions that are strongly implicated fear processing. Considering that acute tianeptine attenuates fear-extinction learning, it might also influence human learning during EBT. Given the bidirectional Fos+ effects that we observed, however, it remains possible that although the drug impairs the initial phase of fear extinction when fear levels are relatively high, contrasting drug effects could emerge after fear levels have partially decreased, especially considering that in a model of post-traumatic stress disorder, chronic tianeptine treatment reduced several indicators of fear and anxiety.62 These results underscore how different aspects of emotional processing may be differentially sensitive to pharmacological interventions, which merit detailed clinical evaluation.
References
Ravindran LN, Stein MB . The pharmacologic treatment of anxiety disorders: a review of progress. J Clin Psychiatry 2010; 71: 839–854.
Bisson J, Andrew M . Psychological treatment of post-traumatic stress disorder (PTSD). Cochrane Database Syst Rev 2007; CD003388.
Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Agustsdottir A et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science 2011; 334: 1731–1734.
Hetrick SE, Purcell R, Garner B, Parslow R . Combined pharmacotherapy and psychological therapies for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev 2010; CD007316.
Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 2004; 61: 1136–1144.
Defrance R, Marey C, Kamoun A . Antidepressant and anxiolytic activities of tianeptine: an overview of clinical trials. Clin Neuropharmacol 1988; 11: S74–S82.
Franciskovic T, Sukovic Z, Janovic S, Stevanovic A, Nemcic-Moro I, Roncevic-Grzeta I et al. Tianeptine in the combined treatment of combat related posttraumatic stress disorder. Psychiatr Danub 2011; 23: 257–263.
Wilde MI, Benfield P . Tianeptine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depression and coexisting anxiety and depression. Drugs 1995; 49: 411–439.
Lepine JP, Altamura C, Ansseau M, Gutierrez JL, Bitter I, Lader M et al. Tianeptine and paroxetine in major depressive disorder, with a special focus on the anxious component in depression: an international, 6-week double-blind study dagger. Hum Psychopharmacol 2001; 16: 219–227.
Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE . The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry 2004; 55: 1171–1178.
Burghardt NS, Bush DE, McEwen BS, LeDoux JE . Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT(2C) receptor antagonist. Biol Psychiatry 2007; 62: 1111–1118.
Alby J, Ferreri M, Cabane J, De Bodinat C, Dagens V . Efficacy of tianeptine for the treatment of major depression and dysthymia with somatic complaints. A comparative study versus fluoxetine. Annales De Psychiatrie 1993; 8: 136–144.
McEwen BS, Chattarji S, Diamond DM, Jay TM, Reagan LP, Svenningsson P et al. The neurobiological properties of tianeptine (Stablon): from monoamine hypothesis to glutamatergic modulation. Mol Psychiatry 2010; 15: 237–249.
Piroli GG, Reznikov LR, Grillo CA, Hagar JM, Fadel JR, Reagan LP . Tianeptine modulates amygdalar glutamate neurochemistry and synaptic proteins in rats subjected to repeated stress. Exp Neurol 2013; 241: 184–193.
Svenningsson P, Bateup H, Qi H, Takamiya K, Huganir RL, Spedding M et al. Involvement of AMPA receptor phosphorylation in antidepressant actions with special reference to tianeptine. Eur J Neurosci 2007; 26: 3509–3517.
Zhang H, Etherington LA, Hafner AS, Belelli D, Coussen F, Delagrange P et al. Regulation of AMPA receptor surface trafficking and synaptic plasticity by a cognitive enhancer and antidepressant molecule. Mol Psychiatry 2013; 18: 471–484.
Reznikov LR, Grillo CA, Piroli GG, Pasumarthi RK, Reagan LP, Fadel J . Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: differential effects of antidepressant treatment. Eur J Neurosci 2007; 25: 3109–3114.
Qi H, Mailliet F, Spedding M, Rocher C, Zhang X, Delagrange P et al. Antidepressants reverse the attenuation of the neurotrophic MEK/MAPK cascade in frontal cortex by elevated platform stress; reversal of effects on LTP is associated with GluA1 phosphorylation. Neuropharmacology 2009; 56: 37–46.
Burghardt NS, Sigurdsson T, Gorman JM, McEwen BS, LeDoux JE . Chronic antidepressant treatment impairs the acquisition of fear extinction. Biol psychiatry 2013; 73: 1078–1086.
Phelps EA, Delgado MR, Nearing KI, LeDoux JE . Extinction learning in humans: role of the amygdala and vmPFC. Neuron 2004; 43: 897–905.
Milad MR, Quirk GJ . Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol 2012; 63: 129–151.
Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 2003; 24: 151–180.
Aggleton J (ed). The Amygdala: a Functional Analysis. Oxford University Press: London, 2000.
Quirk GJ, Garcia R, González-Lima F . Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry 2006; 60: 337–343.
Rocher C, Spedding M, Munoz C, Jay TM . Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb Cortex 2004; 14: 224–229.
Maviel T, Durkin TP, Menzaghi F, Bontempi B . Sites of neocortical reorganization critical for remote spatial memory. Science 2004; 305: 96–99.
Jolkkonen E, Pitkanen A . Intrinsic connections of the rat amygdaloid complex: projections originating in the central nucleus. J Comp Neurol 1998; 395: 53–72.
Paxinos G, Watson C . The Rat Brain in Stereotaxic Coordinates, 4th edn. Academic Press: San Diego, CA, USA, 1998.
Fanselow MS . Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci 1980; 15: 177–182.
Abdi H . Z-scores. In: Salkind NJ (ed). Encyclopedia of Measurement and Statistics, vol. 3. Sage: Thousand Oaks, CA, USA, 2007, pp 1055–1058.
Pare D, Duvarci S . Amygdala microcircuits mediating fear expression and extinction. Curr Opin Neurobiol 2012; 22: 717–723.
Huber D, Veinante P, Stoop R . Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science 2005; 308: 245–248.
Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A . Switching on and off fear by distinct neuronal circuits. Nature 2008; 454: 600–606.
Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 2010; 468: 277–282.
Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 2010; 468: 270–276.
Busti D, Geracitano R, Whittle N, Dalezios Y, Mańko M, Kaufmann W et al. Different fear states engage distinct networks within the intercalated cell clusters of the amygdala. J Neurosci 2011; 31: 5131–5144.
Wolff SB, Gründemann J, Tovote P, Krabbe S, Jacobson GA, Müller C et al. Amygdala interneuron subtypes control fear learning through disinhibition. Nature 2014; 509: 453–458.
Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B . Experience-dependent modification of a central amygdala fear circuit. Nature Neurosci 2013; 16: 332–339.
Sun N, Cassell MD . Intrinsic GABAergic neurons in the rat central extended amygdala. J Comp Neurol 1993; 330: 381–404.
Dong YL, Fukazawa Y, Wang W, Kamasawa N, Shigemoto R . Differential postsynaptic compartments in the laterocapsular division of the central nucleus of amygdala for afferents from the parabrachial nucleus and the basolateral nucleus in the rat. J Comp Neurol 2010; 518: 4771–4791.
McDonald AJ . Cytoarchitecture of the central amygdaloid nucleus of the rat. J Comp Neurol 1982; 208: 401–418.
Chieng BC, Christie MJ, Osborne PB . Characterization of neurons in the rat central nucleus of the amygdala: cellular physiology, morphology, and opioid sensitivity. J Comp Neurol 2006; 497: 910–927.
Nitecka L, Ben-Ari Y . Distribution of GABA-like immunoreactivity in the rat amygdaloid complex. J Comp Neurol 1987; 266: 45–55.
Pitkanen A, Savander V, LeDoux JE . Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci 1997; 20: 517–523.
Bernard JF, Alden M, Besson JM . The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol 1993; 329: 201–229.
McDonald AJ, Mascagni F, Guo L . Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience 1996; 71: 55–75.
Royer S, Pare D . Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience 2002; 115: 455–462.
Vouimba RM, Munoz C, Diamond DM . Differential effects of predator stress and the antidepressant tianeptine on physiological plasticity in the hippocampus and basolateral amygdala. Stress 2006; 9: 29–40.
Pillai AG, Anilkumar S, Chattarji S . The same antidepressant elicits contrasting patterns of synaptic changes in the amygdala vs hippocampus. Neuropsychopharmacology 2012; 37: 2702–2711.
Pare D, Royer S, Smith Y, Lang EJ . Contextual inhibitory gating of impulse traffic in the intra-amygdaloid network. Ann N Y Acad Sci 2003; 985: 78–91.
O'Donnell P, Grace AA . Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci 1995; 15: 3622–3639.
Nicola SM, Surmeier DJ, Malenka RC . Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Ann Revi Neurosci 2000; 23: 185–215.
Rosenkranz JA, Buffalari DM, Grace AA . Opposing influence of basolateral amygdala and footshock stimulation on neurons of the central amygdala. Biolo Psychiatry 2006; 59: 801–811.
Dalton GL, Wang YT, Floresco SB, Phillips AG . Disruption of AMPA receptor endocytosis impairs the extinction, but not acquisition of learned fear. Neuropsychopharmacology 2007; 33: 2416–2426.
Sah P, Faber E, De Armentia ML, Power J . The amygdaloid complex: anatomy and physiology. Physiolo Rev 2003; 83: 803–834.
Sotres-Bayon F, Bush DE, LeDoux JE . Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology 2007; 32: 1929–1940.
Pinard C, Mascagni F, McDonald A . Medial prefrontal cortical innervation of the intercalated nuclear region of the amygdala. Neuroscience 2012; 205: 112–124.
Sotres-Bayon F, Quirk GJ . Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol 2010; 20: 231–235.
Knapska E, Maren S . Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem 2009; 16: 486–493.
Muigg P, Hetzenauer A, Hauer G, Hauschild M, Gaburro S, Frank E et al. Impaired extinction of learned fear in rats selectively bred for high anxiety–evidence of altered neuronal processing in prefrontal-amygdala pathways. Eur J Neurosci 2008; 28: 2299–2309.
Maren S, Quirk GJ . Neuronal signalling of fear memory. Nat Rev Neurosci 2004; 5: 844–852.
Zoladz PR, Fleshner M, Diamond DM . Differential effectiveness of tianeptine, clonidine and amitriptyline in blocking traumatic memory expression, anxiety and hypertension in an animal model of PTSD. Prog Neuropsychopharmacol Biol Psychiatry 2013; 44: 1–16.
Acknowledgements
We thank MJ Sanders and CK Cain for their comments on the manuscript, and A Hambucken, E Davenas and A Eckmier for their technical assistance. BP Godsil was supported by fellowships from INSERM as well as financial support from IRS, Servier Company. B Bontempi was supported by funding from the Fondation pour la Recherche Médicale (FRM: DEQ20130326468), CNRS and University of Bordeaux. This work was partially funded by grants from INSERM and IRS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that the research of BP Godsil, including salary, has been supported by grants from INSERM and IRS. TM Jay is employed by INSERM, and her research has been supported by grants from INSERM and IRS. M Spedding was employed by IRS and Les Labotaoires Servier. P Delegrange is currently employed by IRS and Les Labotaoires Servier. The remaining authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Godsil, B., Bontempi, B., Mailliet, F. et al. Acute tianeptine treatment selectively modulates neuronal activation in the central nucleus of the amygdala and attenuates fear extinction. Mol Psychiatry 20, 1420–1427 (2015). https://doi.org/10.1038/mp.2014.169
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2014.169
- Springer Nature Limited