Abstract
Natural killer (NK) cells are key components of the innate immune system, providing potent antitumor immunity. Here, we show that the tumor growth factor-β (TGF-β)/SMAD signaling pathway is an important mechanism for NK cell immune evasion in childhood B-acute lymphoblastic leukemia (ALL). We characterized NK cells in 50 consecutive children with B-ALL at diagnosis, end induction and during maintenance therapy compared with age-matched controls. ALL-NK cells at diagnosis had an inhibitory phenotype associated with impaired function, most notably interferon-γ production and cytotoxicity. By maintenance therapy, these phenotypic and functional abnormalities partially normalized; however, cytotoxicity against autologous blasts remained impaired. We identified ALL-derived TGF-β1 to be an important mediator of leukemia-induced NK cell dysfunction. The TGF-β/SMAD signaling pathway was constitutively activated in ALL-NK cells at diagnosis and end induction when compared with healthy controls and patients during maintenance therapy. Culture of ALL blasts with healthy NK cells induced NK dysfunction and an inhibitory phenotype, mediated by activation of the TGF-β/SMAD signaling pathway, and abrogated by blocking TGF-β. These data indicate that by regulating the TGF-β/SMAD pathway, ALL blasts induce changes in NK cells to evade innate immune surveillance, thus highlighting the importance of developing novel therapies to target this inhibitory pathway and restore antileukemic cytotoxicity.
Similar content being viewed by others
Introduction
Although cure rates for pediatric acute lymphoblastic leukemia (ALL) approach 90%, outcomes in high-risk subgroups and salvage rates remain poor.1 As conventional chemotherapy is optimized currently to near-maximal tolerable intensity, novel approaches such as immunotherapy are vital to improve outcomes in high-risk disease.
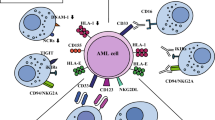
It is well established that natural killer (NK) cells have a critical role in the innate immune response against malignancies, including leukemia.2, 3 The ability of NK cells to kill targets or produce cytokines depends on the balance between signals from activating and inhibitory cell-surface receptors. Activating receptors, which include the natural cytotoxicity receptors NKp46, NKp30, NKp44 and NKG2D,4, 5, 6 recognize stress molecules upregulated on transformed or virally infected targets; however, the cognate ligand for many activating receptors remains unknown.7 Inhibitory receptors, notably the killer immunoglobulin-like receptors (KIRs) and the C-type lectin NKG2A, are specific for different human leukocyte antigen (HLA) molecules on target cells, and transmit signals that inhibit NK cytotoxicity upon engagement.8 Accordingly, NK cells can kill targets that have downregulated surface HLA class-I molecules. Cancer cells can impair NK function through a number of mechanisms including modulation of their surface receptors,9 and release of soluble factors with immunosuppressive properties such as interleukin-10 (IL-10) or tumor growth factor-β (TGF-β).10, 11, 12, 13
Here, we show that mechanisms of tumor escape from NK cell-mediated immunity occur in childhood B-ALL. In a cohort of childhood B-ALL patients sampled at diagnosis, end induction and maintenance therapy, we found evidence of altered NK phenotype and function compared with age-matched controls. The abnormalities only partially corrected during maintenance therapy and could be induced in healthy NK cells following coculture with ALL blasts in vitro via the release of soluble factors, notably TGF-β1. Finally, we report higher expression of phospho-SMAD2/3, the most important signal transducers for transmission of TGF-β1 intracellular signaling,14 in ALL-NK cells at diagnosis and end induction compared with maintenance therapy or healthy controls, thus providing mechanistic insights into the critical role of TGF-β in inducing NK dysfunction in childhood ALL. Taken together, these data suggest that ALL blasts through the release of immunomodulatory factors, critically TGF-β1, induce long-lasting changes in NK cells to evade immune surveillance.
Materials and methods
Samples were collected following informed consent from 50 consecutive patients with newly diagnosed B-ALL at Texas Children’s Cancer Center from September 2012 to March 2014. Peripheral blood (PB) samples were obtained at diagnosis (DX, n=50), day 29 following month-long induction (IND-29, n=50) and during maintenance therapy (n=20) under research protocols approved by the Baylor College of Medicine Institutional Review Board. PB samples were obtained from age-matched (n=20) and adult healthy controls (n=5). PB mononuclear cells (PBMCs) and ALL blasts (from diagnostic bone marrow) were separated using Ficoll density separation (Lymphoprep; Stemcell Technologies, Vancouver, BC, Canada) and cryopreserved.
Phenotyping
PBMCs were immunostained with CD56 and CD3 monoclonal antibodies to identify the NK population (CD56+CD3−), and with CD10/CD19 monoclonal antibodies (BD Biosciences, San Jose, CA, USA) to exclude ALL blasts. NK cells were analyzed for the expression of natural cytotoxicity receptors (NKp30, NKp44, NKp46), activating/inhibitory C-type lectins (NKG2D/NKG2A) and KIRs (KIR2DL1/S1, KIR2DL2/L3, KIR3DL1) (BioLegend, San Diego, CA, USA). Blasts were analyzed for the expression of relevant NK ligands: HLA-A/B/C (ligands for inhibitory KIRs), major histocompatibility complex class-I chain-related genes A/B (MICA/B, ligands for NKG2D), HLA-E (ligand for NKG2A) and HLA-DR4/5 (BioLegend). Controls for blast phenotyping included negatively selected healthy B cells using the B-Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were acquired using an LSRII Cytometer (BD Biosciences) and analyzed using the FlowJo software version 7.6 (Tree Star, San Carlos, CA, USA).
Cytotoxicity studies
Twenty patients had PBMCs available from DX, IND-29 and maintenance therapy. Cells from each time point were thawed, stained and analyzed on the same day to minimize variability. PBMCs were rested in RPMI/FCS 10% (Roswell Park Memorial Institute/fetal calf serum 10%) overnight and incubated with targets for 5 h at an NK:target ratio of 1:1, based on sample NK frequency. Targets included major histocompatibility complex class-I-deficient K562 cells (grown in RPMI/FCS 10%) and autologous blasts. Negative and positive controls included PBMCs cultured alone or stimulated with phorbol 12-myristate 13-acetate (PMA) (50 ng/ml)/ionomycin (2 μg/ml; Sigma-Aldrich, Gillingham, Dorset, UK), respectively. CD107a antibody, monensin (BD GolgiStop, San Jose, CA, USA) and brefeldin A (Sigma, Gillingham, Dorset, UK) were added to cultures at the start of incubation as published previously.15 Cells were stained with CD56 and CD3 monoclonal antibodies (Fisher Scientific, Pittsburgh, PA, USA), fixed/permeabilized (BD Biosciences), followed by intracellular cytokine staining with interferon-γ (IFN-γ) and TNF-α monoclonal antibodies (Fisher Scientific). NK cytotoxicity was compared at all time points, and with age-matched controls. Cells were acquired on a BD LSRFortessa cytometer (BD Biosciences) (non-viable cells excluded using LIVE/DEAD Aqua Dead-Cell Stain Kit; Life Technologies) and analyzed using the FlowJo software. Surface receptor phenotyping and functional assays were performed in triplicate based on sample availability.
As validation, in a subset of patients (n=15) with sufficient PBMCs available, we magnetically negatively selected NK cells using the NK Cell Isolation Kit (Miltenyi Biotech, San Diego, CA, USA) and performed 51chromium release cytotoxicity assays against K562 targets and B-ALL blasts, as published previously.16
Coculture experiments
Healthy NK cells were negatively selected and cultured in 96-well plates at 200 000 cells per well for 24 h, in the presence or absence of ALL blasts or B-ALL cell lines (JM-1/RS4;11/MUTZ5) and 20 IU/ml rhIL-2 (PROLEUKIN; Chiron Corporation, Emeryville, CA, USA). Controls included allogeneic negatively selected healthy B cells (Miltenyi Biotec) and NK cells alone. In a subset of experiments, healthy NK cells were cocultured with ALL blasts in transwell devices (96-well/1 μm micropores; Millipore, Millipore Plate, Fisher Scientific) at a 1:1 ratio, and their effector function analyzed. Following coculture, supernatants were evaluated for cytokine production using the ProcartaPlex Human Cytokine Panel immunoassay with the Luminex-100 reader according to the manufacturer’s instructions. Supernatants were evaluated for TGF-β1 production using the Human TGF-β1 Platinum ELISA assay (ebiosciences, San Diego, CA, USA).
TGF-β1-blocking experiments
ALL blasts and healthy NK cells were cultured in the presence/absence of anti-TGF-β (0.1 μg/μl; TB21) for 24 h. NK cells were then negatively selected and their effector function assessed.
TGF-β1 coculture experiments
Ex vivo selected healthy donor NK cells were cultured in the presence of IL-2 (200 U/ml) for 5 days and TGF-β1 (10 ng/ml) was added to the cells on days 0 and 3 of culture. Surface expression of activating and inhibitory receptors was examined as described above.
P-SMAD-specific phospho-flow cytometry
PBMCs from ALL patients at DX (n=6), IND-29 (n=6), maintenance therapy (n=5) and controls (n=6) were thawed, rested in RPMI/FCS 10% for 4 h and NK cells negatively selected using the NK Cell Isolation Kit. TGF-β1 (Peprotech, Rocky Hill, NJ, USA; Ref100-21) was diluted to 5 ng/μl in HCl and 10 ng/ml added to healthy NK cells for 1 h at 37 °C (positive control). Cells were stained with phospho-SMAD2/3 A647 antibody (BD Biosciences) using the PerFix EXPOSE Phospho-Epitopes Exposure Kit (Beckman Coulter, Brea, CA, USA).
Expansion of NK cells
Cryopreserved PBMCs from ALL samples and controls were thaed, resuspended in RPMI 10%/Click’s Medium plus 100 IU/ml IL-2 and stimulated with irradiated K562 cells transduced with membrane-bound IL-15/41BB ligand (K562-mb15-41BBL, a gift from Cliona Rooney, Center for Cell and Gene Therapy, Houston, TX, USA) at a ratio of 1:2. T cells were magnetically depleted using CD3 microbeads (Miltenyi Biotec). Following 14 days of culture and expansion,17 NK cells were harvested for cytotoxicity assays.
Statistical analyses
Values are expressed as means or medians and standard error of the mean. Statistical significance was assessed with Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA) using unpaired or paired two-tailed t-test and by nonparametric one-way analysis of variance, as appropriate. P-values ≤0.05 were considered significant. To standardize comparisons of median fluorescence intensities (MFIs), we used resolution index (Xpos−Xnegative)/√(SDpos2+SDnegative2).18, 19
Results
Patient and control characteristics
NK cells from pediatric B-ALL patients were analyzed at DX (n=50), IND-29 (n=50) and maintenance therapy. Linked samples from multiple time points were available for 20 patients. Results were compared with age-matched controls (patient characteristics are presented in Table 1). Patients were enrolled on or treated according to Children’s Oncology Group protocols NCI-2011-02599 and NCI-2011-03797, and received appropriate supportive care, including antibiotic, anti-viral and anti-fungal therapy as per institutional protocols.
At diagnosis, ALL-NK cells are reduced in frequency and display an abnormal phenotype
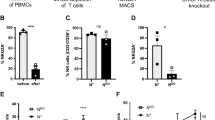
Using flow cytometry, we analyzed NK cell frequencies, and surface expression of NK receptors in 50 newly diagnosed ALL patients compared with 20 age-matched controls (Figure 1). The gating strategy/histograms are presented in Figures 1a and b. The percentage/absolute numbers of NK cells were significantly lower in ALL patients at DX compared with healthy controls (mean 3.3±0.33% vs 6.2±0.71%, P<0.0001 and mean 255±48.9 vs 442±48.3/μl, P=0.016 respectively; Figure 1c), with comparable frequencies of T cells (data not shown). ALL-NK cells at DX had an abnormal phenotype compared with controls, with increased frequencies of NK cells expressing the inhibitory receptor NKG2A (mean 30.1±2.7% vs 16.6±3.5%, P=0.007) (Figure 1d) and lower frequencies of NK cells expressing the activating receptor NKp46 (mean 23.3±3.2% vs 55.3±7.1%, P<0.0001) (Figure 1e). We found no significant difference in the expression of NKp30, NKp44, NKG2D (Supplementary Figure 1A) or KIRs in patients compared with controls (data not shown). Interestingly, we found a higher percentage of the immunoregulatory CD56brightCD16−CD3− ALL-NK cells at DX compared with controls, although this did not reach statistical significance (Supplementary Figure 1B).
NK surface phenotype is abnormal in B-ALL patients compared with healthy pediatric age-matched controls. (a) Gating strategy for NK cell-surface phenotyping. NK cells from pediatric patients were defined by gating on the CD10−CD19−CD3−CD56+ lymphocyte fraction of PBMCs. (b) Histograms depict NK cell-surface phenotype in a representative ALL patient and healthy control. Unshaded histograms represent isotype control and shaded histograms represent staining with specific phycoerythrin (PE)-conjugated monoclonal antibodies. (c) Frequencies and absolute numbers of NK cells in 50 patients with ALL at diagnosis (DX) compared with 20 healthy controls. (d) Frequency of NKG2A+CD56+ and (e) NKp46+CD56+ NK cells in 50 patients with ALL at DX compared with 20 healthy controls. Error bars denote standard error of the mean between individuals within group. P-values report significance of unpaired t-tests.
NK cell phenotypic abnormalities in ALL patients are associated with impaired effector function
We next determined whether these phenotypic abnormalities were associated with altered NK cell functions. Owing to the limited volume of blood available from each patient and the number of assays performed at each time point, we used a flow-based assay and gated on CD56+CD3− NK cells to assess their ability to degranulate (CD107a positivity) and to produce IFN-γ and TNF-α in response to K562 targets. ALL-NK cells at DX had significantly reduced CD107a degranulation (mean CD56+CD3−CD107+ 5.8±1.2% vs 13.3±2.4%, P=0.0027) and IFN-γ production (mean CD56+CD3− IFN-γ+ 1.5±0.4% vs 4.2±0.7%, P=0.0005) compared with NK cells from age-matched controls, with comparable release of TNF-α (mean CD56+CD3− TNF-α+ 2.7±0.9% vs 3.9±0.5%, P=0.26). Further, ALL-NK cells at DX failed to degranulate or produce effector cytokines in response to autologous blasts (Figure 2a). To validate our flow-based functional data and confirm that cytokine secretion and degranulation correlate with target cell killing, 51chromium release assay was used as the gold standard to assess the ability of magnetically purified NK cells (>95% purity) to kill K562 target cells and autologous B-ALL blasts. This approach also allowed us to exclude the contribution of other effector cells such as T cells to target cell killing, as well as exclude the inhibitory effect of immunosuppressive cells such as regulatory T cells on NK cell function. As shown in Figure 2b, magnetically selected NK cells from ALL patients at diagnosis had significant impairment in their ability to kill K562 targets as well as autologous blasts.
NK cell cytotoxicity and effector function in ALL patients at diagnosis compared with pediatric age-matched controls. (a) CD56+CD3− NK cell cytotoxicity (CD107a degranulation), IFN-γ and TNF-α production against K562 leukemia targets, autologous blasts and in response to PMA/ionomycin (P/I; positive control) in 20 patients with ALL compared with 10 healthy controls. (b) Specific lysis (51Cr release assay) of NK cells from ALL patients at DX against K562 targets (circle—solid lines) or autologous blasts (circle—dashed lines) compared with healthy NK cells against K562 targets (inverted triangle) (n=5). Error bars denote standard error of the mean. P-values report significance of paired or unpaired t-tests as appropriate.
Expression of MICA/B and HLA class-I molecules are lower on ALL blasts compared with healthy B cells
NK cell receptor alterations represent a mechanism of leukemia escape only if the affected NK cell receptors are involved in ALL recognition. We measured expression of NK cell receptor ligands including HLA class-I (HLA-A/B/C; ligands for inhibitory KIRs), MICA/B (ligands for NKG2D) and HLA-E (ligand for activating receptor NKG2C/inhibitory receptor NKG2A) on ALL blasts. The MFI of MICA/B was significantly lower on ALL blasts compared with healthy B cells (median MFI 248; range 68–366 vs 419; range 419–426; P=0.01), as was the MFI of HLA-E (median MFI 946; range 636–4064 vs 10000; range 8178–16600; P<0.0001). Interestingly, HLA class-I was expressed on the surface of almost all blasts, although significantly lower compared with healthy B cells (median MFI 5371; range 1067–11 000 vs 10 300; range 7368–12 900; P=0.016; Figure 3 and Supplementary Figure 2). These data indicate the existence of altered expression of NK receptor ligands within certain populations of B-ALL blasts, suggesting that ALL blasts may evade NK cell immune surveillance by downregulating or shedding ligands for activating NK receptors.
ALL cells suppress NK function through the release of soluble factors including TGF-β1
To determine whether ALL blasts induce alterations in allogeneic NK cells, NK cells from five healthy controls were cultured with ALL blasts from 12 patients and three B-ALL cell lines (JM-1, MUTZ5, RS4; 11) at a 1:1 ratio for 24 h. When compared with NK cells cultured alone, coculture of ALL blasts with healthy NK cells induced significant impairment in NK function against K562 targets: namely, CD107a degranulation (mean CD56+CD3−CD107+ 7.9±1.55%; range 0.5–17% vs 22%±2.3; range 18–39%, P=0.0074), IFN-γ (mean CD56+CD3−IFN-γ+ 3.6±0.66%; range 0.2–13.8% vs 15.7±0.37%; range 15–18.4%, P<0.0001) and TNF-α secretion (mean CD56+CD3−TNF-α+ 2.5±0.40%; range 0.35–4.2% vs 14.1±1.53%; range 11.1–16%; P=0.0015) when compared with NK cells cultured alone (Figures 4a and b). Although coincubation of NK cells with healthy allogeneic B cells resulted in some inhibition in NK effector function, the degree of suppression was significantly less than that observed with B-ALL blasts (Figure 4b). In four experiments, sufficient cells were available to test the dose effect of ALL blasts on NK function. At an NK:ALL ratio of 10:1, NK effector abnormalities were still observed but to a lesser extent (Supplementary Figure 3).
ALL cells induce impairment in NK cell effector function in vitro. (a) Healthy donor NK cells were incubated with blasts from ALL patients at 1:1 ratio for 24 h in the presence of IL-2 (20 IU/ml) and their effector response assessed against K562 cells. Representative FACS plots depict NK cell response (CD107a degranulation, IFN-γ and TNF-α) against K562 targets. Plots are gated on CD56+CD3− NK cells. (b) CD107a degranulation and cytokine response against K562 targets were measured in NK cells cultured either alone or with primary ALL blasts or healthy allogeneic B cells at a 1:1 ratio for 24 h under contact-dependent conditions. P-values report significance of paired t-tests. (c) Comparable inhibitory effect of ALL blasts on NK cell cytotoxicity and effector function under both contact-dependent and transwell conditions. Horizontal bars denote mean expression. Error bars denote standard error of the mean between individual values within group. P-values report significance of paired t-tests. (d) Following centrifugation, supernatant from the coculture experiments were harvested for TGF-β by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions using the Human TGF-β1 Platinum ELISA (ebiosciences) assay. Median TGF-β concentration in supernatant of healthy NK cells cocultured for 24 h with B-ALL blasts vs healthy allogeneic B cells, B-ALL blasts alone and healthy NK cells alone was compared. Results are compiled from seven different experiments. Cytokine concentrations in supernatants are expressed as mean plus standard deviation of triplicates. P-values report significance of paired t-tests (or unpaired t-test when comparing ALL blasts alone to healthy NK cells alone). (e) Partial reversal of suppression of degranulation (CD107a) and cytokine release (IFN-γ and TNFα) in the presence of TGF-β blockade (% reversal suppression=% cytokine expression in presence of TGF-β blockade−% cytokine expression in absence of blockade/% cytokine expression in absence of blockade). (f) Healthy donor NK cell degranulation and cytokine production in response to K562 targets following coculture with ALL blasts (in the presence of IL-2 50 IU/ml) in the presence or absence of TGF-β1 blockade. P-values report significance of paired t-tests comparing cytokine release in the presence or absence of TGF-β1 blockade.
To investigate whether the inhibitory effects of ALL blasts on NK function were mediated by soluble or contact-dependent factors, coculture experiments were performed in transwells to separate ALL blasts and NK cells. The inhibitory effect of ALL blasts on CD107a degranulation and IFN-γ production was seen to a similar extent under both transwell and direct cell–cell contact conditions, suggesting that soluble factors released by ALL blasts induce NK dysfunction (Figure 4c). We determined the levels of soluble factors known to be increased in other cancers and to alter lymphocyte functions, such as TGF-β1, GM-CSF (granulocyte–macrophage colony-stimulating factor), IL-10, IL-1β,20 IL-6,21 IL-1822 and IL-21 in supernatants collected from the coculture experiments (Supplementary Table 1). Under both transwell conditions and cell–cell contact, we found increased levels of several cytokines, most notably TGF-β1 in the supernatants collected from ALL blasts cultured alone or with healthy donor NK cells compared with healthy NK cells cultured alone or with healthy donor B cells. These data confirm ALL blasts to be the source of TGF-β1 in the coculture experiments (Figure 4d).
Blockade of TGF-β1 partially corrects ALL-induced NK dysfunction
As TGF-β1 has been shown to mediate immunomodulatory effects in several cancers,23, 24, 25 we further explored its contribution to ALL-induced NK dysfunction. The addition of a TGF-β1-blocking antibody to cocultures of NK cells plus ALL blasts partially restored NK function; CD107a suppression reversed by a median of 95% (range 82–110%); IFN-γ by 50% (range 47–74%); and TNF-α by 51% (range 40–72%; (Figure 4e). Figure 4f summarizes degranulation/cytokine production by healthy NK cells in response to K562 targets following coculture with ALL blasts in the presence or absence of TGF-β1 blockade, compared with healthy NK cells cultured alone.
The TGF-β/SMAD signaling pathway mediates ALL-induced NK dysfunction
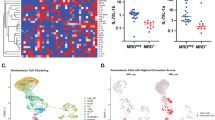
To further examine the mechanisms by which ALL induces NK dysfunction, we assessed the phosphorylation/activation of molecules known to be important for TGF-β signaling in NK cells. We incubated healthy NK cells with primary ALL blasts and observed a strong induction of SMAD2/3 phosphorylation in NK cells. This effect was completely abrogated by the addition of TGF-β-blocking antibody to the cocultures, indicating that ALL cells secrete TGF-β and induce NK dysfunction by activating the TGF-β/SMAD pathway (Figure 5a). Using phospho-flow, we evaluated the expression of SMAD2/3 in NK cells from ALL patients at DX, IND-29 and during maintenance therapy (n=6). Strikingly, the TGF-β/SMAD pathway was constitutively activated in ALL-NK cells at diagnosis (median expression 92.2%; range 65.9–93.1%) compared with that (4.72%; range 1.04–9.15%) in healthy donors (P<0.0001). SMAD2/3 phosphorylation remained considerably elevated in ALL-NK cells at IND-29 but significantly decreased by maintenance therapy (Figures 5b and c).
ALL uses the TGF-β/SMAD pathway to suppress NK cell effector function. (a) Representative histograms showing p-SMAD2/3 expression in healthy donor NK cells cultured either (i) alone, (ii) with exogenous TGF-β, (iii) following coculture for 24 h with primary ALL blasts or (iv) following coculture for 24 h with primary ALL blasts in the presence of TGFβ1-blocking antibody. Unshaded histograms represent isotype control. Shaded histograms represent phospho-SMAD2/3 expression in NK cells. (b) Representative histograms depicting phospho-SMAD2/3 expression in NK cells from an ALL patient at DX, IND-29 and maintenance therapy (Maint). Unshaded histograms represent isotype control. Shaded histograms represent phospho-SMAD2/3 expression in NK cells. (c) Comparison of phospho-SMAD2/3 expression in NK cells from healthy controls (n=4) and ALL patients at DX (n=6), IND-29 (n=6) and Maint (n=6). Error bars denote standard error of the mean. P-values for comparison of NK cells from ALL patients at DX, IND-29 and Maint to healthy control NKs were derived using unpaired t-tests. P-values for comparison of NK cells from ALL patients at DX, IND-29 and Maint were derived using paired t-tests.
Exogenous TGF-β1 modulates the expression of activating and inhibitory receptors on NK cells
To further explore the mechanisms by which TGF-β1/SMAD signaling pathway is involved in NK immune evasion, we analyzed the phenotype of healthy donor NK cells following coculture with exogenous TGF-β1. Five days of coculture with TGF-β1 induced an inhibitory phenotype in healthy NK cells similar to that of ALL-NK cells, with downregulation of activating receptors including NKG2D, NKp30 and NKp46, and upregulation of the inhibitory receptor NKG2A (Supplementary Figure 4). Coculture of primary ALL blasts with healthy NK cells for 24–48 h (n=8) induced a similar inhibitory phenotype with significant upregulation of NKG2A and downregulation of NKp46 (Supplementary Figure 5).
NK cell phenotype and function is partially restored in patients at remission
To investigate the duration of ALL-induced NK cell abnormalities, we examined the NK phenotype of ALL patients at two remission time points, IND-29 and maintenance therapy. To minimize variability, all maintenance samples were collected during cycles 2–4.
At IND-29, we observed partial restoration of phenotype, with an increase in the frequencies of NKp46-expressing NK cells from 24.7±2.9% at DX to 38.6±4.1% (P=0.009). By maintenance therapy, NKp46 expression had normalized (mean 53.1% vs 24.7% at DX and 38.6% at IND-29; P-values <0.0001 and 0.001, respectively), and was comparable to that of age-matched controls (mean 53.1±5.4% vs 55.3%±6, P=0.79). However, the frequencies of NKG2A+CD56+CD3− NK cells remained elevated, even during maintenance therapy (mean 49.2% at maintenance vs 30.1% at DX and 26.8% at IND-29, P=0.0013 and 0.0001, respectively; Figure 6a).
NK cell phenotypic and functional abnormalities are partially restored at end induction and maintenance therapy (Maint). (a) NKp46 and NKG2A expression in 20 patients at Maint compared with DX, IND-29 and healthy controls. Whereas NKp46 expression increased by Maint to levels approaching those of healthy controls, NKG2A expression remained increased compared with healthy controls. (b) NK cell cytotoxicity (CD107a degranulation), IFN-γ and TNF-α production against K562 targets in 25 patients with ALL at Maint compared with DX (n=20), IND-29 (n=20) and 10 healthy controls. Horizontal bars denote mean expression. Error bars denote standard error of the mean between individual values within group. P-values for comparison of ALL patients at DX, IND-29 and Maint to healthy controls were derived using unpaired t-tests. P-values for comparison of ALL patients at DX, IND-29 and Maint were derived using paired t-tests.
Concordant with the phenotypic changes, NK effector function in response to K562 targets was restored in the majority of patients by maintenance therapy (Figure 6b), associated with normalization of phospho-SMAD2/3 expression (Figures 5b and c). We found no significant differences in degranulation/cytokine release of maintenance ALL-NK cells against K562 targets compared with healthy control NK cells (mean CD56+CD3−CD107a+ frequencies 10.3±2.19% vs 13.3±2.38%, P=0.49; mean CD56+CD3−IFN-γ+ 2.88±0.50% vs 4.23±0.67%, P=0.16; mean CD56+CD3−TNF-α+ 3.91±0.710% vs 3.43±0.554%, P=0.59; Figure 6b). The normalization of NK effector responses to K562 targets in the majority of patients corresponded with the morphologic remission and MRD− status observed in 46/50 and 39/50 of patients, respectively.
Ex vivo-expanded and -activated NK cells from ALL patients in remission fail to kill autologous blasts
We next assessed whether NK cells from ALL patients in remission could kill autologous blasts cryopreserved at diagnosis. ALL-NK cells collected at IND-29 or maintenance therapy failed to kill/produce effector cytokines in response to autologous blasts in vitro, although ALL-NK cells from the maintenance time point were able to respond to K562 targets (Figures 7a and b). We therefore explored whether ex vivo expansion/activation of ALL-NK cells collected at IND-29 or maintenance therapy could enhance their cytotoxicity against autologous blasts. NK cells were expanded from PBMCs at IND-29 (median fold expansion 130, range 55–220-fold, n=4) and maintenance therapy (median fold expansion 183, range 73–389-fold, n=8). When compared with non-expanded NK cells, ex vivo-expanded NK cells from ALL patients at IND-29 or maintenance therapy had increased cytotoxicity against K562 targets (Figures 7b and d and Supplementary Figure 6), although the function remained inferior to that of expanded healthy donor NK cells. Ex vivo expansion of ALL-NK cells failed to significantly enhance their cytotoxicity against autologous blasts (Figures 7c and d), suggesting that ALL cells have undergone successful editing and evolved mechanisms to escape recognition by autologous NK cells.
Ex vivo expansion of NK cells from ALL patients in remission restores their cytotoxicity against K562 targets, but not against autologous ALL blasts. (a) NK cell cytotoxicity (CD107a degranulation), IFN-γ and TNF-α production against autologous blasts in patients with ALL (n=10 DX, n=10 IND-29, n=5 Maint). (b) CD107a degranulation, IFN-γ and TNF-α response by unmanipulated vs ex vivo-expanded ALL-NK cells from IND-29 or Maint against K562 leukemia targets (n=4 IND-29; n=8 Maint) or (c) autologous ALL blasts (n=3 IND-29; n=3 Maint). (d) The cytotoxic function of ex vivo-expanded and -activated NK cells from healthy controls (Exp Healthy NK) and ALL patients at maintenance therapy (Exp Maint) is measured by 51Cr release assay against K562 targets or ALL blasts (cryopreserved at diagnosis). Ex vivo-expanded NK cells from ALL patients at maintenance therapy could kill K562 targets (square, solid line) but not autologous blasts (square, dashed line). The cytotoxic function of expanded NK cells from ALL patients at maintenance therapy was inferior to that of expanded healthy NK cells against both K562 targets (inverted triangle, dashed line) or ALL blasts (star, dashed line).
Discussion
Understanding the role of the immune system in the control of cancer and the mechanisms mediating immune evasion remains one of the most challenging questions in tumor immunology. If immunotherapy for childhood ALL is to prove successful, a better understanding of the impact of leukemia on the immune system is critical. Here we report an important role for the TGF-β/SMAD signaling pathway in ALL-induced NK immune evasion.
We showed that pediatric B-ALL cells alter NK cell function through modulation of their surface receptors, a mechanism observed in other malignancies.13, 26, 27, 28 By inducing downregulation of activating receptors and upregulation of inhibitory receptors on NK cells, leukemia cells can escape NK recognition. Phenotypic and functional abnormalities were partially reversed following remission induction, with restoration of NKp46 expression but persistently increased inhibitory NKG2A. A possible explanation for the persistent inhibitory phenotype and decreased cytolytic activity at IND-29 is the use of corticosteroids as a standard component of Induction. By maintenance therapy, however, the majority of phenotypic and functional changes had normalized.
Whereas at diagnosis, NK cells showed impaired cytotoxicity against both K562 targets and ALL blasts, with chemotherapy and achievement of remission, there was a progressive improvement in NK cell function against K562 targets. However, at all time points tested, NK cells were unable to recognize autologous blasts. The use of selected NK cells and purified ALL blasts in our experiments eliminated the contribution of other effector cells such as T cells on target cell killing, as well as the inhibitory effect of immunosuppressive cells such as regulatory T cells on NK cell function. Although ex vivo expansion and activation of NK cells from ALL patients collected at remission significantly enhanced their cytotoxicity against K562 targets, there was minimal cytotoxicity against autologous blasts, further supporting the notion that during the course of the disease, ALL cells undergo successful immune editing, resulting in the selection of blasts with resistance to killing by autologous NK cells. As suggested by the partially retained activity of ALL-NK cells to PMA/ionomycin, NK dysfunction in ALL is likely related to impaired NK receptor–ligand interaction.29 One mechanism of immune editing may be mediated through MICA/B shedding.30, 31 Our observation that MICA/B is downregulated on ALL blasts suggests that through shedding of activating ligands, ALL cells may escape NK cell recognition. Intriguingly, in two of three patients with BCR-ABL+ ALL, we observed higher expression levels of MICA/B. In contrast to studies in adult ALL,32 however, we found no significant differences in the sensitivity of ALL blasts to NK cell killing among patients with different clinical or cytogenetic characteristics, although our study was not powered to address specifically the impact of prognostic factors on susceptibility to NK killing.
The alterations in ALL-NK cells and their reversibility in patients in remission suggest that ALL cells can actively modulate NK cell phenotype and function. Indeed, coculture of ALL blasts with healthy NK cells induced an inhibitory phenotype, as well as significant impairment in NK degranulation and cytokine production against K562 targets. This effect was still present under transwell conditions, suggesting that soluble factors are important in mediating ALL-induced NK dysfunction. Interestingly, coculture of allogeneic B cells with NK cells induced some impairment in NK effector function, albeit to a much lesser extent, likely mediated by regulatory B cells, a subset of B cells with immune suppressive function.33, 34, 35 We performed a comprehensive analysis of cytokines and growth factors reported to exert suppressive effects on immune effectors23, 24, 25 and showed significantly higher levels of ALL-derived TGF-β1 in the supernatant of ALL blasts cultured alone or with NK cells compared with NK cells alone. Although we did not have the opportunity to measure directly TGF-β in the sera of our patients, a number of studies have reported elevated serum levels of TGF-β in patients with other lymphoid malignancies such as hairy cell leukemia and multiple myeloma.36, 37 TGF-β-blocking monoclonal antibodies reversed ALL-mediated NK cell dysfunction, whereas addition of exogenous TGF-β1 to healthy donor NK cells in vitro induced an inhibitory phenotype comparable to that of ALL-NK cells, further supporting a direct role for TGF-β in modulation of NK cell phenotype and function. As a powerful physiological immune suppressant, TGF-β1 suppresses NK effector function through activation of the SMAD signaling pathway.14, 38, 39, 40 To further investigate TGF-β1-induced NK dysfunction in pediatric ALL, we measured the phosphorylation of SMAD2/3 in healthy NK cells following coculture with primary ALL blasts and showed that ALL blasts use the TGF-β1/SMAD pathway to inhibit NK cytotoxicity. Moreover, we found that ALL-NK cells had significantly elevated phospho-SMAD2/3 expression at DX and IND-29, correlating with impaired effector function. Increased phospho-SMAD2/3 in ALL-NK cells at IND-29 may be related to corticosteroid use during induction, considering the steroid receptor coactivator-1 has been shown to potentiate TGF-β1/SMAD signaling.41 Phospho-SMAD2/3 expression had significantly decreased by maintenance therapy, correlating with restoration of NK effector function against K562 targets.
Taken together, our study suggests that ALL actively evades innate immune surveillance by inducing changes in NK cells that limit their ability to kill autologous blasts. Numerous avenues are under investigation to enhance NK function, including engager molecules,42 genetic modification,43 immunomodulatory blockade and epigenetic therapies.43 Indeed, a number of drugs commonly used in salvage therapy for relapsed ALL such as bortezomib or valproate have been shown to sensitize ALL cells to NK-mediated lysis in vitro.44 Studies of NK cell adoptive therapy have shown promise in a number of settings.43, 44 However, our data do not support the use of autologous NK cells for the immunotherapy of ALL; instead, they support the use of allogeneic NK cells and the development of novel strategies to modulate the TGF-β pathway, such as engineered resistance to TGF-β with the dominant-negative TGF-β type II receptor40 to protect NK cells from ALL-induced dysfunction.
References
Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. J Clin Oncol 2012; 30: 1663–1669.
Lotzova E, Savary CA, Herberman RB . Inhibition of clonogenic growth of fresh leukemia cells by unstimulated and IL-2 stimulated NK cells of normal donors. Leuk Res 1987; 11: 1059–1066.
Lowdell MW, Craston R, Samuel D, Wood ME, O'Neill E, Saha V et al. Evidence that continued remission in patients treated for acute leukaemia is dependent upon autologous natural killer cells. Br J Haematol 2002; 117: 821–827.
Lanier LL . Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008; 9: 495–502.
Ljunggren HG, Karre K . In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today 1990; 11: 237–244.
Marsh SG, Parham P, Dupont B, Geraghty DE, Trowsdale J, Middleton D et al. Killer-cell immunoglobulin-like receptor (KIR) nomenclature report, 2002. Tissue Antigens 2003; 62: 79–86.
Molfetta R, Quatrini L, Capuano C, Gasparrini F, Zitti B, Zingoni A et al. c-Cbl regulates. Eur J Immunol 2014; 44: 2761–2770.
Karre K . Immunology. A perfect mismatch. Science 2002; 295: 2029–2031.
Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L . Effect of tumor cells and tumor microenvironment on NK-cell function. Eur J Immunol 2014; 44: 1582–1592.
Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ . Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011; 29: 235–271.
Whiteside TL . The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008; 27: 5904–5912.
Zitvogel L, Tesniere A, Kroemer G . Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol 2006; 6: 715–727.
Stringaris K, Sekine T, Khoder A, Alsuliman A, Razzaghi B, Sargeant R et al. Leukemia-induced phenotypic and functional defects in natural killer cells predict failure to achieve remission in acute myeloid leukemia. Haematologica 2014; 99: 836–847.
Trotta R, Dal CJ, Yu J, Ciarlariello D, Thomas B, Zhang X et al. TGF-beta utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. J Immunol 2008; 181: 3784–3792.
Alter G, Malenfant JM, Altfeld M . CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 2004; 294: 15–22.
Weber G, Caruana I, Rouce RH, Barrett AJ, Gerdemann U, Leen AM et al. Generation of tumor antigen-specific T cell lines from pediatric patients with acute lymphoblastic leukemia—implications for immunotherapy. Clin Cancer Res 2013; 19: 5079–5091.
Lapteva N, Durett AG, Sun J, Rollins LA, Huye LL, Fang J et al. Large-scale ex vivo expansion and characterization of natural killer cells for clinical applications. Cytotherapy 2012; 14: 1131–1143.
Hassett J, Parker J . Laboratory practices in reporting flow cytometry phenotyping results for leukemia/lymphoma specimens: results of a survey. Cytometry 1995; 22: 264–281.
Brando B, Sommaruga E . Nationwide quality control trial on lymphocyte immunophenotyping and flow cytometer performance in Italy. Cytometry 1993; 14: 294–306.
Elkabets M, Ribeiro VS, Dinarello CA, Ostrand-Rosenberg S, Di Santo JP, Apte RN et al. IL-1beta regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol 2010; 40: 3347–3357.
Jewett A, Tseng HC . Tumor induced inactivation of natural killer cell cytotoxic function; implication in growth, expansion and differentiation of cancer stem cells. J Cancer 2011; 2: 443–457.
Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N et al. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res 2011; 71: 5393–5399.
Castriconi R, Dondero A, Bellora F, Moretta L, Castellano A, Locatelli F et al. Neuroblastoma-derived TGF-beta1 modulates the chemokine receptor repertoire of human resting NK cells. J Immunol 2013; 190: 5321–5328.
Lee HM, Kim KS, Kim J . A comparative study of the effects of inhibitory cytokines on human natural killer cells and the mechanistic features of transforming growth factor-beta. Cell Immunol 2014; 290: 52–61.
Yang B, Liu H, Shi W, Wang Z, Sun S, Zhang G et al. Blocking transforming growth factor-beta signaling pathway augments antitumor effect of adoptive NK-92 cell therapy. Int Immunopharmacol 2013; 17: 198–204.
Costello RT, Sivori S, Marcenaro E, Lafage-Pochitaloff M, Mozziconacci MJ, Reviron D et al. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood 2002; 99: 3661–3667.
Epling-Burnette PK, Bai F, Painter JS, Rollison DE, Salih HR, Krusch M et al. Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood 2007; 109: 4816–4824.
Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest 2011; 121: 3609–3622.
Bonnema JD, Karnitz LM, Schoon RA, Abraham RT, Leibson PJ . Fc receptor stimulation of phosphatidylinositol 3-kinase in natural killer cells is associated with protein kinase C-independent granule release and cell-mediated cytotoxicity. J Exp Med 1994; 180: 1427–1435.
Bryceson YT, March ME, Ljunggren HG, Long EO . Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 2006; 107: 159–166.
North J, Bakhsh I, Marden C, Pittman H, Addison E, Navarrete C et al. Tumor-primed human natural killer cells lyse NK-resistant tumor targets: evidence of a two-stage process in resting NK cell activation. J Immunol 2007; 178: 85–94.
Torelli GF, Peragine N, Raponi S, Pagliara D, De Propris MS, Vitale A et al. Recognition of adult and pediatric acute lymphoblastic leukemia blasts by natural killer cells. Haematologica 2014; 99: 1248–1254.
Khoder A, Sarvaria A, Alsuliman A, Chew C, Sekine T, Cooper N et al. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood 2014; 124: 2034–2045.
DiLillo DJ, Weinberg JB, Yoshizaki A, Horikawa M, Bryant JM, Iwata Y et al. Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia 2013; 27: 170–182.
Mauri C, Blair PA . The incognito journey of a regulatory B cell. Immunity 2014; 41: 878–880.
Urashima M, Ogata A, Chauhan D, Hatziyanni M, Vidriales MB, Dedera DA et al. Transforming growth factor-beta1: differential effects on multiple myeloma versus normal B cells. Blood 1996; 87: 1928–1938.
Shehata M, Schwarzmeier JD, Hilgarth M, Hubmann R, Duechler M, Gisslinger H . TGF-beta1 induces bone marrow reticulin fibrosis in hairy cell leukemia. J Clin Invest 2004; 113: 676–685.
Bellone G, Aste-Amezaga M, Trinchieri G, Rodeck U . Regulation of NK cell functions by TGF-beta 1. J Immunol 1995; 155: 1066–1073.
Laouar Y, Sutterwala FS, Gorelik L, Flavell RA . Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat Immunol 2005; 6: 600–607.
Bollard CM, Rossig C, Calonge MJ, Huls MH, Wagner HJ, Massague J et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood 2002; 99: 3179–3187.
Dennler S, Pendaries V, Tacheau C, Costas MA, Mauviel A, Verrecchia F . The steroid receptor co-activator-1 (SRC-1) potentiates TGF-beta/Smad signaling: role of p300/CBP. Oncogene 2005; 24: 1936–1945.
Gleason MK, Verneris MR, Todhunter DA, Zhang B, McCullar V, Zhou SX et al. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther 2012; 11: 2674–2684.
Shimasaki N, Campana D . Natural killer cell reprogramming with chimeric immune receptors. Methods Mol Biol 2013; 969: 203–220.
Jardine L, Hambleton S, Bigley V, Pagan S, Wang XN, Collin M . Sensitizing primary acute lymphoblastic leukemia to natural killer cell recognition by induction of NKG2D ligands. Leuk Lymphoma 2013; 54: 167–173.
Acknowledgements
We are thank the patients and their families for their cooperation. We are grateful to Amos Gaikwad and Tatiana Goltsova for flow cytometry assistance; Drs Cliona M Rooney and Natalia Lapteva for providing critical experimental components. This research was partially performed in the Flow Cytometry and Cellular Imaging Facility at MD Anderson Cancer Center, which is supported, in part, by the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672. This research was funded, in part, by the MD Anderson Chronic Lymphocytic Leukemia Moon Shot Program. We also appreciate the support of shared resources by Dan L Duncan Cancer Center support grant P30CA125123. LLS SCOR (to CMB, KRR), Children’s Leukemia Research Association (to CMB, KRR), St Baldrick Scholar Award (to KRR), MDACC Leukemia SPORE Grant CA 100632 and SINF (to KR), Lymphoma SPORE P50CA126752 (to RHR) and NIH Training Grant T32 HL092332 (to RHR).
Author contributions
RHR designed research, performed research, analyzed data and wrote the paper. HS designed research, performed research and analyzed data. TS performed research and analyzed data. GW performed research. SK performed research. BB performed research. CB performed research. VM performed research. ES analyzed data. CMB designed research, analyzed data and wrote the paper. KRR designed research, analyzed data and wrote the paper. KR designed research, analyzed data and wrote the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Rouce, R., Shaim, H., Sekine, T. et al. The TGF-β/SMAD pathway is an important mechanism for NK cell immune evasion in childhood B-acute lymphoblastic leukemia. Leukemia 30, 800–811 (2016). https://doi.org/10.1038/leu.2015.327
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2015.327
- Springer Nature Limited
This article is cited by
-
Th1 cytokines in pediatric acute lymphoblastic leukemia
Cancer Immunology, Immunotherapy (2023)
-
Circular EZH2-encoded EZH2-92aa mediates immune evasion in glioblastoma via inhibition of surface NKG2D ligands
Nature Communications (2022)
-
Prolactin Acts on Myeloid Progenitors to Modulate SMAD7 Expression and Enhance Hematopoietic Stem Cell Differentiation into the NK Cell Lineage
Scientific Reports (2020)
-
Advances in targeted therapy for malignant lymphoma
Signal Transduction and Targeted Therapy (2020)
-
Medulloblastoma rendered susceptible to NK-cell attack by TGFβ neutralization
Journal of Translational Medicine (2019)