Abstract
Background/Objective:
Prenatal exposure to antibacterials may permanently dysregulate fetal metabolic patterns via epigenetic pathways or by altering maternal microbiota. We examined the association of prenatal exposure to systemic antibacterials with overweight and obesity in schoolchildren.
Subjects/Methods:
We conducted a prevalence study among Danish schoolchildren aged 7–16 years using data from routine school anthropometric evaluations conducted during 2002–2013. Prenatal exposure to antibacterials was ascertained by using maternal prescription dispensations and infection-related hospital admissions during pregnancy. We defined overweight and obesity among the children using standard age- and sex-specific cutoffs. We computed sex-specific adjusted prevalence ratios (aPRs) of overweight and obesity associated with exposure to prenatal antibacterials, adjusting for maternal age at delivery, marital status, smoking in pregnancy and multiple gestation; we also stratified the analyses by birth weight.
Results:
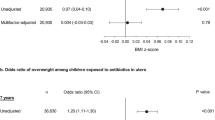
Among 9886 schoolchildren, 3280 (33%) had prenatal exposure to antibacterials. aPRs associated with the exposure were 1.26 (95% confidence interval (CI): 1.10–1.45) for overweight and 1.29 (95% CI: 1.03–1.62) for obesity. Among girls, aPRs were 1.16 (95% CI: 0.95–1.42) for overweight and 1.27 (95% CI: 0.89 to 1.82) for obesity. Among boys, aPRs were 1.37 (95% CI: 1.13–1.66) for overweight and 1.29 (95% CI: 0.96–1.73) for obesity. The aPR for overweight was higher among schoolchildren with birth weight <3500 g (aPR: 1.30, 95% CI: 1.05–1.61) than in schoolchildren with birth weight ⩾3500 g (aPR: 1.18, 95% CI: 0.95–1.46). Inversely, the association for obesity was higher among schoolchildren with birth weight ⩾3500 g (aPR: 1.35, 95% CI: 1.00–1.81) than among those who were <3500 g at birth (aPR: 1.16, 95% CI: 0.82–1.65).
Conclusions:
Prenatal exposure to systemic antibacterials is associated with an increased risk of overweight and obesity at school age, and this association varies by birth weight.
Similar content being viewed by others
Introduction
Childhood obesity has emerged as a challenging global public health problem,1 with adverse health consequences in adulthood.2, 3 It has been hypothesized that prenatal exposure to microbiome-altering agents, such as systemic antibacterials, may lead to postnatal obesity.4, 5
In humans, prenatal infection is associated with a 30% increased risk of overweight in later life, and antibiotic exposure in the neonatal period is associated with a 22% increased risk.6, 7 These findings are consistent with the fetal origins hypothesis, which ascribes a critical role of the perinatal period in the development of childhood obesity.4 Antibacterials act by altering fetal exposure to microbe-associated molecular patterns, which have a role in the programming of development of gut epithelium and of the immune system, through Toll-like receptor stimulation.8 Prenatal antibacterials may also act through endocrine disruption9 and metabolic imprinting,10 both of which program postnatal energy homeostasis, food intake and weight set-point,11 leading to childhood overweight and obesity.12 Additionally, evidence in humans13, 14, 15 and in animals15, 16, 17, 18 indicates that antibacterial exposure during pregnancy changes the composition and function of maternal and placental microbiota, potentially configuring the fetal gut microbiomes in an obesogenic direction. It is therefore plausible that treatment with antibacterials during pregnancy can affect intergenerational transmission of maternal microbiota to the offspring.
Because of the rising prevalence of childhood obesity1 and high antibacterial use among women during pregnancy,19, 20 it is important to understand the interplay between maternal antibacterial use during pregnancy and childhood obesity. Previous studies6, 7 were either restricted to adult men or examined the effect of early-life exposure after birth with different hypothesis. To our knowledge, no study to date has examined the association between prenatal exposure to antibacterials and body mass index (BMI) among adolescent schoolchildren. We conducted a prevalence study to examine the association between prenatal exposure to systemic antibacterials and prevalence of overweight and obesity in schoolchildren.
Materials and methods
Study population and setting
We conducted the study among schoolchildren in the Aalborg municipality of Denmark, linking records from several medical and administrative databases. Our research database included all children (N=9886) from 7 to 16 years of age who were born between 1994 and 1998, attended public or private schools in the Aalborg municipality and who underwent routine school anthropometric evaluation during 2002–2013. The median age at the time of the anthropometric evaluation was 14.5 years (interquartile range 14.1, 14.9 years) among schoolchildren with and without prenatal exposure to systemic antibacterials. The Danish National Health Service provides tax-funded universal access to health care, including access to primary care and hospitals, and a partial reimbursement of costs of prescription medications, including antibacterials.21 Individual-level data from all Danish registries can be linked via the unique central personal registry number, assigned at birth or immigration, and registered in the Danish Civil Registration System.22
Prenatal exposure to systemic antibacterials
We linked the children’s school records to their records in the Danish Medical Birth Registry,23 which has tracked all births in Denmark since 1973 and contains a variable for maternal central personal registry numbers. Prenatal exposure to systemic antibacterials was defined as one of the following: (1) a maternal prescription for a systemic antibacterial agent from 30 days before the estimated conception date until childbirth (this period was chosen to capture exposure between fertilization and diagnosis of pregnancy, which could occur via a prescription that was filled before and lasted at least until conception), and/or (2) a maternal inpatient, emergency or outpatient hospital contact with infection between the estimated conception date and childbirth.
Records of maternal prescriptions were obtained, via the maternal central personal registry number, from the Aarhus University Prescription Database, which electronically tracks reimbursed prescriptions sold at all outpatient pharmacies in northern Denmark, including Aalborg municipality, since 1989.24 The prescription record contains date of sale, the amount purchased and the active substance, coded according to the Anatomical Therapeutic Chemical classification. Similarly, the Danish National Registry of Patients was used to obtain data on maternal hospital contacts.25 The Danish National Registry of Patients records complete hospitalization history of all Danish residents since 1977, including dates of admission and discharge and up to 20 discharge diagnoses, coded according to the eighth revision of the International Classification of Diseases until the end of 1993 and the tenth revision thereafter.25
Women without a prescription for a systemic antibacterial agent or a hospital encounter with an infection in the periods defined above were used as the reference group.
Outcome variables
BMI was calculated from height and weight, as recorded by school physicians and nurses during routine anthropometric examinations.26 Weight was measured to the nearest 0.1 kg on an electronic scale (at most schools) or on a mechanical sliding scale. Height was measured to the nearest 0.5 cm using a stadiometer. BMI (weight (kg)/height (m)2) was classified into three non-overlapping categories of normal weight, overweight and obesity, using the International Obesity Task Force age- and sex-specific cutoffs.27
Covariates
Potential confounders measured in the linked data sources were identified a priori and included multiple gestation (yes/no), maternal marital status at the time of childbirth (married/cohabiting or single/divorced/widowed), maternal age at delivery (⩽20/21–35/⩾35 years), maternal smoking during pregnancy (yes/no), maternal diabetes (yes/no), maternal gestational diabetes (yes/no) and child’s gestational age (full-term (⩾37 weeks)/preterm (<37 weeks)).23
All diagnostic and medication codes are listed in the Supplementary Appendix.
Statistical analyses
We described the distributions of the study variables among the schoolchildren according to prenatal exposure to systemic antibacterials. We calculated sex-specific means with s.d. and medians with range of BMI and sex-specific prevalences of overweight and obesity. We computed sex-specific crude and adjusted prevalence ratios (aPRs) of overweight and obesity with associated 95% confidence intervals (CIs) using log-binomial regression28 with generalized estimating equations to model correlated outcomes between children of the same mothers.29 We adjusted the analysis for maternal age at delivery, marital status, smoking during pregnancy and multiple gestation. We did not include gestational age in the regression model, because we worked from the hypothesis that gestational age is a causal intermediary on the pathway between prenatal antibiotic exposure (maternal infection) and obesity; furthermore, we did not include the diabetes variables in the final model, as their prevalence did not differ by the prenatal antibiotic exposure status. We examined the association for exposure in each trimester of pregnancy, for the number of filled prescriptions (0 (reference), 1, 2 and ⩾3) and type of antibacterials. Chi-square test for trend was used to evaluate the dose–response relation between the number of antibiotic prescriptions and the study outcomes. We lacked information on maternal obesity, which could be a potential confounder. Maternal obesity confers a relative risk of 3.1 for childhood obesity and 2.7 for childhood overweight.30, 31 Reported prevalence of obesity is 33% among women taking antibacterials and 32% among women not taking antibacterials. Based on this information, we computed estimates externally adjusted for maternal obesity and compared it with our crude estimates to assess the impact of confounding by maternal obesity.32 Additionally, we examined effect modification by birth weight and mode of delivery.
Data were managed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) and analyzed using STATA version 12 (StataCorp. 2011. Stata Statistical Software: Release 12; College Station, TX, USA: StataCorp LP). This study was approved by the Danish Data Protection Agency (number 2009-41-4051). As this registry-based study did not include human biological material, approval by the Danish Scientific Ethical Committee was not needed according to Danish legislation.
Results
Characteristics of the study population
Anthropometric records were available for 9886 schoolchildren, of whom 33% (3280) had prenatal exposure to systemic antibacterials. Median age at the time of anthropometric evaluations was 14 years (range: 7–16 years), and 48% (4718) of the children were girls. Compared with unexposed schoolchildren, exposed children were more likely to be boys, were less likely to be first-born and were more likely to be born to a married or cohabiting mother. In addition, prenatal exposure to systemic antibacterials was associated with a higher prevalence of maternal smoking, caesarean delivery and preterm birth (Table 1). Distributions of exposure to specific antibacterial types and specific hospital infection diagnoses are provided in Supplementary Table S1.
Prevalence of overweight
Among all schoolchildren, 7.8% (768/9886) were overweight, including 7.6% (390/5168) of boys and 8.0% (378/4718) of girls (Table 2). The crude PR for overweight was 1.31 (95% CI: 1.14–1.51) and aPR was 1.26, 95% CI: 1.10–1.45). Among boys, the aPR was 1.37 (95% CI: 1.13–1.66), whereas among girls the aPR was 1.16 (95% CI: 0.95–1.42) (Table 3). Stratification by birth weight suggested higher prevalence of overweight in schoolchildren born with weight <3500 g and exposed to prenatal antibacterials compared with non-exposed (aPR: 1.30, 95% CI: 1.05–1.61) than in schoolchildren with birth weight ⩾3500 g (aPR: 1.18, 95% CI: 0.95–1.46). The aPRs were similar in those delivered by vaginal and Caesarean delivery (Table 3). Second-trimester exposure (aPR: 1.39, 95% CI: 1.11–1.73) and third-trimester exposure (aPR: 1.43, 95% CI: 1.17–1.76) were associated with increased prevalence of overweight among schoolchildren but not first-trimester exposure (aPR: 1.09, 95% CI: 0.91–1.31). Prevalence of overweight increased with the increasing number of maternal prescriptions for antibacterials (P=0.001 for trend) (Table 4). PRs did not vary according to antibacterial type (Supplementary Table S2). External adjustment for unmeasured maternal obesity changed the crude PR from 1.31 to 1.33 for the ‘overweight’ outcome.
Prevalence of obesity
The overall prevalence of obesity was 3.1% (309/9886): 3.5% (181/5168) among the boys and 2.7% (128/4718) among the girls (Table 2). The crude PR for obesity was 1.39 (95% CI: 1.11–1.73) and the aPR was 1.29 (95% CI: 1.03–1.62). We observed no difference in aPRs among boys and girls (aPR: 1.29, 95% CI: 0.96–1.73 for boys; and aPR: 1.28, 95% CI: 0.90–1.82 for girls). Among schoolchildren who weighed ⩾3500 g at birth, the association between prenatal antibacterials and obesity was higher (aPR: 1.35, 95% CI: 1.00–1.81) than in those who weighed <3500 g at birth (aPR: 1.16, 95% CI: 0.82–1.65). The aPRs for obesity did not differ across mode of delivery and trimesters of exposure (Tables 3 and 4). We observed an increasing prevalence of obesity with higher numbers of maternal antibacterial prescriptions (P=0.016 for trend; Table 4). There was no difference associated with exposure to specific antibacterials (Supplementary Table S2). The external adjustment for unmeasured maternal obesity did not change the crude PR for obesity among their children.
We further analyzed the effect of prepregnancy antibacterial use on the overweight and obesity among schoolchildren. For overweight, the crude PR was 1.14 (95% CI: 0.99–1.30) and the aPR was 1.08 (95% CI: 0.94–1.24), and for obesity, the crude PR was 1.31 (95% CI: 1.05–1.63) and the aPR was 1.24 (95% CI: 1.00–1.55).
Discussion
Prenatal exposure to antibacterials was associated with a 26–29% increased prevalence of overweight and obesity at school age after adjustment for measured confounding. This association varied by birth weight.
The primary strength of our study was its population-based design and well-defined population receiving care within a uniform health care system. We used independent and highly valid population-based registries.23, 24 The registries prospectively collect data on dispensed prescriptions, and all other variables used in this analysis, years before collection of anthropometric data among the schoolchildren, thus ruling out investigator and recall biases.33 Selection bias based on socioeconomic status can be ruled out, as school anthropometric evaluations were performed at all public and private schools. The prevalence of overweight and obesity among schoolchildren observed in our study was similar to that reported in another recent Danish report,34 indicating that our study population was representative of the source population.
Study limitations include potential unmeasured confounding by maternal gestational weight gain and genetic predisposition to obesity and infection. Second, we did not have information on maternal obesity. The risk of obesity in early childhood is twofold to fourfold higher in children born to obese women compared with children born to non-obese women.31, 35 Obese women have an increased risk of genital tract infections (odds ratio (OR): 1.30, 95% CI: 1.07–1.56) and urinary tract infections (OR: 1.39, 95% CI: 1.18–1.63)36 and are therefore more likely to receive systemic antibacterials. However, external adjustment for maternal obesity had little effect on our estimates. Third, the fact and the timing of maternal medication intake may be misclassified by use of dispensations data, with the extent of misclassification depending on individual adherence to treatment. A recent Danish study reported 37% prevalence of antibacterial use during pregnancy based on self-report, which is close to the 33% prevalence identified in our study and other studies,37 indirectly indicating strong adherence. Fourth, because we used a prevalence design and examined schoolchildren who survived to the age at school anthropometric evaluation, we were able to measure only prevalence instead of incidence of overweight and obesity. However, only 1% of children die between birth and reaching school age in Denmark, suggesting that such mortality is a negligible source of selection bias.38 Finally, confounding by the underlying infection might have attenuated the effect of exposure in our study. We tried to address this issue by exploring the effect of specific antibacterial agents, as they may differ in their effect on normal microbiota (Supplementary Table S2). The number of children with maternal-dispensed prescriptions for antifungal and antiviral agents was too low to address the class effect. Nor did we have data on untreated prenatal infections.
Our results accord with the finding by Cocoros et al.6 of an increase in the prevalence of obesity at age 17–24 years among Danish conscripts born to mothers after hospitalization with an infection during pregnancy (adjusted OR: 1.34, 95% CI: 0.82–2.19). In a recent study, Mueller et al.39 observed that the risk of obesity among children at 7 years of age was increased by 84% (95% CI: 33–154) among those who were exposed to antibacterials during second or third trimester compared with those who were not exposed and by 46% (8–98) among those who were delivered by caesarean section compared with vaginally delivered children. The higher risk of obesity among schoolchildren born by Caesarean delivery observed in our study corroborates findings by Blustein et al.40 who reported 1.83 times higher odds of obesity (95% CI: 1.24–2.70) at age 11 years among children delivered by caesarean section. Additionally, early-life exposure to antibacterials was associated with consistent increase in BMI in later life according to a longitudinal birth cohort study of 11 532 children.7
Plausible mechanisms underlying the observed association may involve altered maternal microbiota through use of antimicrobials during pregnancy. In humans, maternal gut microbiota provides newborns with their first natural microbial exposure,41, 42 while human placenta harbors commensal organisms responsible for antenatal infections.14 Exposure to antimicrobials during gestation15 and in early life43 may affect the postnatal metabolism by altering the composition of the ‘pioneer’ microbiota, ultimately leading, in some persons, to overweight or obesity.40, 44 In addition, use of antibiotics in childhood was associated with an increased risk of obesity in a recent large cohort study.43, 44 Prenatal antibacterial exposure impacts intergenerational transfer of microbiota to infants, which leads to persistent alteration in host immunity45 and can make newborns more susceptible to infections and results in early exposure to antibiotics.46
Another possible mechanism for the findings of this study involves epigenetic pathways. The relevant evidence comes primarily from animal studies.15, 16, 47, 48, 49 In adult rats, prenatal infection-associated exposure to interleukin-6 and tissue necrosis factor-alpha can induce neuroendocrine upregulation of adipose tissue.50 In mice, Cho et al.16 and Cox et al.15 recently demonstrated that antibiotics disrupt early-life metabolic homeostasis and can alter gene expression in ileal tissue, causing microbe-induced obesity in later life. Prenatal exposure to antibiotics may differentially alter methylation at regulatory regions of imprinted genes and may be associated with low birth weight in humans,30 lending support to our hypothesis that somatic epigenetic changes may occur in response to prenatal exposure to antibacterials. This could lead to overweight and obesity at school age mediated by altered development and long-term dysfunction in various organs, including the liver.51 The role of prenatal antibacterial exposure as a possible source of abnormal imprinting raises the issues of its potential effects on postnatal BMI and on other key imprinted genes controlling growth and development.52, 53 However, similar estimates were observed for prepregnancy antibacterial use and most different antibacterial classes increased the risk of obesity (Supplementary Table S2), which favors the hypothesis of intergenerational transmission of maternal microbiota. Alternatively, antibacterial use might be a marker of other risk factors, for instance, lifestyle; otherwise the effect would not have been similar with antibacterial exposure outside pregnancy.
Conclusions
Prenatal exposure to systemic antibacterials was associated with an increased risk of both overweight and obesity at school age. The role of the underlying infection in this association cannot be ruled out. This association varied by birth weight. In addition to lifestyle intervention necessary to reduce obesity epidemic among adolescent, we should also try to find early-life links that may help in finding the key to reduce the epidemic further. There is urgent need to explore early-life modifiable risk factors that can help to reduce later burden on health care as well as on community.
References
Lobstein T, Baur L, Uauy R, IASO International Obesity Task Force. Obesity in children and young people: a crisis in public health. Obes Rev 2004; 5: 4–104.
Salwen JK, Hymowitz GF, Vivian D, O'Leary KD . Childhood abuse, adult interpersonal abuse, and depression in individuals with extreme obesity. Child Abuse Negl 2014; 38: 425–433.
Lloyd LJ, Langley-Evans SC, McMullen S . Childhood obesity and risk of the adult metabolic syndrome: a systematic review. Int J Obes (Lond) 2012; 36: 1–11.
van Dijk SJ, Molloy PL, Varinli H, Morrison JL, Muhlhausler BS,, members of EpiSCOPE. Epigenetics and human obesity. Int J Obes (Lond) 2014; 39: 85–97.
Ajslev TA, Andersen CS, Gamborg M, Sorensen TI, Jess T . Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes (Lond) 2011; 35: 522–529.
Cocoros NM, Lash TL, Norgaard M, Farkas DK, DeMaria A Jr, Sorensen HT . Hospitalized prenatal and childhood infections and obesity in Danish male conscripts. Ann Epidemiol 2013; 23: 307–313.
Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ . Infant antibiotic exposures and early-life body mass. Int J Obes (Lond) 2013; 37: 16–23.
Nylund L, Satokari R, Salminen S, de Vos WM . Intestinal microbiota during early life - impact on health and disease. Proc Nutr Soc 2014; 73: 457–469.
Romano M, Savitz D, Braun J . Challenges and future directions to evaluating the association between prenatal exposure to endocrine-disrupting chemicals and childhood obesity. Curr Epidemiol Rep 2014; 1: 57–66.
Sullivan EL, Grove KL . Metabolic imprinting in obesity. Forum Nutr 2010; 63: 186–194.
Keith SW, Redden DT, Katzmarzyk PT, Boggiano MM, Hanlon EC, Benca RM et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes (Lond) 2006; 30: 1585–1594.
Perkins E, Murphy SK, Murtha AP, Schildkraut J, Jirtle RL, Demark-Wahnefried W et al. Insulin-like growth factor 2/H19 methylation at birth and risk of overweight and obesity in children. J Pediatr 2012; 161: 31–39.
Stokholm J, Schjorring S, Eskildsen CE, Pedersen L, Bischoff AL, Folsgaard N et al. Antibiotic use during pregnancy alters the commensal vaginal microbiota. Clin Microbiol Infect 2013; 20: 629–635.
Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J . The placenta harbors a unique microbiome. Sci Transl Med 2014; 6: 237.
Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 2014; 158: 705–721.
Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012; 488: 621–626.
Cox LM, Blaser MJ . Pathways in microbe-induced obesity. Cell Metab 2013; 17: 883–894.
Bernardeau M, Vernoux JP . Overview of differences between microbial feed additives and probiotics for food regarding regulation, growth promotion effects and health properties and consequences for extrapolation of farm animal results to humans. Clin Microbiol Infect 2013; 19: 321–330.
Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernandez-Diaz S et al. Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am J Obstet Gynecol 2011; 205: 51.
Persaud RR, Azad MB, Chari RS, Sears MR, Becker AB, Kozyrskyj AL et al. Perinatal antibiotic exposure of neonates in Canada and associated risk factors: a population-based study. J Matern Fetal Neonatal Med 2014. e-pub ahead of print 14 August 2014 doi:10.3109/14767058.2014.947578.
Danish Health and Medicines Authority. Reimbursement of medicines, 2012. Available at: http://sundhedsstyrelsen.dk/en/medicines/reimbursement/central-reimbursement-register.
Schmidt M, Pedersen L, Sorensen HT . The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014; 29: 541–549.
Kristensen J, Langhoff-Roos J, Skovgaard LT, Kristensen FB . Validation of the Danish Birth Registration. J Clin Epidemiol 1996; 49: 893–897.
Ehrenstein V, Antonsen S, Pedersen L . Existing data sources for clinical epidemiology: Aarhus University Prescription Database. Clin Epidemiol 2010; 2: 273–279.
Lynge E, Sandegaard JL, Rebolj M . The Danish National Patient Register. Scand J Public Health 2011; 39: 30–33.
Rasmussen S, Petersen TA, Madsen M . Body height of 6-15-year-old school children measured in the period 1986/1987 to 1996/1997. Compared with Danish measurements in 1971/1972. Ugeskr Laeger 2002; 164: 5011–5015.
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH . Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000; 320: 1240–1243.
Reichenheim ME, Coutinho ES . Measures and models for causal inference in cross-sectional studies: arguments for the appropriateness of the prevalence odds ratio and related logistic regression. BMC Med Res Methodol 2010; 10: 66.
Zeger SL, Liang KY, Albert PS . Models for longitudinal data: a generalized estimating equation approach. Biometrics 1988; 44: 1049–1060.
Vidal AC, Murphy SK, Murtha AP, Schildkraut JM, Soubry A, Huang Z et al. Associations between antibiotic exposure during pregnancy, birth weight and aberrant methylation at imprinted genes among offspring. Int J Obes (Lond) 2013; 37: 907–913.
Whitaker RC . Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics 2004; 114: e29–e36.
Schneeweiss S . Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf 2006; 15: 291–303.
Sorensen HT . Regional administrative health registries as a resource in clinical epidemiology: a study of options, strengths, limitations and data quality provided with examples of use. Int J Risk Saf Med 1997; 10: 1–22.
Schmidt MC, Rokholm B, Sjoberg BC, Schou AC, Geisler AL, Rasmussen M et al. Trends in prevalence of overweight and obesity in Danish infants, children and adolescents—are we still on a plateau? PLoS One 2013; 8: e69860.
Kleiser C, Schaffrath Rosario A, Mensink GB, Prinz-Langenohl R, Kurth BM . Potential determinants of obesity among children and adolescents in Germany: results from the cross-sectional KiGGS Study. BMC Public Health 2009; 9: 46.
Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord 2001; 25: 1175–1182.
Bjorn AM, Norgaard M, Hundborg HH, Nohr EA, Ehrenstein V . Use of prescribed drugs among primiparous women: an 11-year population-based study in Denmark. Clin Epidemiol 2011; 3: 149–156.
Ehrenstein V, Pedersen L, Grijota M, Nielsen GL, Rothman KJ, Sorensen HT . Association of Apgar score at five minutes with long-term neurologic disability and cognitive function in a prevalence study of Danish conscripts. BMC Pregnancy Childbirth 2009; 9: 14.
Mueller NT, Whyatt R, Hoepner L, Oberfield S, Dominguez-Bello MG, Widen EM et al. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes (Lond) 2015; 39: 665–670.
Blustein J, Attina T, Liu M, Ryan AM, Cox LM, Blaser MJ et al. Association of caesarean delivery with child adiposity from age 6 weeks to 15 years. Int J Obes (Lond) 2013; 37: 900–906.
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA 2010; 107: 11971–11975.
Ganu RS, Harris RA, Collins K, Aagaard KM . Early origins of adult disease: approaches for investigating the programmable epigenome in humans, nonhuman primates, and rodents. ILAR J 2012; 53: 306–321.
Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA . Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr 2014; 168: 1063–1069.
Azad MB, Bridgman SL, Becker AB, Kozyrskyj AL . Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int J Obes (Lond) 2014; 38: 1290–1298.
Zeissig S, Blumberg RS . Life at the beginning: perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat Immunol 2014; 15: 307–310.
Hooper LV, Littman DR, Macpherson AJ . Interactions between the microbiota and the immune system. Science 2012; 336: 1268–1273.
Makino H, Kushiro A, Ishikawa E, Kubota H, Gawad A, Sakai T et al. Mother-to-infant transmission of intestinal bifidobacterial strains has an impact on the early development of vaginally delivered infant's microbiota. PLoS One 2013; 8: e78331.
Fak F, Ahrne S, Molin G, Jeppsson B, Westrom B . Microbial manipulation of the rat dam changes bacterial colonization and alters properties of the gut in her offspring. Am J Physiol Gastrointest Liver Physiol 2008; 294: G148–G154.
Pantoja-Feliciano IG, Clemente JC, Costello EK, Perez ME, Blaser MJ, Knight R et al. Biphasic assembly of the murine intestinal microbiota during early development. ISME J 2013; 7: 1112–1115.
Dahlgren J, Nilsson C, Jennische E, Ho HP, Eriksson E, Niklasson A et al. Prenatal cytokine exposure results in obesity and gender-specific programming. Am J Physiol Endocrinol Metab 2001; 281: E326–E334.
Carter R, Mouralidarane A, Soeda J, Ray S, Pombo J, Saraswati R et al. Non-alcoholic fatty pancreas disease pathogenesis: a role for developmental programming and altered circadian rhythms. PLoS One 2014; 9: e89505.
Isles AR, Holland AJ . Imprinted genes and mother-offspring interactions. Early Hum Dev 2005; 81: 73–77.
Robertson KD . DNA methylation and human disease. Nat Rev Genet 2005; 6: 597–610.
Acknowledgements
We thank Jasper Kondrup Christensen, Preben Selgen Pedersen, IT-Centre, and Aalborg Kommune for assistance with data collection. This research was supported by the Clinical Epidemiology Research Foundation, Aarhus University Hospital, Aarhus, Denmark. The study also received support from the Danish Centre for Strategic Research in Type 2 Diabetes (DD2) project, which is supported by the Danish Agency for Science (grant no. 09-067009 and 09-075724), the Danish Health and Medicines Authority, the Danish Diabetes Association and an unrestricted donation from Novo Nordisk A/S. The Department of Clinical Epidemiology, Aarhus University Hospital, receives funding for other studies from companies in the form of research grants to (and administered by) Aarhus University. The Novo Nordisk Foundation Center for Basic Metabolic Research is an independent Research Center at the University of Copenhagen partially funded by an unrestricted donation from the Novo Nordisk Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Mor, A., Antonsen, S., Kahlert, J. et al. Prenatal exposure to systemic antibacterials and overweight and obesity in Danish schoolchildren: a prevalence study. Int J Obes 39, 1450–1455 (2015). https://doi.org/10.1038/ijo.2015.129
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2015.129
- Springer Nature Limited
This article is cited by
-
Systemic antibiotics increase microbiota pathogenicity and oral bone loss
International Journal of Oral Science (2023)
-
Sex-specific associations between prenatal antibiotics exposure and offspring’s body mass index
International Journal of Obesity (2020)
-
Maternal infection and antibiotic use in pregnancy and the risk of childhood obesity in offspring: a birth cohort study
International Journal of Obesity (2020)
-
Association of prenatal antibiotics with measures of infant adiposity and the gut microbiome
Annals of Clinical Microbiology and Antimicrobials (2019)
-
From conception to infancy — early risk factors for childhood obesity
Nature Reviews Endocrinology (2019)