Abstract
Recombinant vectors based on adeno-associated virus (AAV) are proving to be powerful tools for genetic manipulation of the liver, for both discovery and therapeutic purposes. The system can be used to deliver transgene cassettes for expression or, alternatively, DNA templates for genome editing via homologous recombination. The replicative state of target cells is known to influence the efficiency of these processes and knowledge of the host–vector interactions involved is required for optimally effective vector deployment. Here we show, for the first time in vivo, that in addition to the known effects of hepatocellular replication on AAV-mediated gene transfer, the vector itself exerts a potent, albeit transient suppressive effect on cell cycle progression that is relieved on a time course that correlates with the known rate of clearance of input single-stranded vector DNA. This finding requires further mechanistic investigation, delineates an excellent model system for such studies and further deepens our insight into the complexity of interactions between AAV vectors and the cell cycle in a clinically promising target tissue.
Similar content being viewed by others
Introduction
The liver is an attractive target for gene therapy due to the large number of genetic disorders in which specific functions of the liver are lost or impaired. Among existing gene delivery technologies, vectors based on adeno-associated virus (AAV) show special promise for liver-targeted applications, with the potential to be exploited in both conventional gene addition and targeted genome editing strategies. Numerous disease phenotypes in small and large animal models have been corrected using the former approach1, 2, 3, 4, 5, 6 and clinical benefit has been reported in a human clinical trial.7, 8 Reports of successful correction of the coagulation defect in mouse models of haemophilia B (factor IX deficiency) using AAV-mediated gene targeting in vivo have also been recently published, further underscoring the exciting potential of AAV vectors in the liver.9, 10
Realisation of the full therapeutic potential of AAV vectors in the liver requires a thorough understanding of host–vector interactions. A host parameter of particular importance is the replicative state of the target cell population. For example, in vitro studies of AAV-mediated transduction and gene targeting efficiencies in primary human fibroblasts have shown that both are favoured by active proliferation.11, 12 Conversely, in vivo studies in the mouse have shown that hepatocellular proliferation is accompanied by loss of episomal AAV genomes, the predominant molecular species responsible for transgene expression in the liver following AAV transduction.3, 13 Further complicating these host cell cycle-related effects is the potential of the vector itself to influence the cell cycle. This phenomenon was first reported for wild-type AAV, with infection interfering with transit through the cell cycle even after inactivation of the virus by UV light exposure.14 This effect has subsequently been linked to the capacity of wild-type AAV genomes to provoke a DNA-damage response that is independent of viral gene expression.15, 16 The underlying mechanism by which this DNA-damage response is provoked remains incompletely understood. One hypothesis is that the single-stranded viral genome and flanking T-hairpin shaped inverted terminal repeats induce a DNA-damage response by mimicking a stalled replication fork.15 If this hypothesis is correct, recombinant AAV genomes would be predicted to be similarly potent in provoking DNA-damage responses. However, the same group have subsequently reported that recombinant viral genomes are deficient in provoking a DNA-damage response, and presented evidence that sequences lying within the p5 promoter region (AAV2 nucleotides 250–304), absent from recombinant AAV (rAAV) genomes, are involved.17, 18
We and others have previously shown that gender influences the level of rAAV gene transfer to the liver, with male mice exhibiting higher levels of transgene expression than females.1, 19, 20 Extending this observation, we have also observed that the patterns of long-term persistence of episomal vector genomes and associated transgene expression differ across the hepatic lobule in male and female mice, and presented early evidence of a correlation with hepatocellular proliferation.20 Given the potential clinical significance of these observations, the current study was undertaken to further examine the levels of hepatocellular proliferation in adult male and female mice following AAV-mediated gene transfer. Although we anticipated basal rates of hepatocellular proliferation would influence rAAV transduction, we unexpectedly observed a converse effect. Transduction was associated with a marked, albeit transient and suppression of hepatocellular proliferation in both male and female mice. We further show that this effect is vector genome-dependent and likely involves triggering of DNA-damage checkpoints leading to cell cycle arrest.
Results and discussion
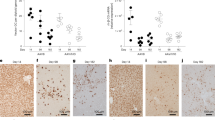
To detect hepatocyte replicative activity a 5-Bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich, St Louis, MO, USA) labelling strategy was used. This nucleotide analogue is incorporated into newly synthesised DNA allowing nuclei that have transited S phase to be detected by immunohistochemical staining (Figure 1a). Mononucleated hepatocytes with labelled nuclei (singlets) are indicative of cells that have transited S phase, but not progressed to mitosis. Two closely spaced labelled nuclei (doublets) are indicative of either a mononucleated hepatocyte that has transited S phase and progressed through G2 to mitosis, or alternatively a binucleated hepatocyte that has transited S phase, but not progressed to mitosis (with or without cytokinesis).21 Cohorts of young adult (8–10-weeks-old) male and female mice were injected with BrdU daily for 1 week immediately before liver harvest at 2 weeks, 3 months and 6 months after AAV vector delivery (Figure 1b). The initial rAAV2/8 vector used encoded enhanced green fluorescent protein (eGFP) and was delivered at a dose of 1 × 1011 vg per mouse via the portal vein as previously described.20 Control mice received phosphate buffered saline (PBS) via the same route. Following harvest at the specified time-points, livers were processed for immunohistochemical analysis of BrdU labelling. The total number of labelling events per 104 hepatocytes, and the relative proportion of singlet and doublet labelling events were then counted.
Detection of hepatocyte proliferation in vivo. BrdU incorporation was determined by immunohistochemistry of liver sections (a) with BrdU positive nuclei (brown; arrow heads) and central veins labelled for glutamine synthetase (purple) indicated. Singlet and doublet nuclei are shown. Images represent × 200 magnification (left) and × 400 magnification (right). Nuclei have been counter-stained with haematoxylin. Scale bars=100 μM. The experimental regime (b) for dosing mice prior to harvest is indicated. Mice were injected ip with 100 mg kg−1 BrdU twice a day (10 h apart) for 7 days before harvest at 2 weeks, 3 and 6 months post-injection of rAAV2/8 or control transduction.
At 2 weeks post-transduction the total number of labelling events (singlet and doublet) were dramatically reduced by ∼11-fold in both male and female vector treated mice relative to PBS-treated controls (Figure 2a), whereas the relative proportion of singlet and doublet labelling events did not change significantly (data not shown). By 3 months post-transduction this suppression was completely relieved, and replaced with an opposite trend, albeit not statistically significant, towards increased labelling in both vector-treated male and female mice (Figure 2b). A similar result was also obtained 6 months post-transduction, but against lower basal levels of BrdU labelling, and with the result in males achieving statistical significance (Figure 2c). When data from both genders were combined to increase statistical power, the increased labelling observed in vector-treated mice achieved statistical significance at both 3 month and 6 month, post-transduction (Figure 2d).
Quantitation of BrdU positive hepatocytes in mouse liver over time. The total numbers of BrdU positive hepatocyte nuclei were counted across liver sections from mice harvested at (a) 2 weeks, (b) 3 months and (c) 6 months after injection with rAAV2/8-LSP1-eGFP or PBS. The BrdU events for each mouse were normalised per 10 000 hepatocyte nuclei. Error bars represent mean±s.e.m. of n=6 (AAV groups) or n=3 (PBS controls) of female (white bars) and male (black bars) mice. (d) represents the combined analysis of male and female mice with AAVeGFP (white bars) and PBS controls (grey bars). Statistical significance (P⩽0.05) between groups is indicated (*) and was determined using the Mann–Whitney test.
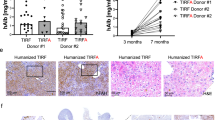
We next sought to determine which component of the rAAV virion is responsible for the transient suppression of BrdU labelling observed 2 weeks post-transduction. To address this question cohorts of young adult male mice were injected via the portal vein with either (i) AAV8 empty capsids, (ii) an rAAV2/8 vector encoding argininosuccinate synthetase, a urea cycle enzyme native to the mouse liver, (iii) the rAAV2/8-eGFP vector used in initial experiments or (iv) PBS. As in the initial experiment, a vector dose of 1 × 1011 vg per mouse was used, and the equivalent dose of empty capsids determined by enzyme-linked immunosorbent assay (ELISA). The Livers were harvested 2 weeks or 3 months later with BrdU labelling over the preceding week. Consistent with the previously acquired results, the livers from mice receiving rAAV2/8 vectors encoding either argininosuccinate synthetase or eGFP showed significantly suppressed levels of BrdU labelling relative to PBS-treated control mice, whereas mice receiving empty capsids showed no suppression of labelling relative to control mice (Figure 3a). At the 3 month time-point there was no statistically significant difference in the level of labelling between any of the treatment groups, although the previously observed trend towards increased labelling in AAV2/8 vector-treated mice was again observed (Figure 3b). In order to show that mice treated with empty capsid were indeed exposed to AAV8 capsid protein, an ELISA was performed on serum collected 2 weeks post-injection (Figure 3c). All groups exposed to AAV8 capsids (whether empty or containing vector genome) produced significantly higher absorbance values than PBS-treated control mice, confirming capsid exposure.
The presence of single-stranded DNA (ssDNA) rather than the AAV capsid influences the level of BrdU incorporation in hepatocytes. Liver was harvested 2 weeks (a) or 3 months (b) post transduction after labelling with BrdU. The relative number of BrdU positive nuclei counted across liver sections after normalisation per 10 000 hepatocyte nuclei is shown. In order to show that mice receiving the empty capsid preparation were indeed exposed to capsid, serum from all groups was collected and anti-AAV8 capsid antibodies detected by ELISA (c). Error bars represent mean±s.e.m. of n=4 per group. Statistical significance between groups is indicated (*) and was determined using the Mann-Whitney test.
Taken together these data support the conclusion that transduction of the adult mouse liver with AAV vectors is accompanied by marked transient suppression of hepatocellular proliferation. Observation of similar levels of reduction in the number of BrdU-labelled events (singlet and doublet nuclei) 2 weeks following vector exposure is consistent with cell cycle arrest at the G1/S checkpoint and possibly also at the G2/M checkpoint. Although numerically probable, G2/M checkpoint arrest cannot be definitively concluded as doublet labelling events can be produced by arrest at either checkpoint, depending on the initial number of nuclei present in a replicating hepatocyte.
The failure of empty AAV capsids to exert a similar effect is consistent with a mechanism involving the vector genome. Although the data presented do not directly define which properties of the vector genome might trigger hepatocyte cell cycle arrest, there are multiple lines of indirect evidence favouring hepatocyte sensing of input single-stranded rAAV genomes. The involvement of vector-encoded transgene products is improbable given the relatively long term persistence of transgene expression in the adult mouse liver following rAAV-mediated gene transfer3, 22 and transient nature of the suppression observed. Similarly, long-term transgene expression is supported predominantly by the persistence of double-stranded rAAV episomes, and to a lesser extent by integrated vector genomes. In contrast, we have previously shown that the number of input single-stranded rAAV genomes present in the adult mouse liver declines rapidly between 3 days and 2 weeks after delivery, a time-course matching the transient suppression of hepatocellular proliferation observed.22
Although there is an extensive body of literature exploring the effects of DNA-damage-response proteins, such as the Mre11, Rad50 and Nbs1 complex, on rAAV transduction,23, 24, 25 this report is the first to provide in vivo evidence of efficient AAV vector-mediated triggering of cell cycle arrest. Moreover, our findings are difficult to reconcile with previously published data suggesting that AAV vectors are deficient in provoking a DNA-damage response due to the absence of cis-acting elements mapped to the p5 promoter region of the wild-type AAV genome.17, 18 Resolution of these apparent discrepancies requires more extensive analyses than we have undertaken in this brief descriptive report. Certainly our central observation of potent transient AAV vector-mediated inhibition of cell cycle progression in primary murine hepatocytes in vivo defines a potentially powerful model system for detailed dissection of the mechanisms by which cells sense incoming AAV vector genomes and the properties of the AAV vector genome involved. It will also be important to establish whether similar transient inhibition of hepatocellular proliferation occurs in the growing juvenile liver where the basal rates of hepatocellular proliferation are higher.
One further observation of interest from a host–vector interaction perspective is the small but statistically significant increase in the levels of hepatocellular proliferation seen at 3 months and 6 months following vector exposure when compared with PBS controls. One possible explanation in the context of the current study is a subtle toxic effect of eGFP expression driving increased cell turnover.26, 27, 28 Alternatively, the increased levels of hepatocellular proliferation observed at later time points may be the result of compensatory liver growth once the initial replicative suppression is relieved. Again, further studies are required to definitively define the mechanism involved.
In summary, we show for the first time that recombinant AAV vectors are capable of causing transient cell cycle arrest in vivo in the adult mouse liver, and present initial evidence favouring a mechanism involving the input single-stranded vector genome. Our findings support the conclusion that AAV vectors can indeed stimulate DNA-damage responses in a clinically relevant primary target cell type, and add a further level of complexity to the nexus of bidirectional host–vector interactions involving the cell cycle. More extensive studies are required to explore the mechanisms involved and to document the effects of age-dependent differences in basal rates of hepatocellular proliferation on the levels of transient replicative suppression observed.
Materials and methods
Vector production and animal studies
The vector construct containing a liver-specific promoter (LP1) driving the expression of eGFP has been previously described.22 The rAAV vector stocks were prepared by calcium phosphate transfection of HEK293 cells and purified by CsCl centrifugation.22 Vector was titred by quantitative PCR using primers to the woodchuck post-regulatory element as previously described.29 Empty capsid vector stock was prepared as above with omission of the plasmid containing the vector genome. Location of empty genomes in the CsCl gradient was determined by ELISA with capsid detected using serum from AAV8 immunised mice and a polyvalent goat anti-mouse horseradish peroxidase conjugate (Sigma-Aldrich) detection antibody. Titre of empty capsid vector stock was established by ELISA. A standard curve was prepared using vector genome containing virus stocks (with titre established by quantitative PCR) from which an equivalent capsid titre for the empty capsid stock was interpolated. All animal ethics procedures were evaluated and approved by the Children's Medical Research Institute and The Children's Hospital at Westmead Animal Care and Ethics Committee. Male and female C57bl/6 mice were injected with 1 × 1011 vg via the portal vein.29 To label proliferating hepatocytes mice were injected with 100 mg kg−1 BrdU twice a day by intra-peritoneal injection for 7 days before harvest. Liver collected for immunohistochemistry analysis was fixed in 10% neutral buffered formalin before embedding in paraffin. Liver collected for eGFP, vector genome and immunofluorescence analysis was prepared as previously described.29
Quantification of BrdU positive hepatocytes
Paraffin sections (0.5 μM) were cut onto SuperFrost Plus (Menzel-Gläser, Braunschweig, Germany), dewaxed in xylene and rehydrated through ethanol gradient. Antigen was retrieved in sodium citrate buffer before endogenous peroxidase was blocked in 0.3% (v/v) H2O2, prepared in methanol, for 30 min. Slides were rinsed in PBS before denaturation of DNA was carried out by incubation in 1.5 M HCl for 15 min. Slides were washed 3 times in 2 × 0.3 M sodium chloride, 0.03 M sodium citrate dehydrate, pH7.0, buffer before endogenous avidin and biotin was blocked using Avidin/Biotin Blocking Kit (VectorLabs, Burlingame, CA, USA). Slides were incubated with sheep anti-BrdU antibody (Abcam, Melbourne, NSW, Australia) in donkey serum block (8.6% (v/v) fetal calf serum (FCS)/13% (v/v) donkey serum in PBS) for 1 h at room temperature. This primary antibody was detected with donkey anti-sheep biotin conjugated secondary (Jackson Immuno Research, West Grove, PA, USA) and the strepavidin-horseradish peroxidase complex, ready-to-use Elite ABC reagent (VectorLabs). Positive staining was visualised with 3,3'-diaminobenzidine substrate solution (Sigma-Aldrich). To label central veins sections underwent a second avidin/biotin blocking step and proceeded through the steps described above using a primary rabbit anti-glutamine synthetase antibody (Abcam) followed by a biotin conjugated donkey anti-rabbit secondary (Jackson Immuno Research). Detection of the second label was performed using VIP purple peroxidase substrate solution (VectorLabs). Sections were subsequently counterstained with haematoxylin before dehydration and cover slipping.
For each mouse two separate sections of liver were analysed for the number and location of BrdU hepatocytes. Samples were de-identified before analysis. Using an upright light microscope the number of positive hepatocyte nuclei (identified based on size and morphology) were counted (section areas contained between 1.2 × 104 and 26 × 104 nuclei). To account for any bias due to sample size the area of each section was determined using ImagePro Plus version 4.5 software (Media Cybernetics Inc., Rockville, MD, USA). The total hepatocyte nuclei in two representative sections (with known area) were counted to determine the number of nuclei per unit area. Counts of all sections were then normalised to area and expressed per 104 nuclei. Mean basal rates of proliferation in control mice ranged from 0.27–0.6%, consistent with previous reports30, 31 supporting the validity of the method.
Detection of anti-capsid 8 antibodies in mouse serum by ELISA
Serum was collected from mice at time of harvest. ELISA plates (96-well Microtest; BD Biosciences, North Ryde, NSW, Australia) were coated with 50 μL rAAV2/8 viral stock diluted in PBS (1:200, 1:1000, 1:10 000 or 1:60 000), plates were incubated at 4 °C overnight. Plates were blocked with 100 μL 5% (w/v) skim milk powder in PBS for 1 h at room temperature. After washing 50 μL of mouse serum samples diluted 1:400 with PBS was added and plates incubated for 1 h at room temperature. To detect the mouse serum antibodies bound to viral capsid a polyvalent goat anti-mouse horseradish peroxidase conjugate (Sigma-Aldrich) was diluted at 1:2000 in 5% skim milk powder, incubated on the plate for 1 h at room temperature before detection with 75 μL 3,3′,5,5′ Tetramethylbenzidine substrate solution. The reaction was stopped with an equal volume of 1 M H2SO4 before absorbance was read at 450 nm.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA). Data were analysed using the non-parametric Mann–Whitney test. Significance was interpreted as P⩽0.05.
References
Sun B, Zhang H, Franco LM, Young SP, Schneider A, Bird A et al. Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II. Mol Ther 2005; 11: 57–65.
Jiang H, Lillicrap D, Patarroyo-White S, Liu T, Qian X, Scallan CD et al. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood 2006; 108: 107–115.
Cunningham SC, Spinoulas A, Carpenter KH, Wilcken B, Kuchel PW, Alexander IE . AAV2/8-mediated correction of OTC deficiency is robust in adult but not neonatal spfash mice. Mol Ther 2009; 17: 1340–1346.
Nathwani AC, Rosales C, McIntosh J, Rastegarlari G, Nathwani D, Raj D et al. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol Ther 2011; 19: 876–885.
Kok CY, Cunningham SC, Carpenter KH, Dane AP, Siew SM, Logan GJ et al. Adeno-associated virus-mediated rescue of neonatal lethality in argininosuccinate synthetase deficient mice. Mol Ther 2013; 21: 1823–1831.
Hinderer C, Bell P, Gurda BL, Wang Q, Louboutin JP, Zhu Y et al. Liver-directed gene therapy corrects cardiovascular lesions in feline mucopolysaccharidosis type I. Proc Natl Acad Sci USA 2014; 111: 14894–14899.
Nathwani AC, Tuddenham EG, Rangaraja S, Rosales C, McIntosh J, Linch DC et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011; 365: 2357–2365.
Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014; 371: 1994–2004.
Li H, Haurigot V, Doyon Y, Li T, Wong SY, Bhagwat AS et al. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature 2011; 475: 217–221.
Barzel A, Paulk NK, Shi Y, Huang Y, Chu K, Zhang F et al. Promoterless gene targeting without nucleases ameliorates haemophilia B in mice. Nature 2015; 517: 360–364.
Russell DW, Miller AD, Alexander IE . Adeno-associated virus vectors preferentially transduce cells in S phase. Proc Natl Acad Sci USA 1994; 91: 8915–8919.
Trobridge G, Hirata RK, Russell DW . Gene targeting by adeno-associated virus vectors is cell-cycle dependent. Hum Gene Ther 2005; 16: 522–526.
Nakai H, Yant SR, Storm TA, Fuess S, Meuse L, Kay MA . Extrachromosomal recombinant adeno-associated virus vector genomes are primarily responsible for stable liver transduction in vivo. J Virol 2001; 75: 6969–6976.
Winocour E, Callaham MF, Huberman E . Perturbation of the cell cycle by adeno-associated virus. Virology 1988; 167: 393–399.
Jurvansuu J, Raj K, Stasiak A, Beard P . Viral transport of DNA damage that mimics a stalled replication fork. J Virol 2005; 79: 569–580.
Raj K, Ogston P, Beard P . Virus-mediated killing of cells that lack p53 activity. Nature 2001; 412: 914–917.
Fragkos M, Breuleux M, Clement N, Beard P . Recombinant adeno-associated viral vectors are deficient in provoking a DNA damage response. J Virol 2008; 82: 7379–7387.
Francois A, Guilbaud M, Awedikian R, Chadeuf G, Moullier P, Salvetti A . The cellular TATA binding protein is required for rep-dependent replication of a minimal adeno-associated virus type 2 p5 element. J Virol 2005; 79: 11082–11094.
Davidoff AM, Ng CY, Zhou J, Spence Y, Nathwani AC . Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood 2003; 102: 480–488.
Dane AP, Cunningham SC, Graf NS, Alexander IE . Sexually dimorphic patterns of episomal rAAV genome persistence in the adult mouse liver and correlation with hepatocellular proliferation. Mol Ther 2009; 17: 1548–1554.
Duncan AW . Aneuploidy, polyploidy and ploidy reversal in the liver. Semin Cell Dev Biol 2013; 24: 347–356.
Cunningham SC, Dane AP, Spinoulas A, Alexander IE . Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol Ther 2008; 16: 1081–1088.
Schwartz RA, Palacios JA, Cassell GD, Adam S, Giacca M, Weitzman MD . The Mre11/Rad50/Nbs1 complex limits adeno-associated virus transduction and replication. J Virol 2007; 81: 12936–12945.
Cervelli T, Palacios JA, Zentilin L, Mano M, Schwartz RA, Weitzman MD et al. Processing of recombinant AAV genomes occurs in specific nuclear structures that overlap with foci of DNA-damage-response proteins. J Cell Sci 2008; 121: 349–357.
Lentz TB, Samulski RJ . Insight into the mechanism of inhibition of recombinant adeno-associated virus by the Mre11/Rad50/Nbs1 complex. J Virol 2015; 89: 181–194.
Liu HS, Jan MS, Chou CK, Chen PH, Ke NJ . Is green fluorescent protein toxic to the living cells? Biochem Biophys Res Commun 1999; 260: 712–717.
Huang WY, Aramburu J, Douglas PS, Izumo S . Transgenic expression of green fluorescence protein can cause dilated cardiomyopathy. Nat Med 2000; 6: 482–483.
Klein RL, Dayton RD, Leidenheimer NJ, Jansen K, Golde TE, Zweig RM . Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Mol Ther 2006; 13: 517–527.
Dane AP, Wowro SJ, Cunningham SC, Alexander IE . Comparison of gene transfer to the murine liver following intraperitoneal and intraportal delivery of hepatotropic AAV pseudo-serotypes. Gene Ther 2013; 20: 460–464.
Soames AR, Lavender D, Foster JR, Williams SM, Wheeldon EB . Image analysis of bromodeoxyuridine (BRDU) staining for measurement of S-phase in rat and mouse liver. J Histochem Cytochem 1994; 42: 939–944.
Magami Y, Azuma T, Inokuchi H, Kokuno S, Moriyasu F, Kawai K et al. Cell proliferation and renewal of normal hepatocytes and bile duct cells in adult mouse liver. Liver 2002; 22: 419–425.
Acknowledgements
We thank Margot Latham (The Children’s Hospital at Westmead) for assistance in manuscript preparation. This work was supported by an Australian National Health and Medical Research Council (NHMRC) project grant (1008021). APD was supported by an NHMRC Postgraduate Research Scholarship (477110) and a Children’s Medical Research Institute PhD stipend.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Dane, A., Cunningham, S., Kok, C. et al. Transient suppression of hepatocellular replication in the mouse liver following transduction with recombinant adeno-associated virus. Gene Ther 22, 917–922 (2015). https://doi.org/10.1038/gt.2015.66
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2015.66
- Springer Nature Limited