Abstract
Background/objectives
Kidney dialysis patients with sarcopenia have increased mortality. Screening for low muscle mass may allow interventions at an earlier stage to help improve outcomes. We wished to determine the prevalence of low muscle mass in a cohort of peritoneal dialysis (PD) patients.
Subjects/methods
We measured lean body mass index (LBMI) in 490 PD patients by bioimpedance, grading patients using two different classifications of sarcopenia.
Results:
LBMI was 9.7±1.9 kg/m2, mean age was 55.3±16.4 years, 53.1% were male, 33.7% were diabetic and 51% were Caucasoid. 98.5% of patients were classified as having sarcopenia based on LBMI cutoffs from NHANES data, whereas 28.8% had moderate and 6.3% severe sarcopenia using a grading correlated with functional disability. Lower muscle mass was associated with increasing co-morbidity (β=0.34, P=0.02) and age (β=0.01, P=0.006), and negatively with body mass index (β=−0.23, P<0.001), log serum creatinine (β=−0.231, P<0.001), normalised protein nitrogen appearance (β=−1.33, P<0.001) and log urine volume (β=−0.28, P=0.002). There was no association with duration of PD, dialysis prescription, residual renal function or solute clearances.
Conclusions:
There is currently no agreed universal definition for sarcopenia, and prevalence varied markedly depending on the scoring system. Prevalence was not associated with small solute clearances, but was associated with sex, age co-morbidity, BMI and ethnicity. There was an association with dietary protein intake and urine volume, which may allow for dietary interventions and strategies to preserve urine output to reduce muscle loss in PD patients.
Similar content being viewed by others
Introduction
Body composition is typically divided into fat mass and fat free mass, predominantly skeletal muscle, which contributes to around 40% of total body weight and 50% of body protein.1 Skeletal muscle mass acts as a reserve of protein stores for the body, and muscle can be catabolised to release proteins and amino acids at times of need. Patients with chronic kidney disease are potentially at greater risk of muscle wasting because of increased urinary protein losses and reduced dietary protein intake,2 and dialysis patients may also have protein and amino acid losses in the dialysate.3 In addition, patients with chronic kidney disease are more likely to have increased muscle loss because of a combination of factors, including metabolic acidosis, vitamin D deficiency, insulin resistance, anaemia, reduced physical activity, steroid therapy and treatment with other immune suppressants, testosterone deficiency in men and depression.2, 4, 5 It has been suggested that the uraemic state causes changes in muscle bioenergetics, due to a mitochondrial energy deficit,4 although on routine light microscopy muscle biopsies generally show nonspecific changes with type II muscle fibre atrophy.6
Muscle mass typically starts to decline after the age of 50, with estimates of 0.5–1.0% loss per year. Sarcopenia encompasses both loss of muscle mass and a loss of muscle function.7 However, there is no universally agreed definition of sarcopenia, particularly for patients with chronic kidney disease.8 Baumgartner et al.9 proposed a pragmatic definition of sarcopenia based on the measurement of lean body mass by dual-energy X-ray absorptiometry (DEXA), defining sarcopenia as >2 s.d. below the mean lean body mass for gender-specific healthy young adults. Subsequently, the European Society on Clinician Nutrition and Metabolism Special Interest Groups on geriatric nutrition and on cachexia–anorexia in chronic wasting diseases published a consensus definition,10 combining both reduced muscle mass, again >2 s.d. below the mean measured in young adults (aged 18–39 years from the 3rd National Health and Nutrition Examination Survey (NHANES) population) of the same sex and ethnic background, and a reduced gait speed.7 However, as most muscle mass is in the limbs, adjusting skeletal muscle mass for height permits comparison between patients,11 and a lowered skeletal muscle index below an accepted normal range for men and women has also been used to diagnose sarcopenia.7 Although the earlier definitions of sarcopenia used lean body mass measured by DEXA, more recent reports have used bioimpedance assessments of lean body mass and skeletal muscle.7 We have previously reported that there is a strong correlation between lean body mass measured by multi-frequency bioelectrical impedance assessments (MFBIA) and DEXA in dialysis patients.12, 13 We wished to determine the prevalence of low muscle mass in patients with chronic kidney disease established on peritoneal dialysis using MFBIA assessments of body composition. We employed two definitions of sarcopenia; lean body mass index (LBMI) >2 s.d. below normal adult values,14 and a graded classification derived from functional assessments, in which the degree of sarcopenia correlated with normal, moderate and severe disability.15
Methods
We measured body composition in 490 adult peritoneal dialysis outpatients attending for routine assessment of peritoneal dialysis adequacy using MFBIA, with an eight electrode multi-frequency segmental bioimpedance device (InBody 720, Seoul, South Korea). Patients did not have peritonitis or other infections, or hospital admissions within the previous 6 weeks. MFBIA16 was measured in a previously reported standardised manner; first patients were asked to empty the bladder and then peritoneal dialysate was drained out, as ascites and peritoneal dialysate can potentially alter bioimpedance-derived body composition measurements.17, 18 Patients with amputations, pregnancy and those who were wheelchair bound were excluded.19
We determined residual renal function by measuring 24 -h urinary urea and creatinine and taking the mean clearance adjusted to 1.73 m2 from the urinary collection as part of the peritoneal dialysis adequacy assessment. Similarly, urea, creatinine and protein were measured in 24 -h spent peritoneal dialysate effluents, with corresponding serum biochemistry (Roche Integra, Roche Diagnostics, Lewes, UK), using the bromocresol green method for albumin determination and module P for enzymatic creatinine measurements. Peritoneal dialysis adequacy was determined by standard methods.19 Normalised protein nitrogen appearance rate was calculated using the Randerson equation,20 adjusted for body weight on the day bioimpedance was measured.
Patient demographics and ethnicity were obtained from the Royal Free Hospital computerised records. Patient co-morbidity was determined using the Davies-Stoke co-morbidity scoring system.21 All patients who passed more than 200 ml urine/day were prescribed 250 mg frusemide.
Muscle loss was defined by LBMI >2 s.d. below normal adult values (Supplementary Table 1),14 and also using a graded classification derived from functional assessments, with skeletal muscle cut points of 8.51–10.75 and ⩽8.50 kg/m2 for men, and 5.76–6.75 and ⩽5.75 kg/m2 for women to denote moderate and high physical disability risk, respectively.15
This retrospective audit complied with the UK National Health Service guidelines for clinical audit and service development (UK National Health Service guidelines for clinical audit and service development, available at http://www.hra.nhs.uk/documents/2013/09/defining-research.pdf, and http://www.gov.uk/government/publications/health-research-ethics-committees-governancearrangements).
Statistical analysis
Data are presented as mean±s.d., median (interquartile range), or percentage. Standard statistical tests were used to analyse data, (t-test, Mann–Whitney U-test, ANOVA, Kruskal–Wallis, or χ2-test) with appropriate post hoc corrections made for multiple testing (Tukey or Dunn), where appropriate. Correlation was by Pearson or Spearman analysis, depending upon whether variables were normally distributed. Nonparametric data was log transformed for multivariable step forward linear analysis, using all variables with a P<0.1 correlation and those variables considered to be of clinical significance, and then variables were excluded if not statistically significant, unless they improved the model fit. The model was checked for collinearity. Statistical analysis used Prism 6.0 (Graph Pad, San Diego, CA, USA) and SPSS 22 (University Chicago, Chicago, IL, USA). Statistical significance was taken as P<0.05.
Results
Body composition was measured by MFBIA in 490 patients out of a possible total cohort of 525 patients. Patients with amputations, those who were wheelchair bound and those with clinical volume overloaded were excluded. The mean age of the patients studied was 55.3±16.4 years, 53.1% were male and 33.7% diabetic. Median Davies co-morbidity score was 1.0 (0–2). 25.1% patients were treated by continuous ambulatory peritoneal dialysis, 20.4% by overnight automated peritoneal dialysis with no day time exchange and 54.5% automated peritoneal dialysis with a day time exchange for a median of 3 (2–17) months. In all, 30.4% of patients used hypertonic glucose exchanges (22.7 g/l dextrose), 23.0% used neutral pH glucose dialysates and 77% patients used 7.5% icodextrin dialysate.
Patient body composition, standard laboratory investigations and urinary and peritoneal dialysis clearances are set out in Table 1.
We divided patients into those with and without loss of muscle mass based on normative values from the NHANES cohort14 as recommended by the European and International consensus expert groups.7, 10 Using this metric, 98.9% of our patients had loss of muscle mass.
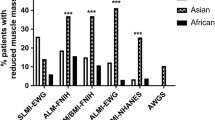
We then divided patients according to LBMI with cutoff points which have been shown to correlate with the degree of functional disability (normal muscle mass, moderate loss of muscle mass and severe loss of muscle mass) (Table 2). In all, 64.9% of patients had loss of muscle mass, 28.8% moderate and 6.3% had severe loss of muscle mass. We found greater loss of muscle mass in diabetic patients and older patients and those of South Asian ethnicity (Figure 1). As expected, a higher degree of loss of muscle mass was associated with lower body weight and body mass index. However, for men increasing sarcopenia grade was associated with a significant increase in percentage body fat (grade 1: 23.8±10.1%, grade 2: 27.8±10.1% and grade 3: 32.1±10.1%, P<0.01).
Loss of muscle mass according to LBMI cutoffs according to functional assessments15 and racial origins, grades 0 (normal), 1 (moderate) and 2 (severe). χ2=60.6, P<0.001.
Serum urea, creatinine and albumin concentrations were higher in patients without loss of muscle mass. There was no association between the degree of loss of muscle mass and duration of PD therapy, PD modality, or measures of clearance. Urine output, however, was lower in those with severe loss of muscle mass (Table 2). There were no differences in muscle mass when patients were compared according to duration of peritoneal dialysis therapy (Table 3). The ratio of extracellular water (ECW) to total body water and co-morbidity score both increased with more severe loss of muscle mass (Figures 2 and 3).
LBMI cutoffs according to functional assessments grades 0 (normal), 1 (moderate) and 2 (severe),15 and ECW as a percentage of total body water (TBW). ***P<0.001 vs normal (grade 0).
A univariate analysis was undertaken (Table 4), and all variables with a P<0.1 value were then included along with variables considered to be of clinical relevance in a multivariate step forward regression model. Nonparametric data were log transformed if appropriate to improve distribution. LBMI was positively associated with male gender, BMI, urine output, serum creatinine, nPNA, and negatively associated with age and co-morbidity grade (Table 5).
Discussion
Sarcopenia is associated with increased risk of mortality.22 We used bioimpedance to assess body composition and measure muscle mass. Previous studies have validated bioimpedance techniques against DEXA scanning in haemo- and peritoneal dialysis patients,12, 13, 23 and reported on the reproducibility of bioimpedance measurements.17, 24 However, the presence of peritoneal dialysate does have an effect on measurements of muscle and fat,16 and as such measurements should preferably be made when dialysate has been drained out.25 As with DEXA scans, muscle mass many be overestimated by bioimpedance if patients are overhydrated.26
Currently, many definitions of sarcopenia depend on cutoff points derived from the US NHANES data, with some definitions based on muscle mass measurements from a healthy young population, and others using cutoff points derived from local populations.9, 14, 15, 27 When we used a definition of loss of muscle mass based on normative values from the NHANES data set, almost all our patients fulfilled the criteria for loss of muscle mass. Previous reports using the NHANES data set have reported up to a 20-fold difference in the prevalence of sarcopenia depending on the cutoff point used by different societies and study groups.28 Others have reported varying prevalence for sarcopenia, when using different definitions.23 Other definitions of sarcopenia have attempted to include a functional element in addition to a measurement of muscle mass.7, 10 When we used a grading system developed from a functional assessment of disability, the prevalence of loss of muscle mass fell, with 64.9% of patients being classified as normal, 28.8% as moderate and 6.3% as severe loss of muscle mass.15 This highlights the need for an agreed definition to allow for interventional studies to assess treatments to reverse or prevent sarcopenia.
Muscle mass naturally declines with older age. Although there are many potential risk factors for muscle wasting in patients with chronic kidney disease,2, 4 because of the differences in definitions of sarcopenia, few studies have demonstrated more severe sarcopenia in dialysis patients compared with age- and sex-matched patients.29 We found an association between loss of muscle mass grade and age and sex, with a much greater prevalence for men. This may be explained using higher cutoff levels for women compared with that in some other studies.30 We also noted a greater prevalence in patients from the South Asian subcontinent, and patients from this ethnic background have been previously noted to have lower muscle mass than Caucasoids and African-Afro-Caribbeans.31 Similarly, we noted a greater prevalence in diabetic patients, and again there are reports of changes in body composition in patients with diabetes, particularly those with type 2 diabetes.32 In keeping with previous observations, we noted that BMI appeared protective, and studies have shown that body size is significantly associated with improved physical functioning and quality of life.33 Our data showed an association with higher serum creatinine concentrations and normalised urea nitrogen appearance rates being protective against loss of muscle mass. Thus would suggest that these patients not only had greater dietary protein intake but also were more active with greater metabolic turnover. This is in keeping with recent reports that patients starting dialysis with higher serum urea and creatinine concentrations have higher survival rates.34 We found that co-morbidity had a strong correlation with the prevalence of loss of muscle mass, supporting previous observations, linking co-morbidity and inflammation.35, 36 This association with inflammatory conditions links changes in ECW and intracellular water,37 with an increased ECW/total body water ratio.36, 38 Although we found no association between residual renal function and loss of muscle mass, which is in keeping with previous studies in patients with chronic kidney disease after adjusting for age and other factors,29 we did note that greater daily urine volume appeared to be protective against loss of muscle mass. Urine output in peritoneal dialysis patients is associated with lower ECW/total body water ratios because of a combination of both lower ECW and also greater ICW and cell mass.39 In addition to finding no association between loss of muscle mass and measured residual renal function, we also observed no association with peritoneal dialysis prescriptions, dialysate prescriptions or delivered dose of dialysis measured by urea clearance (Kt/V) or litres of creatinine cleared adjusted for body surface area. This may be due to clinical practice guidelines advising dialysis-dosing targets for patients, and repeated studies have not shown any survival benefit for higher targets in dialysis patients.40
The term sarcopenic obesity has been used to describe the coexistence of increased fat mass and low skeletal muscle mass. The definition varies between studies.15, 28 In keeping with previous reports, we noted particularly for males that increasing sarcopenia grade was associated with increased percentage body fat.41, 42 Although body composition changes with age, there has been speculation as to whether increased fat mass is a risk factor for low muscle mass, or whether chronic inflammatory conditions lead to increased body fat and muscle loss.42
We performed a cross-sectional analysis, and as such do not have longitudinal follow-up data to determine whether changes in body composition occur with duration of peritoneal dialysis therapy, or track or precede changes in inflammatory markers, such as CRP or hospital admissions. Although previous studies have reported reproducible body composition results in peritoneal dialysis patients using the InBody bioimpedance device and validated this against dual electron X ray absorption measurements,13 not all bioimpedance devices are equal, and as with dual electron X ray absorption measurements then peritoneal dialysate must be drained out, to ensure more accurate measurements.17
We examined the prevalence of loss of muscle mass in a cohort of almost 500 adult patients established on peritoneal dialysis and noted a wide variation in prevalence depending upon the definition used. Definitions or sarcopenia vary, ranging from those based simply on lean body mass, to those adding a functional component, such as testing muscle strength, or sit-to-stand testing. When we used a definition that combined LBMI, which we measured by bioimpedance,43 and included a grading system derived from functional assessment, then the prevalence was much lower than that simply based solely on muscle mass alone. As expected, loss of muscle mass was more prevalent with increasing age and co-morbidity. However, loss of muscle mass was less prevalent for patients with greater body mass index, and estimated dietary protein intake, based on peritoneal dialysate effluent and urinary urea, and higher serum creatinine concentrations. However, the association with dietary protein intake, urinary urea and serum creatinine may simply reflect greater muscle mass, physical activity and dietary protein intake. We found that patients from the South Asian subcontinent had a greater prevalence of loss of muscle mass; this may be due to the low representation of this ethnic group in the NHANES study population, but these patients are more likely to eat vegetarian diets and have diabetes.
References
Griffiths RD . Muscle mass, survival, and the elderly ICU patient. Nutrition 1996; 12: 456–458.
Wang XH, Mitch WE . Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol 2014; 10: 504–516.
Rajakaruna G, Caplin B, Davenport A . Peritoneal protein clearance rather than faster transport status determines outcomes in peritoneal dialysis patients. Perit Dial Int 2015; 35: 216–221.
Fahal IH . Uraemic sarcopenia: aetiology and implications. Nephrol Dial Transplant 2014; 29: 1655–1665.
Cigarrán S, Pousa M, Castro MJ, González B, Martínez A, Barril G et al. Endogenous testosterone, muscle strength, and fat-free mass in men with chronic kidney disease. J Ren Nutr 2013; 23: e89–e95.
Davenport A, King RF, Ironside JW, Will EJ, Davison AM . The effect of treatment with recombinant human erythropoietin on the histological appearance and glycogen content of skeletal muscle in patients with chronic renal failure treated by regular hospital haemodialysis. Nephron 1993; 64: 89–94.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–423.
Reid J, Noble H, Davenport A, Farrington K, Fouque D, Porter S et al. Defining cachexia in a renal population. J Ren Care 2015; 41: 79–80.
Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147: 755–763.
Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM, Biolo G et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases" and "nutrition in geriatrics". Clin Nutr 2010; 29: 154–159.
Kyle UG, Schutz Y, Dupertuis YM, Pichard C . Body composition interpretation. Contributions of the fat-free mass index and the body fat mass index. Nutrition 2003; 19: 597–604.
Fürstenberg A, Davenport A . Comparison of multifrequency bioelectrical impedance analysis and dual-energy X-ray absorptiometry assessments in outpatient haemodialysis patients. Am J Kidney Dis 2010; 57: 123–129.
Fürstenberg A, Davenport A . Assessment of body composition in peritoneal dialysis patients using bioelectrical impedance and dual-energy x-ray absorptiometry. Am J Nephrol 2011; 33: 150–156.
Kelly TL, Wilson KE, Heymsfield SB . Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS One 2009; 4: e7038.
Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R . Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 2004; 159: 413–421.
McCafferty K, Fan S, Davenport A . Extracellular volume expansion, measured by multifrequency bioimpedance, does not help preserve residual renal function in peritoneal dialysis patients. Kidney Int 2014; 85: 151–157.
Davenport A . Does peritoneal dialysate affect body composition assessments using multi-frequency bioimpedance in peritoneal dialysis patients? Eur J Clin Nutr 2013; 67: 223–225.
Davenport A, Argawal B, Wright G, Mantzoukis K, Dimitrova R, Davar J et al. Can non-invasive measurements aid clinical assessment of volume in patients with cirrhosis? World J Hepatol 2013; 5: 433–438.
NKF-K/DOQI Clinical practice guidelines for peritoneal dialysis adequacy: Clinical practice recommendations for peritoneal dialysis adequacy. Am J Kid Dis 2006; 48: S98–S158.
Randerson DH, Chapman GV, Farell PC . Amino acid and dietary status in CAPD patients Atkins RC, Farell PC, Thomson N Peritoneal Dialysis. Churchill-Livingstone: Edinburgh, Scotland, 1981; 180–191.
Davies SJ, Phillips L, Naish PF, Russell GI . Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant 2002; 17: 1085–1092.
Carrero JJ, Chmielewski M, Axelsson J, Snaedal S, Heimbürger O, Bárány P et al. Muscle atrophy, inflammation and clinical outcome in incident and prevalent dialysis patients. Clin Nutr 2008; 27: 557–564.
Lamarca F, Carrero JJ, Rodrigues JC, Bigogno FG, Fetter RL, Avesani CM . Prevalence of sarcopenia in elderly maintenance haemodialysis patients: the impact of different diagnostic criteria. J Nutr Health Aging 2014; 18: 710–717.
Davenport A, Willicombe M . Comparison of fluid status in patients treated by different modalities of peritoneal dialysis using multi-frequency bioimpedance. Int J Artif Organs 2009; 32: 779–786.
Davenport A . Effect of intra-abdominal dialysate on bioimpedance-derived fluid volume status and body composition measurements in peritoneal dialysis patients. Perit Dial Int 2013; 33: 578–579.
Panorchan K, Nongnuch A, El-Kateb S, Goodlad C, Davenport A . Changes in muscle and fat mass with haemodialysis detected by multi-frequency bioelectrical impedance analysis. Eur J Clin Nutr 2015; 69: 1109–1112.
Patel HP, Syddall HE, Jameson K, Robinson S, Denison H, Roberts HC et al. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS). Age Ageing 2013; 42: 378–384.
Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ . Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc 2013; 61: 974–980.
Stenvinkel P, Carrero JJ, von Walden F, Ikizler TA, Nader GA . Muscle wasting in end-stage renal disease promulgates premature death: established, emerging and potential novel treatment strategies. Nephrol Dial Transplant 2015; 31: 1070–1077.
Yu S, Appleton S, Adams R, Chapman I, Wittert G, Visvanathan T et al. The impact of low muscle mass definition on the prevalence of sarcopenia in older Australians. Biomed Res Int 2014; 2014: 361790.
Davenport A, Hussain Sayed R, Fan S . The effect of racial origin on total body water volume in peritoneal dialysis patients. Clin J Am Soc Nephrol 2011; 6: 2492–2498.
Davenport A, Willicombe MK . Does diabetes mellitus predispose to increased fluid overload in peritoneal dialysis patients? Nephron Clin Pract 2010; 114: c60–c66.
Martinson M, Ikizler TA, Morrell G, Wei G, Almeida N, Marcus RL et al. Associations of body size and body composition with functional ability and quality of life in haemodialysis patients. Clin J Am Soc Nephrol 2014; 9: 1082–1090.
Floege J, Gillespie IA, Kronenberg F, Anker SD, Gioni I, Richards S et al. Development and validation of a predictive mortality risk score from a European haemodialysis cohort. Kidney Int 2015; 87: 996–1008.
Isoyama N, Qureshi AR, Avesani CM, Lindholm B, Bàràny P, Heimbürger O et al. Comparative associations of muscle mass and muscle strength with mortality in dialysis patients. Clin J Am Soc Nephrol 2014; 9: 1720–1728.
Demirci MS, Demirci C, Ozdogan O, Kircelli F, Akcicek F, Basci A et al. Relations between malnutrition-inflammation-atherosclerosis and volume status. The usefulness of bioimpedance analysis in peritoneal dialysis patients. Nephrol Dial Transplant 2011; 26: 1708–1716.
Booth J, Pinney J, Davenport A . N-terminal proBNP–marker of cardiac dysfunction, fluid overload, or malnutrition in haemodialysis patients? Clin J Am Soc Nephrol 2010; 5: 1036–1040.
John B, Tan BK, Dainty S, Spanel P, Smith D, Davies SJ . Plasma volume, albumin, and fluid status in peritoneal dialysis patients. Clin J Am Soc Nephrol 2010; 5: 1463–1470.
Fan S, Sayed RH, Davenport A . Extracellular volume expansion in peritoneal dialysis patients. Int J Artif Organs 2012; 35: 338–345.
Daugirdas JT . Kt/V (and especially its modifications) remains a useful measure of haemodialysis dose. Kidney Int 2015; 88: 466–473.
Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L . Sarcopenic obesity—definition, aetiology and consequences. Curr Opin Clin Nutr Metab Care 2008; 11: 693–700.
Dominguez LJ, Barbagallo M . The cardiometabolic syndrome and sarcopenic obesity in older persons. J Cardiometab Syndr 2007; 2: 183–189.
Davies SJ, Davenport A . The role of bioimpedance and biomarkers in helping to aid clinical decision-making of volume assessments in dialysis patients. Kidney Int 2014; 86: 489–496.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website
Supplementary information
Rights and permissions
About this article
Cite this article
Greenhall, G., Davenport, A. Screening for muscle loss in patients established on peritoneal dialysis using bioimpedance. Eur J Clin Nutr 71, 70–75 (2017). https://doi.org/10.1038/ejcn.2016.202
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2016.202
- Springer Nature Limited
This article is cited by
-
Low muscle mass and early hospital readmission post-kidney transplantation
International Urology and Nephrology (2022)
-
Gender-specific associations of skeletal muscle mass and arterial stiffness among peritoneal dialysis patients
Scientific Reports (2018)