Abstract
Background/Objectives:
The impact of nutritional status on survival among community-dwelling older adults is unclear. We aimed to investigate the prevalence and association of poor nutritional status, including malnutrition and risk for malnutrition defined by the Mini-Nutritional Assessment-Short Form (MNA-SF) with survival, and to explore the role of relevant biomarkers (hemoglobin, albumin and C-reactive protein) in this association.
Subjects/Methods:
This study included 3041 participants aged ⩾60 in the Swedish National study on Aging and Care-Kungsholmen. On the basis of the total score in MNA-SF, nutritional status for each participant was assessed as normal (score 12–14), risk for malnutrition (8–11) or malnutrition (<8). Over an 11-year follow-up, survival status was observed. Data were analysed using logistic regression, flexible parametric survival and Laplace models.
Results:
Of all the participants, 51 (1.7%) had malnutrition and 751 (24.7%) were at risk for malnutrition. The multi-adjusted hazard ratio (95% confidence interval) of mortality was 2.40 (1.56−3.67; P<0.001) for malnutrition and 1.49 (1.29−1.71; P<0.001) for risk for malnutrition. The median ages at death of participants with malnutrition and risk for malnutrition were ~3 and 1.5 years shorter than those with normal nutritional status, respectively, whereas malnutrition or risk for malnutrition together with abnormal biomarker (hemoglobin and albumin) levels was related to 1 year more shortened survival.
Conclusions:
Malnutrition and risk for malnutrition are highly prevalent and significantly associated with a shorter survival. Poor nutritional status in combination with abnormalities in the biomarkers is associated with even more shortened survival.
Similar content being viewed by others
Introduction
Malnutrition is generally defined as adverse health outcome due to an imbalance between the intake of different nutrients and physiological requirements.1 Today malnutrition in developed countries mainly implies disease-related undernutrition.1 Because various physiological and psychological alterations in older ages may lead to decreased hunger, insufficient food intake and metabolic inefficiency, impaired nutritional status is becoming increasingly common among older adults.2, 3 This condition may be present before disease manifestation; however, it is frequently unrecognized.4
Prevalence of malnutrition is generally higher among older adults at health care facilities than in the community.5, 6 In a combined data set including 4507 participants from 12 countries (80.6% of the data is from Europe), the average prevalence was ~22.8%, ranging from 5.8% in the community to 50.5% in clinical settings.6 Malnutrition may adversely affect disease prognosis and delay recovery, leading to early death in older populations.7 Different nutritional assessment tools have been used to identify people at risk for malnutrition. Several clinical studies using the Mini Nutritional Assessment (MNA) tool in older adults have reported an association between malnutrition and increased mortality among hospitalized elderly patients.8, 9 However, fewer population-based studies using the same assessment tool in community elderly populations have suggested that malnutrition is associated with mortality.10, 11
In recent years, the Mini-Nutritional Assessment-Short Form (MNA-SF), as a nutritional risk screening tool, has been widely used to detect malnutrition and risk for malnutrition among elderly people.12 Besides, as no diagnostic tool has proven conclusive in the diagnosis of malnutrition, biochemical measurements such as serum albumin (Alb) and inflammatory markers have been suggested to help identify malnutrition, though these biomarkers are more likely to reflect the health than nutritional status.2 To date, no long-term population-based longitudinal studies have examined the association between nutritional status and survival taking biochemical measures into account among older community dwellers. This assessment of biomarkers could help in prioritizing the clinical care needed after nutritional risk screening. In the present study, we aimed to: (1) investigate the prevalence of malnutrition and risk for malnutrition defined by the MNA-SF among the Swedish older adults; (2) confirm the association of nutritional status with survival; and (3) explore the role of relevant biomarkers (hemoglobin (Hb), Alb and C-reactive protein (CRP)) in this association.
Materials and methods
Study population
The study population was derived from the Swedish National study on Aging and Care-Kungsholmen (SNAC-K), an ongoing longitudinal study that is one of four subprojects of the Swedish National study on Aging and Care.13 The SNAC-K study population consists of a random sample of individuals aged ⩾60 years living at home or in institutions in the Kungsholmen district of Stockholm. The sampling was stratified by age and year of assessment. A total of 5111 persons were initially selected for participation, of which 200 were deceased, 262 unreachable, 32 had moved, 23 could not speak Swedish and 4 were deaf. Of the 4590 living eligible persons, 1227 refused to participate. The remaining 3363 participants (73.3%) underwent baseline examinations during March 2001 and June 2004. Out of the 3363 participants, 322 persons had missing information on at least one of the MNA-SF items, leaving 3041 persons for the current analyses.
SNAC-K was approved by the Ethics Committee at Karolinska Institutet and the Regional Ethical Review Board in Stockholm. Written informed consent was collected from the participants at baseline, or, if the participant had cognitive impairment, from a proxy (for example, a close family member).
Data collection
The baseline survey included collection of information about present status and past events through interviews, clinical examinations and tests by trained staff following a structured protocol (available at http://www.snac.org). The interviews covered demographic data, socioeconomic status, living arrangement, physical functioning and use of medical and social care facilities.
During the baseline nurse interview, the highest level of formal education was recorded as one of the three categories: elementary school, high school or university. Living arrangement was defined on the basis of participants’ living situations and categorized as living at home with someone, living at home alone or living in an institution.
Data on chronic diseases, including vascular disorders (hypertension, stroke and heart diseases including heart failure, coronary heart disease and arrhythmia), diabetes and cancer were ascertained based on clinical examinations, self-reported medical histories, medications use and the Swedish inpatient registry that covers all hospitalizations in Sweden since 1969. The ninth and tenth revisions of the International Classification of Diseases (ICD-9 and ICD-10) were used in the registry. Global cognitive functioning was tested using the Mini-Mental State Examination (MMSE).14 Weights and heights were measured with a standard scale in light clothing and no shoes.
Blood samples were taken from the participants for laboratory tests. Hb was measured using the Sodium Lauryl Sulphate method (Sysmex XE-5000, Sysmex Corp., Kobe, Japan); serum Alb was measured by Bromcresol Purple dye method (DXC800, Beckman Coulter, Brea, CA, USA); and CRP was measured by a turbidimetric method (DXC800, Beckman Coulter). Hb levels were dichotomized according to the World Health Organization criteria for the diagnosis of anemia (Hb concentration <130 g/l in men and <120 g/l in women).15 Hypoalbuminemia was defined as Alb concentrations <37 g/l.16 CRP levels were divided into three groups: normal (0–5 mg/l, lab reference value), high (6–20 mg/l) and very high (>20 mg/l) according to its distribution.
Assessment of nutritional status
Nutritional status of each participant was assessed using the MNA-SF, which is a six-item nutritional risk assessment questionnaire that was developed and validated specifically for old people.5, 17 The MNA-SF considers: (1) decline in food intake during the last 3 months (score 0–2); (2) weight loss during the last 3 months (score 0–3); (3) mobility (score 0–2); (4) psychological stress or acute diseases in the last 3 months (score 0 or 2); (5) psychological problems (that is, dementia and depression; score 0–2); and (6) body mass index (score 0–3). Possible total MNA-SF scores range between 0 and 14. On the basis of the total score, the nutritional status for each participant was defined as normal (score of 12–14), risk for malnutrition (score of 8–11) or malnutrition (score of <8).17 Details on the MNA-SF components assessment is presented in the Supplementary Text File.
Ascertainment of death during the 11-year follow-up
For all participants, death certificates from the Swedish Cause of Death Register at the National Board of Health and Welfare were used to ascertain deaths from the baseline survey (27 August 2001) until 15 October 2012. It is estimated that 93% of all deaths in Sweden are reported within 10 days, and 100% are reported within 30 days.18
Statistical analyses
Characteristics of the participants by nutritional status were compared using Chi-square or Fisher’s exact tests for categorical variables and Mann–Whitney test for continuous variables with non-normal distributions. Standardized estimates of prevalence were calculated by the direct method based on the age- and sex-specific structure of the adult population aged ⩾60 years in the Kungsholmen area in 2001 (65.6% women and 34.4% men). Multinomial logistic regression models were used to assess the indicators (age, sex, education, living arrangement and biomarkers) of nutritional status (normal nutritional status was used as the reference group).
Flexible parametric survival models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the associations between nutritional status and mortality rates. Potential statistical interactions between age, sex, education and nutritional status in predicting mortality rates were examined by incorporating the independent variables and cross-product terms in the same model. Moreover, we evaluated the association between mortality and the combination of nutritional status and biomarkers levels. The variables of biomarkers were coded as having normal or having at least one low biomarker level. A dummy variable was created based on every possible combination of the two variables, and people with normal nutritional status and normal biomarkers level were used as reference group. In this categorization, as the number of malnourished participants in the very high CRP group was very low (n=5), only Hb and Alb levels were considered. To provide a time dimension to the combined association between nutritional status and biochemical markers, we used Laplace regression to model the median survival time.19, 20
Because the youngest (60–66 years old) and the oldest (⩾90 years) age groups in SNAC-K were oversampled, the sampling weight method with age group stratification was used to weight each age group sample back to the original population from which the samples were drawn. The weighted variable was created as the number of people in each age strata in the original population divided by the number of individuals in relevant age strata in the study sample. Probability weight was used in the analyses.
Additional statistical analyses are presented in the Supplementary Text File. All the statistical analyses were performed using Stata SE 12 for Windows (StataCorp, College Station, TX, USA).
Results
Characteristics of the study population and prevalence of poor nutritional status
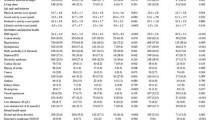
Of the 3041 participants at baseline, 1933 (63.6%) were women and 1108 (36.4%) were men. The mean age (s.d.) of the population was 73.7 (10.7) years. Among all the participants, 51 (1.7%) had malnutrition and 751 participants (24.7%) were at risk for malnutrition. Participants at risk for malnutrition and those with malnutrition were more likely to be women, older, less educated, living alone or in an institution, and to have poorer functional and health conditions compared with those having a normal nutritional status (Table 1). Weighted prevalence of malnutrition in the study population was 1.9% (0.7% in men and 2.6% in women) and of risk for malnutrition was 26.1% (21.3% in men and 28.6% in women). Figure 1 shows the weighted prevalence of malnutrition and risk for malnutrition by age groups.
In multivariable multinomial logistic regression, old age (80–89, OR (odds ratio) 1.72, 95% CI: 1.39−2.13 and ⩾90, OR 3.52, 95% CI: 2.71−4.57), living alone (OR 1.81, 95% CI: 1.47−2.22) and living in an institution (OR 4.71, 95% CI: 2.42−9.13) were independently associated with increased odds of poor nutritional status.
Table 2 shows the association of biomarkers with malnutrition and risk for malnutrition. Anemia, hypoalbuminemia and very high CRP (>20 mg/l) were significantly related to both malnutrition and risk for malnutrition (Table 2). The sensitivity and specificity of low Hb, low Alb and high CRP in predicting poor nutritional status were 46 and 77% for Hb, 43 and 76% for Alb, and 48 and 75% for CRP, respectively.
Nutritional status, all-cause mortality and survival
During the 11-year follow-up period (24 575.4 person-years; median per person=9.1 years; maximum=11.6 years), 1073 participants (35.3%) died. Of them, 680 (63.4%) were women. Age-, sex- and education-standardized mortality rates (per 1000 persons-years) in different nutritional status groups are shown in Table 3. After adjusting for age, sex, education and living arrangement, the mortality rates for people with malnutrition and at risk for malnutrition were 1.5-fold and 3.4-fold higher than those with normal nutritional status, respectively (Table 3). There were no statistically significant interactions of age, sex and education with poor nutritional status on mortality in flexible parametric survival analysis (P ranged between 0.411 and 0.979).
In Laplace regression analysis, the median age at death for participants with malnutrition was about 3 years shorter than those with normal nutritional status (difference in median age at death −2.75, 95% CI: −4.67 to −0.83, P=0.005) and almost 1.5 years shorter for those at risk for malnutrition (−1.48, 95% CI: −1.84 to −1.11, P<0.001) after adjustment for age at baseline.
Role of biomarkers in the association between nutritional status and survival
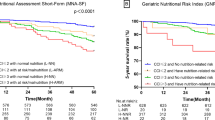
Figure 2 shows the adjusted median age at death for people with different combination of nutritional status and biomarkers levels based on Laplace regression analysis. Compared with people having both normal nutritional status and normal levels of biomarkers, people with malnutrition or at risk for malnutrition and low level of any biomarker had on average 4 (difference in median age at death: −4.31, 95% CI: −5.34 to −3.27, P<0.001) and 2.5 (difference in median age at death: −2.49, 95% CI: −3.15 to −1.83, P<0.001) years younger age at death, respectively.
Median age at death in six groups according to combinations of nutritional status and biomarker levels. Results were from Laplace regression, adjusted for age, sex, education and living arrangement. Biomarker levels were categorized as: normal and at least one low biomarker level (Hb <130 g/l in men and <120 g/l in women, and/or Alb <37 g/l). As the number of malnourished participants in the very high CRP group was very low (n=5), CRP was not included in this analysis.
Discussion
In this large-scale population-based longitudinal study of older adults, we found that: (1) the prevalence of malnutrition was 1.7% and the prevalence of the risk for malnutrition was 24.7%; (2) during the 11-year follow-up, malnutrition and risk for malnutrition were significantly associated with all-cause mortality and shortened survival by 3 and 1.5 years, respectively; and (3) poor nutritional status together with abnormal biomarker (Hb and Alb) levels was related to even more shortened survival.
The prevalence of poor nutritional status defined on the basis of MNA scores varies greatly in different countries, ranging from 0 to 26% for malnutrition, and 0 to 76% for risk for malnutrition among community-dwelling older adults.21 In European countries, several studies have reported that the prevalence of malnutrition was in the range of 0 to 13% and the prevalence of risk for malnutrition was in the range of 16 to 58%.21 In Sweden, a community-based study in 2009, on 258 old people aged 71 to 80 years living at home, showed that the prevalence of malnutrition was 0.4% and the prevalence of risk for malnutrition was 16%.22 Compared with these studies, our study is a relatively larger community-based cohort of older people with long-term follow-up, and the study population included people with a wider age range living either at home or in institutions. Results from our study confirm the high prevalence of poor nutritional status among older adults, and underscore the importance of nutritional risk screening in old age. As several studies reported,23, 24, 25, 26, 27 we also found that old age, living alone and living in an institution are related to poor nutritional status.
Malnutrition may negatively affect the health of older adults, as it can reinforce the development of adverse outcomes. Malnutrition can lead to increased infections occurrence, electrolyte imbalances, altered skin integrity, anemia, weakness and fatigue, which can result in increased morbidity, length of hospital stays, functional disabilities and mortality.2 The association between malnutrition and death has been reported in several studies of elderly patient populations.9, 28 In these studies, malnutrition was associated with ~2-fold higher mortality rates within short follow-up periods. Few population-based studies have examined this association. A community-based study of 1170 Brazilian adults aged ⩾60 years showed that poor nutritional status, especially malnutrition, was associated with death during a 7-year follow-up period.11 In another population-based study on 584 old women in Sweden, malnutrition was associated with about two times higher mortality during 10 years of follow-up.10 During an 11-year follow-up period, we verified that malnutrition and even risk for malnutrition were associated with elevated mortality and significantly shortened survival.
Although the MNA was introduced and validated years ago (1994),12, 17 it has received new attention since the 2009 structural revision that improved the form by decreasing the time burden on both those who administer and those who respond to the questionnaire.12 With regard to nutritional and inflammatory biochemical makers, there are still debates about using these biomarkers to assess nutritional status, as metabolic stress may also affect their levels.29, 30 They are, however, still considered relative markers of chronic nutritional status.31, 32, 33 In our study, poor nutritional status, defined by the MNA-SF, was significantly associated with lower Hb and Alb and higher CRP indicating that poor nutritional status detected by the MNA-SF could reflect some biological alterations. Further, we found that poor nutritional status together with the abnormalities in these markers was related to more shortened survival suggesting that using MNA-SF together with assessment of these biomarkers may better predict survival in older adults.
The main strength of this study is its population-based prospective design with a large sample of the Swedish older adults, living either at home or in institutions providing a more complete picture of nutritional status among the general elderly population in an urban center in Sweden. Most of the information (such as dementia and depression diagnosis) that was required for the MNA-SF was gained from comprehensive clinical examinations by physicians and structured interviews by nurses, therefore potential information biases were limited. In addition, 11-year-long survival status was observed for all the participants in this study. However, some limitations need to be pointed out. First, some of the items within the short MNA, such as food intake decline and weight loss were scored based on self-reports. However, in testing validation of the MNA-SF based on self-reports, Rubenstein et al.17 have found a high diagnostic accuracy (98.7%) of MNA-SF for predicting undernutrition among a sample of European and US older populations (n=881, 73.8% community dwellers, mean age 76.4 years), as compared with clinically assessed nutritional status. Second, 1227 refusers at baseline, and 322 participants with missing information on MNA-SF were excluded. There was no difference in sex distribution between participants and non-participants, but the non-participants were on average slightly older.13As the prevalence of malnutrition increases with age, our results might have underestimated the association between poor nutritional status and survival. In addition, unequal distribution of the missing values of biomarkers among MNA-SF groups might have led to an underestimation of the association between biomarker abnormalities and poor nutritional status, and consequently the association with survival. However, when the analyses were repeated including participants with imputed values, the results did not differ substantially from the initial analyses. Therefore, selection bias might not have a significant role in the given associations. We acknowledge that cautions are needed when generalizing the major findings from this study. SNAC-K is a prospective study of community-based cohorts, which includes individuals who have been living in a geographically defined central area of Stockholm (Kungsholmen). The study population is a random sample of the local general population at the time the project started, and is representative of the urban population in Sweden. Therefore, the major findings from this study may be generalizable to populations aged ⩾60 living in Western societies with similar characteristics to Kungsholmen population (that is, urban and well-educated). Finally, the constant interactions between nutritional status and diseases in old ages may make the nutritional assessment in these vulnerable groups very challenging. Decreased food intake and weight loss may be the consequence of acute or chronic conditions; while in return, malnutrition state may boost the development of physiological dysfunctions.21 Thus, the ‘pure’ effect of malnutrition on mortality may not be clearly disentangled. In our study, the association between poor nutritional status and mortality seems to be independent of chronic diseases suggesting that subclinical changes may have a role in the association between poor nutritional status and mortality.
In conclusion, our results confirm that the prevalence of malnutrition and risk for malnutrition defined by MNA-SF, is high among older adults, and may significantly shorten survival. Biomarkers (such as Hb and Alb) may have an important role in the association between nutritional status and survival. These findings highlight the need for regular nutritional risk screening for the timely detection and management of malnutrition and risk for malnutrition in old age. Biochemical measures of nutritional status could help in prioritizing the clinical care needed after nutritional risk screening.
References
Norman K, Pichard C, Lochs H, Pirlich M . Prognostic impact of disease-related malnutrition. Clin Nutr 2008; 27: 5–15.
Furman EF . Undernutrition in older adults across the continuum of care: nutritional assessment, barriers, and interventions. J Gerontol Nurs 2006; 32: 22–27.
Meijers JM, van Bokhorst-de van der Schueren MA, Schols JM, Soeters PB, Halfens RJ . Defining malnutrition: mission or mission impossible? Nutrition 2010; 26: 432–440.
Wells JL, Dumbrell AC . Nutrition and aging: assessment and treatment of compromised nutritional status in frail elderly patients. Clin Interv Aging 2006; 1: 67–79.
Guigoz Y . The Mini Nutritional Assessment (MNA) review of the literature—What does it tell us? J Nutr Health Aging 2006; 10: 466–485; discussion 485–487.
Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc 2010; 58: 1734–1738.
Sullivan DH . The role of nutrition in increased morbidity and mortality. Clin Geriatr Med 1995; 11: 661–674.
Correia MI, Waitzberg DL . The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr 2003; 22: 235–239.
Persson MD, Brismar KE, Katzarski KS, Nordenstrom J, Cederholm TE . Nutritional status using mini nutritional assessment and subjective global assessment predict mortality in geriatric patients. J Am Geriatr Soc 2002; 50: 1996–2002.
Lundin H, Saaf M, Strender LE, Mollasaraie HA, Salminen H . Mini nutritional assessment and 10-year mortality in free-living elderly women: a prospective cohort study with 10-year follow-up. Eur J Clin Nutr 2012; 66: 1050–1053.
Ferreira LS, do Amaral TF, Marucci Mde F, Nascimento LF, Lebrao ML, Duarte YA . Undernutrition as a major risk factor for death among older Brazilian adults in the community-dwelling setting: SABE survey. Nutrition 2011; 27: 1017–1022.
Kaiser MJ, Bauer JM, Ramsch C, Uter W, Guigoz Y, Cederholm T et al. Validation of the Mini Nutritional Assessment short-form (MNA-SF): a practical tool for identification of nutritional status. J Nutr Health Aging 2009; 13: 782–788.
Lagergren M, Fratiglioni L, Hallberg IR, Berglund J, Elmstahl S, Hagberg B et al. A longitudinal study integrating population, care and social services data. The Swedish National study on Aging and Care (SNAC). Aging Clin Exp Res 2004; 16: 158–168.
Folstein MF, Folstein SE, McHugh PR . 'Mini-mental state'. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–198.
Patel KV . Epidemiology of anemia in older adults. Semin Hematol 2008; 45: 210–217.
Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF et al. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA 1998; 279: 585–592.
Rubenstein LZ, Harker JO, Salva A, Guigoz Y, Vellas B . Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). J Gerontol A Biol Sci Med Sci 2001; 56: M366–M372.
Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A . The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009; 24: 659–667.
Bottai M, Zhang J . Laplace regression with censored data. Biom J 2010; 52: 487–503.
Bottai M, Orsini N . A command for Laplace regression. Stata J 2013; 13: 1–13.
Cereda E . Mini nutritional assessment. Curr Opin Clin Nutr Metab Care 2012; 15: 29–41.
Johansson L, Sidenvall B, Malmberg B, Christensson L . Who will become malnourished? A prospective study of factors associated with malnutrition in older persons living at home. J Nutr Health Aging 2009; 13: 855–861.
Serrano-Urrea R, Garcia-Meseguer MJ . Malnutrition in an elderly population without cognitive impairment living in nursing homes in Spain: study of prevalence using the Mini Nutritional Assessment test. Gerontology 2013; 59: 490–498.
Morley JE . Undernutrition in older adults. Fam Pract 2012; 29: i89–i93.
Vandewoude MF, Alish CJ, Sauer AC, Hegazi RA . Malnutrition-sarcopenia syndrome: is this the future of nutrition screening and assessment for older adults? J Aging Res 2012; 2012: 651570.
Rendall MS, Weden MM, Favreault MM, Waldron H . The protective effect of marriage for survival: a review and update. Demography 2011; 48: 481–506.
Cowan DT, Roberts JD, Fitzpatrick JM, While AE, Baldwin J . Nutritional status of older people in long term care settings: current status and future directions. Int J Nurs Stud 2004; 41: 225–237.
Saletti A, Johansson L, Yifter-Lindgren E, Wissing U, Osterberg K, Cederholm T . Nutritional status and a 3-year follow-up in elderly receiving support at home. Gerontology 2005; 51: 192–198.
Soeters PB, Reijven PL, van Bokhorst-de van der Schueren MA, Schols JM, Halfens RJ, Meijers JM et al. A rational approach to nutritional assessment. Clin Nutr 2008; 27: 706–716.
Fuhrman MP . The albumin-nutrition connection: separating myth from fact. Nutrition 2002; 18: 199–200.
Fuhrman MP, Charney P, Mueller CM . Hepatic proteins and nutrition assessment. J Am Diet Assoc 2004; 104: 1258–1264.
Mitrache C, Passweg JR, Libura J, Petrikkos L, Seiler WO, Gratwohl A et al. Anemia: an indicator for malnutrition in the elderly. Ann Hematol 2001; 80: 295–298.
Carmel R . Nutritional anemias and the elderly. Semin Hematol 2008; 45: 225–234.
Acknowledgements
The Swedish National study on Aging and Care, SNAC, (www.snac.org) is financially supported by the Ministry of Health and Social Affairs, Sweden, the participating County Councils and Municipalities, and the Swedish Research Council for Health, Working Life and Welfare (grant 825-2011-6243 to LF; and grant 2012-0022 to WX). In addition, specific grants were obtained from the Gun och Bertil Stohnes Foundation; Demensfonden; Tornspiran Foundation; and Ragnhild och Einar Lundströms Minne Foundation (grant LA2012-0508 to BS; Sweden). We specially thank the staff of the SNAC-K project for their collaboration in data collection and management.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website
Supplementary information
Rights and permissions
About this article
Cite this article
Shakersain, B., Santoni, G., Faxén-Irving, G. et al. Nutritional status and survival among old adults: an 11-year population-based longitudinal study. Eur J Clin Nutr 70, 320–325 (2016). https://doi.org/10.1038/ejcn.2015.109
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2015.109
- Springer Nature Limited
This article is cited by
-
Is the Utility of the GLIM Criteria Used to Diagnose Malnutrition Suitable for Bicultural Populations? Findings from Life and Living in Advanced Age Cohort Study in New Zealand (LiLACS NZ)
The Journal of nutrition, health and aging (2023)
-
Health management of malnourished elderly in primary health care: a scoping review
BMC Primary Care (2022)
-
Mortality risks of body mass index and energy intake trajectories in institutionalized elderly people: a retrospective cohort study
BMC Geriatrics (2022)
-
Nutritional risk screening in malignant tumors: a study of 375 cancer inpatients
Supportive Care in Cancer (2022)
-
County-Level Characteristics Driving Malnutrition Death Rates among Older Adults in Texas
The Journal of nutrition, health and aging (2021)