Abstract
Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) for severe aplastic anemia (SAA) is mainly limited by the high incidence of graft failure and GvHD. Mesenchymal stem cells (MSCs) have been shown to support hematopoiesis in vivo and to display potent immunosuppressive effects to prevent or treat GvHD after HSCT. In a multicenter phase II trial, we developed an approach with co-transplantation of MSCs in patients undergoing haplo-HSCT. Forty-four patients with SAA were included. The conditioning regimen included busulfan, cyclophosphamide and thymoglobulin (ATG). The recipients received cyclosporin A (CsA), mycophenolate mofetil and short-term methotrexate for GvHD prophylaxis. Three out of 44 patients, who died early before hematopoietic engraftment, were not assessed. Evaluable patients (97.6%; 40/41) achieved hematopoietic reconstitution and sustained full donor chimerism. The median time for myeloid engraftment was 12 days (range 8–21 days) and for platelet engraftment was 19 days (range 8–154 days). The incidence was 29.3% for grade II–IV acute GvHD and 14.6% for chronic GvHD. The overall survival was 77.3% with a median 12-month (range 0.9–30.8) follow-up for surviving patients. These data suggest that co-transplantation of MSCs could reduce the risk of graft failure and severe GvHD in haplo-HSCT for SAA.
Similar content being viewed by others
Introduction
Nowadays, with advances in transplant technology, haploidentical hematopoietic stem cell transplantation (haplo-HSCT) has achieved good clinical results.1, 2, 3 Data from Xu et al.3 indicated that patients who received haplo-HSCT achieved 100% donor myeloid engraftment and 64.6±12.4% of overall survival (OS) with a median time of 746-day follow-up. Compared with malignant diseases, haplo-HSCT for severe aplastic anemia (SAA) involves distinct challenges mainly associated with high graft failure and incidence of GvHD.3, 4, 5 Consequently, if we can find a way to promote while reducing the incidence of GvHD, the efficacy of haplo-HSCT may improve.
Mesenchymal stem cells (MSCs), multi-potent non-hematopoietic progenitors, could support hematopoiesis, enhance engraftment of HSCs and reduce the incidence of GvHD following HSCT.6, 7, 8 A clinical trial involving infusion of MSCs from healthy donors to patients with AA was approved in 2009 in our center (NCT 01305694). In a preliminary study, we demonstrated that infusion of MSCs resulted in 33.3% refractory AA patients achieving complete response or partial response,9 which strengthened our confidence in MSCs as a promising therapeutic strategy for patients with AA. In very recent years, there have been few reports on co-transplantation of haplo-HSCT with MSCs for SAA achieving promising clinical results.10, 11, 12, 13 To date, there has been no multi-center clinical study with larger number of patients in this area. We first launched a multi-center clinical study to examine the safety and feasibility of co-transplantation of MSCs from third party donors in haplo-HSCT for SAA patients. We further investigated the rates and kinetics of hematopoietic engraftment and the incidence and severity of GvHD. The trail was registered at ClinicalTrials.gov, identifier NCT02247973.
Subjects and methods
Study setting and participants
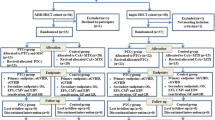
Consecutively acquired SAA patients who underwent haplo-HSCT at the Depatment of Hematology, General Hospital of Guangzhou Military Command, Guangzhou First People’s Hospital, Second Affiliated Hospital of Sun Yat-Sen University, Affiliated Nanfang Hospital of Southern Medical University or the Affiliated Zhujiang Hospital of Southern Medical University between 6 March 2013 and 24 August 2015 were enrolled in this study. Informed consent had been obtained from all patients. This study was approved by the Ethics Committee of General Hospital of Guangzhou Military Command.
Patients met the following inclusion criteria: (1) in line with the 2009 Edition (UK), aplastic anemia diagnostic criteria for SAA or very severe SAA; (2) age <50 years old; (3) no HLA-identical sibling donor; (4) HLA-mismatched related donors with ⩾5/10 HLA-matched loci; (5) no serious infection or acute hemorrhage; (6) cardiac ultrasound examination showed left ventricular ejection fraction >50%; (7) both transaminase and serum creatinine level no more than twice the upper limit of normal value; (8) no acute contagious disease; (9) ability to understand and willingness to sign a written informed consent document; and (10) Eastern Cooperative Oncology Group score of 0–2 points. Patients were excluded if they were diagnosed with (1) severe infection or active bleeding; (2) severe cardiac insufficiency; left ventricular ejection fraction <50%; (3) severe liver dysfunction, alanine transaminase and total bilirubin, higher than three times the upper limit of normal value (ULN); (4) severe renal insufficiency, renal function (measured by creatinine) twice or more the ULN, or 24 h urine creatinine clearance rate lower than 50 mL/min; (5) active tuberculosis, severe acute hepatitis or other contagiously diseases in the active period; (6) Eastern Cooperative Oncology Group (ECOG) score >3 points; (7) concomitant malignant tumors or other clonal disease; and (8) poor compliance and the researchers considered them unsuitable for MSC infusion. HLA compatibility was determined by high-resolution DNA techniques for HLA-A, B, C, DRB1 and DQB1 loci. Donors were ranked on the basis of HLA match, age (younger preferred), gender (same preferred) and health status (better preferred).
Conditioning regimen
Patients who underwent haplo-HSCT were treated with a regimen that consisted of the following: busulfan: 3.2 mg/kg IV in divided doses daily for 2 days (total dose 6.4 mg/kg) on days −7 and −6; cyclophosphamide: 50 mg/kg once daily IV for 4 consecutive days on days −5, −4, −3 and −2 (total dose 200 mg/kg); and ATG (rabbit, Genzyme Polyclonals SAS, Lyon, France): 3.125 mg/kg once daily IV, for 4 consecutive days on day −5 to −2 (total dose 12.5 mg/kg) (Figure 1).
Schematic diagram of conditioning procedures. Regimen for patients who received haplo-HSCT. Busulfan (BU): 3.2 mg/kg IV in divided doses daily for 2 days (total dose 6.4 mg/kg) on days −7 and −6; cyclophosphamide (CY): 50 mg/kg/day once daily IV for 4 consecutive days on days −5, −4, −3 and −2 (total dose 200 mg/kg); and ATG (rabbit, Genzyme Polyclonals SAS): 3.125 mg/kg once daily IV for 4 consecutive days on day −5 to −2 (total dose 12.5 mg/kg).
Stem cell collection
All donors were injected with G-CSF (Filgrastim, Kirin, Japan) subcutaneously at 5 μg/kg/day for 5–6 consecutive days. A total of 200–400 mL of bone marrow was collected at the posterior superior iliac spine after injection on day 4. On the fifth day of mobilization, PBSCs were collected with a Fresenius blood cell separator. An additional collection of PBSCs was required the next day if the cell numbers from the previous 2 days were insufficient. The target mononuclear cell yield from BM and peripheral blood was ⩾5 × 108/kg and CD34+ cells ⩾2 × 106/kg of recipient weight. This corresponded to Day 0 in the recipient cycle. The first day of stem cell infusion was designated as Day ‘01’; the second day of infusion was Day ‘02’.
MSCs preparation and transfusion
BM (20–30 mL), which was used for growing MSCs, was aspirated at the posterior superior iliac spine from the same related healthy donor when collecting G-CSF primed BM at d01. MSCs were cultured and supplied by the Center for Cell Therapy and Research, General Hospital of Guangzhou Military Command. MSCs were cultured and expanded in vitro by the serum-decremental method as their previous report.14 It will take around 7–10 days to grow, to be the second generation of MSCs. Then, the MSCs were divided into two to three infusion doses and were frozen and stored in a ‘MSCs bank’ waiting to be resuscitated and cultured to be a final product. The third-generation MSCs with a quantity of 3.6 (range 3.2–4.1) million per kilogram per dose were collected and suspended in 10 mL saline for transfusion. The first dose of MSCs was administered by venous infusion within 6 h before stem cell infusion on Day 01. At Day+14, a second dose of MSCs was infused immediately as a fresh preparation (Figure 1). Patients were monitored for vital signs and symptoms of allergy during the transfusion. Considering that MSCs had been tested for effectiveness on poor graft function and GvHD, MSCs could also be applied weekly for 4 weeks as a complementary treatment when poor graft function or severe GvHD occurred.
GvHD prophylaxis
Acute GvHD (aGvHD) prophylaxis for haplo-HSCT included CsA, mycophenolate mofetil and methotrexate. CsA was administered twice, IV from day −7 at a dose of 2.5 mg/kg/day until bowel function returned to normal, at which time patients received oral CsA. A target trough blood concentration of 200–250 ng/ml was requested up to 9 months after haplo-HSCT and was gradually tapered thereafter; then CsA was withdrawn completely over the next 2–3 months. Methotrexate was administered at a dose of 15 mg/m2 IV on day +1 and 10 mg/m2 on days +3, +6 and +11. Mycophenolate mofetil was administered orally (0.5 g, every 12 h, 0.25 g for children) from day −9 to +30 and subsequently 0.25 g from days +30 to +90. In cases if GvHD occurred in any organ, CsA and mycophenolate mofetil were continued and adjusted up to the therapeutic concentration.
Definitions and evaluation of engraftment and chimerism
Myeloid engraftment was defined as the first of three consecutive days with an absolute neutrophil count above 0.5 × 109/L and platelet engraftment was defined as the day the platelet count met or exceeded 20 × 109/L without transfusion for a week. Complete donor chimerism was defined as the presence of only donor-type hematopoietic cells after allogeneic HSCT. Primary graft failure was defined by the absence of hematological recovery in patients surviving +21 days after transplantation and late graft failure was defined as graft loss after initial graft function.15, 16 Secondary poor graft function was defined as per our previous report.17 The incidence and severity of GvHD were based on a National Institutes of Health consensus conference to determine GvHD grade.18 CMV infection and CMV pneumonia were defined according to the reference literature19, 20 and the definition of EBV infection and EBV-associated post-transplant lymphoproliferative disorders (PTLDs) were as previously reported.21, 22
Infection prevention and supportive care
All patients were hospitalized in laminar flow clean wards and received oral antibiotics for gastrointestinal decontamination (norfloxacin capsules for adults and gentamycin for children) from day −9 to myeloid recovery. Infection and complications prophylaxis around the transplant period has been described by Xiao et al.17 in our previous paper. G-CSF (Filgrastim; 5 μg/kg/day) was injected subcutaneously into all recipients from day +6 after transplantation until myeloid recovery. A blood transfusion was performed once hemoglobin reached <60 g/L and/or platelets reached <20 × 109/L. Human Ig (10 g) from healthy volunteer donors was IV administered each week during the first month.
Statistical analysis
The primary endpoint of the study was the OS rate from transplantation. Secondary endpoints included engraftment rate, incidence and severity of aGvHD and chronic GvHD (cGvHD). In evaluation of engraftment, patients who died before engraftment were not considered evaluable and were excluded from analyses of aGvHD and cGvHD. Patients who survived >100 days were analyzed for cGvHD. The OS rate was evaluated using the Kaplan–Meier estimate. The incidences of aGvHD and cGvHD were evaluated by the Kaplan–Meier estimate. The date of the last follow-up for all surviving patients was 26 November 2015. A statistical software packages (SPSS 16.0, IBM Inc, Chicago, IL, USA, 1989–2007) was used for analyses.
Results
Characteristics of patients and their donors
Data are shown in Table 1. A total of 44 patients who were diagnosed with SAA were enrolled in the study. Of these, 13 patients (29.5%) were considered to have very severe SAA. The patients’ median age was 24 years (range 8–47). Thirty-three (75%) patients had failed to respond to previous immunosuppressive therapy including one or more courses of CsA±ATG±stanozolol±steroids and all were transfusion dependent. The median time from diagnosis to transplantation was 31.2 months (ranging from 1 to 249). The median mononuclear cell and CD34+ cell counts were 12.2 × 108/kg and 5.5 × 106/kg, respectively. The median age of the donors was 34 (11–63) years.
Engraftment
Primary graft failure occurred in one patient (2.4%). Myeloid recovery and full donor chimerism were both achieved in the other 40 evaluable patients (97.6%). The median time to achieve neutrophil engraftment was 12 days (range 8–21 days). The median time of platelet engraftment was 19 (range 8–154) days (as shown in Table 2). Further, the 40 patients sustained complete stable donor chimerism without late graft failure. After day +28 post transplant, 2 out of 40 (5%) patients developed secondary poor graft function. Platelet count in one patient slowly rose to over 20 × 109/L at day+154 and stabilized around 20–30 × 109/L. His RBCs relied on infusion. This patient received supplemental infusions of MSCs once a week for 4 consecutive weeks at +5 months and +9 months. As a result his hemoglobin gradually recovered to over 100 g/L and his platelet count recovered to normal. The other patient experienced delayed platelet and RBC recovery, and his platelet count recovered to over 20 × 109/L up to day +61 then gradually recovered to normal. In addition, two patients suffered from recurrent cytopenia also in the presence of complete donor chimerism. One patient infected by CMV on day +60. After anti-CMV therapy, her hemoglobin and platelets dropped remarkably on day+81. She was treated with an infusion of MSCs from the original donor once a week for 4 weeks and her blood cells gradually recovered. The other patient developed secondary thrombocytopenia with the platelet count falling to 1 × 109/L on day +112 after suffering from several days of EBV antigenemia. This patient was excluded with a diagnosis of thrombotic microangiopathy without other clinical manifestations including microangiopathic hemolytic anemia, microvascular thrombosis and multiple organ failure. He had received methylprednisolone, rituximab (Mabthera, Roche Pharma, Basel, Switzerland) and thrombopoietin booster without infusion of HSCs and MSCs, and the platelet count also gradually recovered (Table 2).
Severity of GvHD
Acute GvHD
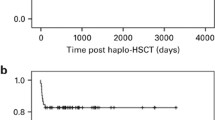
Table 2 indicates the incidence and severity of GvHD. Of the 41 patients, 23 (56.1%) experienced aGvHD after transplantation, including 11 (26.8%) with grade I, 10 (24.4%) with grade II, 2 (4.9%) with grade III aGvHD and none with grade IV aGvHD (0%). At 100 days after transplantation, the cumulative incidence of grades II–IV aGvHD was 29.3% and that of grades II–IV was 4.9% (Figure 2).
The two patients with grade III aGvHD received additional treatment with methylprednisolone (2 mg/kg/day for 5 days), CD25 monoclonal antibody and CsA was changed to FK-506. One patient was cured and the other one showed improvement.
Chronic GvHD
Of 35 patients surviving longer than 100 days after transplantation, 29 patients remained disease-free and 6 (17.1%) developed cGvHD. Among these patients, four (11.4%) showed limited cGvHD and two (5.7%) showed extensive cGvHD (as shown in Table 2 and Figure 3). One patient who had extensive moderate cGvHD with the skin, mouth and eye involvement at day +116 received additional long time methylprednisoline treatment and showed no obvious improvement. Immunodeficiency secondary to corticosteroids was followed by pulmonary aspergillosis with death finally occurring on day +332. The other patient with extensive moderate cGvHD involving skin and intestine received methylprednisoline treatment and additional MSCs infusion once a week for four consecutive weeks and showed improvement.
OS and treatment-related mortality
Three patients died before engraftment. One died of cerebral hemorrhage at day 0; one died at day 7 after 3 days of septicemia due to phialophora infection; the third died of respiratory failure caused by severe pneumonia at day 15. Through 26 November 2015, 7 patients died within the follow-up period. Causes of death and general condition are summarized in Table 3. Infections were major causes of death. A total of 23 patients were monitored for CD3 and CD4 cell recovery in the blood for 6 months after SCT. Data are shown in Table 4. A total of 34 patients have survived to date with an OS rate of 77.3% (Figure 4). All of the surviving patients achieved a hematologic complete response.
CMV reactivation
Twenty-nine out of 44 patients (65.9%) experienced CMV reactivation detected by antigen or DNA testing, with onset averaging 35 (range, 16–83) days after HSCT. Fortunately, nobody progressed to CMV-associated pneumonia or enteritis (as shown in Table 2). Most patients (27/29) with CMV reactivation after HSCT exhibited complete resolution with antiviral treatment. Two out of 10 patients whose clinical outcome was death had CMV viremia when they died.
EBV reactivation
Of the 44 patients, 14 (31.8%) experienced EBV reactivation detected by antigen or DNA testing, with onset averaging 52 (range, 13–221) days after HSCT. Four (9.1%) patients progressed to EBV-associated PTLD (as shown in Table 2). Any viral infection in the remaining patients was treated with ganciclovir or foscarnet, gamma globulin and all patients with PTLD received treatment with rituximab (Mabthera, Roche Pharma, Schweiz). Most patients without PTLD demonstrated full recovery. Two patients with PTLD died at day +77 and day +86, respectively. Two patients recovered from PTLD with enlarged lymph nodes which disappeared and copies of EBV declined to normal. However, one patient finally died of septic shock at day +325 after HSCT.
Complications
All patients received the conditioning regimen on schedule without encountering any significant transplantation-related, bowel, renal or liver toxicity. No patients experienced infusional toxicity during the infusion of MSCs. Hemorrhagic cystitis was observed in 14 patients (31.8%) at 23–70 (median 40) days after transplantation. After hydration, alkalization of urine and bladder flushing, all of them recovered within 2–3 weeks. Seizures occurred in three cases (6.8%) at day −4, −2 and+30, respectively. Two patients did not undergo magnetic resonance imaging and other imaging examinations, owing to inconvenient events in the laminar flow ward. They received treatment with sodium vedproate and both recovered and there was never any recurrence. Reversible posterior leukoencephalopathy syndrome was considered in one patient who developed seizures at day +30 after magnetic resonance imaging examination. At the time of occurrence, her serum CsA concentration was at a maximum of 772.5 ng/mL. After the discontinuation of intravenous CsA and treated with sodium vedproate, the seizures were resolved. Capillary leak syndrome was observed in one patient (2.3%) at day +86 accompanied with pulmonary infection and CMV viremia; the patient eventually gave up treatment and was discharged at day +88. One patient (2.3%) suffered from cerebral hemorrhage a few hours after transfusion of BM-derived stem cells at day 0. Bilateral femoral head aseptic necrosis was observed in one patient (2.3%) at +12 month after HSCT and she received a total hip replacement surgery at +13 month. In addition, one patient (2.3%) developed thrombembolism at day +32 involving left subclavian vein and basilic vein. He finally recovered after thrombolytic therapy. Glaucosis was diagnosed in one (2.3%) 8-year-old boy at day +36 after HSCT and he improved after withdrawal glucocorticoid and dehydration with mannite (Table 2).
Discussion
In our study, we used busulfan+ cyclophosphamide+ATG conditioning regimen as previous reported by Xu et al.3 and 90.9% (40/44) patients had at least 2 and at most 5 mismatched loci. Among the evaluated 41 patients, only one patient did not achieve hematopoietic engraftment. The OS in our study was 77.3% with incidence rates of grade II–IV aGvHD and cGvHD of 29.3% and 14.6%, respectively. The risk of GvHD in our study was significant lower than the risk reported in Xu et al.3 (aGvHD 42.1% and cGvHD 56.2%). Furthermore, none of our study patients had severe GvHD resulting in death. The above data demonstrate our hypothesis that adding MSCs in transplantation increases the engraftment rate; lowers the incidence of GvHD and improves the OS.
The possible mechanisms by which MSCs improve the outcomes of haplo-HSCT for patients with SAA are the following. First, during the transplant procedure MSCs may create a more favorable BM microenvironment favoring engraftment of hematopoietic progenitor cells.23 Second, MSCs could prevent and treat GvHD by inhibiting the activation and proliferation of activated T cells that have been induced by a variety of stimuli24, 25 and downregulating inflammatory cytokine expression such as TNF-α, IL2R-α, elafin and interferon-γ.26, 27
From 2007 to 2011, several case reports, usually involving fewer than five cases, involving co-transplantation of MSCs with PBSCs or BM in haplo-HSCT or unrelated donor (UD)—HSCT for patients with SAA have been reported.28, 29, 30, 31 Results from these attempts indicate that co-transplantation of MSCs might, as theoretically speculated, enhance engraftment of HSCs and taper the incidence of GvHD. Gradually, a larger number of patients, a maximum of 21 cases in the current report, began to enter clinical research in different centers. Nearly all patients achieved rapid and sustained hematopoietic reconstitution. The mean time for neutrophil and platelet recovery ranged from 11 to 16 and 13 to 22 days, respectively.32, 33, 34, 35, 36 In our study, the median time for myeloid engraftment was 12 days and for platelet engraftment was 19 days, which is consistent with previous reports.
Reports involving the incidence of aGvHD after adding MSCs are at variance. Fu et al.34 reported five young patients with a long history of SAA, who had not responded to initial CSA treatment and who then underwent co-transplantation of unrelated donor PBSCs and umbilical cord (UC)-MSCs. No cases of severe aGvHD or cGvHD were observed.34 In other reports, the incidence of aGvHD grade II-–IV was around 23.5–40.7%35, 36 and cGvHD was documented in 14.2%35 and 21.25%36 of patients, respectively. These limited promising data, together with our results, suggest that combined transplantation of haplo-HSCs and MSCs in cases of SAA without an HLA-identical sibling donor is safe, effectively reduces the incidence of severe GvHD and improves patient survival. The differences between our trial and other studies lie in the following aspects: (1) the source of MSCs (BM-MSCs versuss UC-MSCs); (2) frequency of MSCs infusions (twice versus once); (3) a prospective multicenter clinical trial versus single center study. The rigorous prospective design of our study makes our data objective and convincing.
A relatively higher incidence of EBV (31.8%) and CMV (65.9%) infection in our study has aroused our concern. Among these patients, four (9.1%) developed EBV-associated PTLD and three died finally. Zhang et al.37 analyzed risk factors of PTLD associated with EBV infection following haplo-HSCT and concluded patients with delayed immune reconstitution are at higher risk of developing PTLD after allo-HSCT. In addition, a conditioning regimen including ATG,38 HLA mismatch, mismatch in EBV serology and splenectomy33 have been reported in other studies to be risk factors associated with PTLD. As for CMV infection, Peking University researchers39 retrospectively analyzed 260 patients who received allo-HSCT. Among these patients, 188 (72.3%) patients were infected with CMV, 34% in the related donor-HSCT group and 81.9% in the haplo-HSCT group. Reduction in CMV infection after haplo-SCT will only be achieved by hastening post-transplant immune reconstitution. However, immune reconstitution after haplo-SCT is usually slower than that after matched-sibling donor or matched-unrelated donor transplants.40 Post-transplant complications, including GvHD and its associated treatment with high-dose steroid,41 should also be considered.
These limited data from our trial give encouraging results and suggest that co-transplantation of MSCs from related donors might be an attractive option for SAA patients receiving haplo-HSCT. Despite the lack of control subjects and the inadequate number of cases in this study, our survival data are encouraging and support previous studies showing that alternative donor HSCT combined with MSCs infusion could be an effective approach to treat SAA.33, 34, 35, 36
References
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 2006; 38: 291–297.
González-Vicent M, Molina B, Andión M, Sevilla J, Ramirez M, Pérez A et al. Allogeneic hematopoietic transplantation using haploidentical donor vs unrelated cord blood donor in pediatric patients: a single-center retrospective study. Eur J Haematol 2011; 87: 46–53.
Xu LP, Liu KY, Liu DH, Han W, Chen H, Chen YH et al. A novel protocol for haploidentical hematopoietic SCT without in vitro T-cell depletion in the treatment of severe acquired aplastic anemia. Bone Marrow Transplant 2012; 47: 1507–1512.
Lacerda JF, Martins C, Carmo JA, Lourenco F, Juncal C, Ismail S et al. Haploidentical stem cell transplantation with purified CD34+ cells after a chemotherapy-alone conditioning regimen in heavily transfused severe aplastic anemia. Biol Blood Marrow Transplant 2005; 11: 399–400.
Woodard P, Cunningham JM, Benaim E, Chen X, Hale G, Horwitz E et al. Effective donor lymphohematopoietic reconstitution after haplo-identical CD34+/− selected hematopoietic stem cell transplantation in children with refractory severe aplastic anemia. Bone Marrow Transplant 2004; 33: 411–418.
Guo M, Sun Zh, Sun QY, Han Q, Yu CL, Wang DH et al. A modified haploidentical nonmyeloablative transplantation without T cell depletion for high-risk acute leukemia: successful engraftment and mild GVHD. Biol Blood Marrow Transplant 2009; 15: 930–937.
Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant 2005; 11: 389–398.
Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I . The role of mesenchymal stem cells in haemopoiesis. Blood Rev 2006; 20: 161–171.
Xiao Y, Jiang ZJ, Pang Y, Li L, Gao Y, Xiao HW et al. Efficacy and safety of mesenchymal stromal cell treatment from related donors for patients with refractory aplastic anemia. Cytotherapy 2013; 15: 760–766.
Wang H, Wang Z, Zheng X, Ding L, Yan H, Guo Z . Hematopoietic stem cell transplantation with umbilical cord multipotent stromal cell infusion for the treatment ofaplastic anemia—a single-center experience. Cytotherapy 2013; 15: 1118–1125.
Fu Y, Wang Q, Zhou J, Liu S, Fang B, Wei X et al. Reduced intensity conditioning and co-transplantation of unrelated peripheral stem cells combined with umbilicalcord mesenchymal stem/stroma cells for young patients with refractory severe aplastic anemia. Int J Hematol 2013; 98: 658–663.
Li XH, Gao CJ, Da WM, Cao YB, Wang ZH, Xu LX et al. Reduced intensity conditioning,combined transplantation of haploidentical hematopoietic stem cells andmesenchymal stem cells in patients with severe aplastic anemia. PLoS ONE 2014; 9: 1–7.
Wu Y, Cao Y, Li X, Xu L, Wang Z, Liu P et al. Contransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells for severe aplasticanemia: successful engraftment and mild GVHD. Stem Cell Res 2014; 12: 132–138.
Li L, Pang Y, Zhang H, Kuang LP, Xiao ZF, Wang YC et al. Human umbilical cord mesenchymal stem cells cultured in serum-decremental medium in vitro. Chinese J Tissue Eng Res 2012; 16: 989–993.
Bacigalupo A, Brand R, Oneto R, Bruno B, Socie G, Passweg J et al. Treatment of acquired severe aplastic anaemia: bone marrow transplantation compared with immunosuppressive therapy - The European Group for Blood and Marrow Transplantation experience. Semin Hematol 2000; 37: 69–80.
Fuhrer M . Risk-adapted procedures for HSCT from alternative donor in children with severe aplastic anemia. Bone Marrow Transplant 2008; 42 (S2): S97–S100.
Xiao Y, Song JY, Jiang ZJ, Li YH, Gao Y, Xu WN et al. Risk-factor analysis of poor graft function after allogeneic hematopoietic stem cell transplantation. Int J Med Sci 2014; 11: 652–657.
Cho BS, Min CK, Eom KS, Kim YJ, Kim HJ, Lee S et al. Feasibility of NIH consensus criteria for chronic graft-versus-host disease. Leukemia 2009; 23: 78–84.
Ljungman P, Griffiths P, Paya C . Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34: 1094–1097.
Luo XH, Chang YJ, Huo MR, Li D, Huang XJ . Cytomegalovirus-specific T cells immune reconstitution after human leukocyte antigen matched sibling donor allogeneic bone marrow plus peripheral blood hematopoietic stem cell transplantation. Zhonghua Xue Ye Xue Za Zhi 2012; 33: 605–609.
Styczynski J, Reusser P, Einsele H, de la Camara R, Cordonnier C, Ward KN et al. Management of HSV, VZV and EBV infections in patients with hematological malignancies and after SCT: guidelines from the Second European Conference on Infections in Leukemia. Bone Marrow Transplant 2009; 43: 757–770.
Han TT, Xu LP, Liu DH, Liu KY, Zhang XH, Chen H et al. Prevalence of EBV infection in patients with allogeneic hematopoietic stem cell transplantation. Zhonghua Xue Ye Xue Za Zhi 2013; 34: 651–654.
de Lima M, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med 2012; 367: 2305–2315.
Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC . Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 2003; 75: 389–397.
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002; 99: 3838–3843.
Aggarwal S, Pittenger MF . Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105: 1815–1822.
Dander E, Lucchini G, Vinci P, Introna M, Bonanomi S, Balduzzi A et al. Understanding the immunomodulatory effect of mesenchymal stem cell infused in transplanted patients with steroid-refractory GVHD. Blood (ASH Annu Meet Abstracts) 2010; 116: 2306.
Le Blanc K, Samuelsson H, Gustafsson B, Remberger M, Sundberg B, Arvidson J et al. Transplantation of mesenchymal stem cells to enhance engraftment of hematopoietic stem cells. Leukemia 2007; 21: 1733–1738.
Fang B, Song Y, Li N, Li J, Zhao RC . Cotransplantation of haploidentical mesenchymal stem cells to reduce the risk of graft failure in a patient with refractory severe aplastic anemia. Acta Haematol 2008; 119: 162–165.
Fang B, Li N, Song Y, Li J, Zhao RC, Ma Y . Cotransplantation of haploidentical mesenchymal stem cells to enhance engraftment of hematopoietic stem cells and to reduce the risk of graft failure in two children with severe aplastic anemia. Pediatr Transplant 2009; 13: 499–502.
Jaganathan BG, Tisato V, Vulliamy T, Dokal I, Marsh J, Dazi F et al. Effects of MSC co-injection on the reconstitution of aplastic anemia patient following hematopoietic stem cell transplantation. Leukemia 2010; 24: 1791–1795.
Wang H, Yan H, Wang Z, Zhu L, Liu J, Guo Z . Cotransplantation of allogeneic mesenchymal and hematopoietic stem cells in children with aplastic anemia. Pediatrics 2012; 129: e1612–e1615.
Wu Y, Cao Y, Li X, Xu L, Wang Z, Liu P et al. Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells for severe aplastic anemia: successful engraftment and mild GVHD. Stem Cell Res 2014; 12: 132–138.
Fu Y, Wang Q, Zhou J, Liu S, Fang B, Wei X et al. Reduced intensity conditioning and co-transplantation of unrelated peripheral stem cells combined with umbilical cord mesenchymal stem/stroma cells for young patients with refractory severe aplastic anemia. Int J Hematol 2013; 98: 658–663.
Li XH, Gao CJ, Da WM, Cao YB, Wang ZH, Xu LX et al. Reduced intensity conditioning, combined transplantation of haploidentical hematopoietic stem cells and mesenchymal stem cells in patients with severe aplastic anemia. PLoS ONE 2014; 9: 1–7.
Wu YM, Cao YB, Li XH, Xu LX, Liu ZY, Liu B et al. Clinical effect of cotransplantation of haploidentical hematopoietic stem cells and umbilical cord mesenchymal stem cells for 27 patients with severe aplastic anemia. Jie Fang Jun Yi Xue Yuan Xue Bao 2014; 35: 1191–1195.
Zhang C, Huang X, Liu D, Mo X, Zhang X, Chen H et al. The risk factors of post-transplant lymphoproliferative disorders following haploidentical hematopoietic stem cell transplantation. Zhonghua Nei Ke Za Zhi 2014; 53: 527–531.
Xu LP, Huang XJ, Liu DH, Chen YH, Shi HX, Chen DB . A clinical study of lymphoproliferative disorders following allogeneic hematopoietic stem cell transplantation. Zhonghua Nei Ke Za Zhi 2007; 46: 996–999.
Lin Z, KongY, Wang Y, Zhang X, Liu D, Xu L et al. Analysis of risk factors for secondary cytopenia after allogeneic hematopoietic stem cell transplantation. Zhonghua Xue Ye Xue Za Zhi 2014; 35: 4–8.
Fu YW, de Wu P, Cen JN, Feng YF, Chang WR, Zhu ZL et al. Patterns of T-cell reconstitution by assessment of T-cell receptor excision circle and T-cell receptor clonal repertoire after allogeneic hematopoietic stem cell transplantation in leukemia patients-a study in Chinese patients. Eur J Haematol 2007; 79: 138–145.
Sayer HG, Longton G, Bowden R, Pepe M, Storb R . Increased risk of infection in marrow transplant patients receiving methylprednisolone for graft-versus-host disease prevention. Blood 1994; 84: 1328–1332.
Acknowledgements
This work was supported by funding from the Guangzhou Health and Medical Collaborative Innovation Major Program, Project number 201400000003-1 and 201400000003-4; the Army Medical, Science and Technology Research Program (12.5 Program), Project number BWS11J071; the State Natural Sciences Fund, Project number 81570107; and Natural Science Foundation of Guangdong Province, Project number 2014A030311006.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Liu, Z., Zhang, Y., Xiao, H. et al. Cotransplantation of bone marrow-derived mesenchymal stem cells in haploidentical hematopoietic stem cell transplantation in patients with severe aplastic anemia: an interim summary for a multicenter phase II trial results. Bone Marrow Transplant 52, 704–710 (2017). https://doi.org/10.1038/bmt.2016.347
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.347
- Springer Nature Limited
This article is cited by
-
First-line treatment of severe aplastic anemia: immunosuppressive therapy plus eltrombopag versus haploidentical hematopoietic stem cell transplantation, a multicenter prospective study
Bone Marrow Transplantation (2024)
-
A review of the application of mesenchymal stem cells in the field of hematopoietic stem cell transplantation
European Journal of Medical Research (2023)
-
Mesenchymal stromal cells as prophylaxis for graft-versus-host disease in haplo-identical hematopoietic stem cell transplantation recipients with severe aplastic anemia?—a systematic review and meta-analysis
Stem Cell Research & Therapy (2021)
-
Combination of haploidentical haematopoietic stem cell transplantation with an unrelated cord-blood unit in patients with severe aplastic anemia: a report of 146 cases
Bone Marrow Transplantation (2020)
-
Current insights into the treatments of severe aplastic anemia in China
International Journal of Hematology (2020)