Abstract
Mycophenolate mofetil (MMF) is an important immunosuppressant used in renal transplantation, and mycophenolic acid (MPA) is the active component released from the ester prodrug MMF. The objective of this study was to investigate the population pharmacokinetics of mycophenolic acid (MPA) following oral administration of MMF in Chinese adult renal transplant recipients and to identify factors that explain MPA pharmacokinetic variability. Pharmacokinetic data for MPA and covariate information were retrospectively collected from 118 patients (79 patients were assigned to the group for building the population pharmacokinetic model, while 39 patients were assigned to the validation group). Population pharmacokinetic data analysis was performed using the NONMEM software. The pharmacokinetics of MPA was best described by a two-compartment model with a first-order absorption rate with no lag time. Body weight and serum creatinine level were positively correlated with apparent clearance (CL/F). The polymorphism in uridine diphosphate glucuronosyltransferase gene, UGT2B7, significantly explained the interindividual variability in the initial volume of distribution (V1/F). The estimated population parameters (and interindividual variability) were CL/F 18.3 L/h (34.2%) and V1/F 27.9 L (21.3%). The interoccasion variability was 13.7%. These population pharmacokinetic data have significant clinical value for the individualization of MMF therapy in Chinese adult renal transplant patients.
Similar content being viewed by others
Introduction
Mycophenolate mofetil (MMF, CellCept®; Roche Laboratories, Nutley, NJ, USA) has been widely accepted as the antimetabolite immunosuppressant of choice in the immunosuppressive regimen of solid organ transplantation. Mycophenolic acid (MPA) is the active component released from the ester prodrug MMF. MPA acts as a potent specific inhibitor of human lymphocyte proliferation by inhibiting inosine monophosphate dehydrogenase (IMPDH), the key enzyme in the de novo purine biosynthesis of proliferating T and B lymphocytes1. MMF has been used in immunosuppressive regimens in combination with corticosteroids and calcineurin inhibitors (CNIs) or other nephrotoxicity-free immunosuppressants, such as sirolimus in renal transplant patients, and its efficacy has been widely confirmed2,3,4,5.
The pharmacokinetic/pharmacodynamic relationships have been well established for MPA in renal transplant recipients3,6,7,8. Both under- and over-exposure to MPA may lead to serious consequences: patients with low MPA area under the concentration-time curve from 0 to 12 h (MPA AUC0-12h) will be at high risk of experiencing graft rejection, whereas patients with a high MPA AUC0-12h may be at an increased risk of toxic side effects such as leucopenia or infections3,6,9. MMF exhibits nonlinear pharmacokinetics; MPA exposure will increase less than proportionally with increasing MMF doses, and this should be taken into account when performing therapeutic drug monitoring10. More than a 10-fold variability in MPA AUC was found when MMF was given at a fixed dose of 1.0 g twice daily to renal and cardiac transplant patients, emphasizing the importance of incorporating the therapeutic drug monitoring of MPA into routine clinical practice11,12,13,14. Individualizing the MMF dosage to achieve a target MPA AUC within the range of 30–60 mg·h/L has been shown to reduce the risk of acute rejection and hematological side effects, providing effective MMF dose individualization in transplant patients3,6,7,8,11,15,16.
Trough concentration (C0) is often used as a surrogate marker of AUC in therapeutic drug monitoring, but there is a poor correlation between MPA AUC0-12h and MPA C0, with r2 values of 0.23 to 0.6511,17,18,19,20,21,22. Therefore, monitoring MPA AUC has been proposed for the individualization of MMF therapy in view of the relationship between MPA AUC and clinical results. However, measuring MPA AUC0-12h is impractical in the clinical setting. Limited sampling strategies (LSSs) have therefore been developed18,20,23,24,25. Bayesian estimation of individual pharmacokinetic parameters involves more complex calculations than limited sampling but the sampling times are less strict26. Furthermore, this approach is based on a population pharmacokinetic analysis that permits the quantification of patient characteristics (covariates) that contribute to interindividual pharmacokinetic variability27,28.
To date, no information on the population pharmacokinetics of MPA in Chinese renal transplant patients has been published. The objective of this study was to investigate the population pharmacokinetics of MPA in Chinese adult renal transplant patients, to quantify the population pharmacokinetic parameters, inter-individual and inter-occasion variability, and to identify factors (covariates) that explain pharmacokinetic variability in this population.
Materials and methods
Patients and data collection
Data were collected from 118 adult renal transplant patients (71 males, 47 females) who had undergone renal transplantation at the Organ Transplantation Centre of Ruijin Hospital, Shanghai JiaoTong University School of Medicine (Shanghai, China). The investigational protocol was approved by the Regional Ethics Committee (Ruijin Hospital, Shanghai JiaoTong University School of Medicine) and performed in accordance with the legal requirements, with the Declaration of Helsinki of 1975 (as revised in 1983), and with current Chinese guidelines for good clinical practice. All patients provided informed consent before inclusion.
Pharmacokinetic, demographic and covariate data were collected retrospectively from patient medical records, clinical trial case folders, and a therapeutic drug monitoring database. For all patients, a physical examination and standard laboratory analyses, including hematological and biochemical tests, had been performed during the study period.
A total of 97 full concentration-time profiles (10 sampling times between two drug intakes) were obtained from 93 patients, 3–41 d after the start of MMF treatment. A total of 90 patients had one full concentration-time profile, 2 patients had two full concentration-time profiles and 1 patient had three full concentration-time profiles. Among these patients, 19 had additional blood samples collected at limited sampling times, 3–363 d after the start of MMF treatment. For 25 patients, only limited samples were drawn, 5–1460 d after the start of MMF treatment. Thus, one pharmacokinetic evaluation was performed in all patients, 2 pharmacokinetic evaluations were performed in 31 patients and 3 pharmacokinetic evaluations were performed in 4 patients.
Drug administration
All patients received oral MMF (Cellcept®, Roche Pharmaceuticals, Basel, Switzerland) therapy as part of a triple immunosuppressive regimen that also included cyclosporine A (CsA, Neoral®; Novartis Pharma, Beijing, China) (or tacrolimus for 12 patients) and corticosteroids. MMF was administered at a dose of 1.0 g within 6 h before renal transplantation and thereafter at a daily dose of 2.0 g divided in two 12-hourly doses, starting 12 h after transplantation and adjusted empirically on the basis of clinical evidence of efficacy and toxicity. CsA was initially administered at 7 mg·kg−1·d−1 in two divided doses, starting 3 days after surgery when the renal function of the patients was at a stage of rapid recovery. During the first month of treatment, doses of CsA were adjusted to achieve 12-h trough concentrations of 200–250 μg/L and 2-h concentrations (C2h) of 1200–1500 μg/L. Thereafter, morning blood trough concentrations of 150–200 μg/L were targeted. Tacrolimus (Prograf®, Astellas Pharma Inc) was initiated at an oral dose of 0.1 mg·kg−1·d−1 and then adjusted to achieve steady-state trough concentrations of 10–15 μg/L during the first week of treatment and 5–10 μg/L thereafter. CsA was given 2 h after the administration of MMF. Tacrolimus and MMF were administered at the same time in the morning and in the evening in all patients. Methylprednisolone sodium succinate (Pfizer Manufacturing Belgium NV, 500 mg) was given intravenously during surgery. The dosage was progressively tapered over one week and then maintained at a low oral dose of prednisone (5–20 mg). All concomitant medications were recorded for each patient.
Sample collection and bioanalytical assay
Blood samples were collected in EDTA-coated tubes and centrifuged at room temperature (3000×g for 10 min) to separate plasma, which was kept frozen at -35 °C until analysis. Full pharmacokinetic profiles were evaluated using 10 blood samples drawn before (C0) and 0.5, 1, 1.5, 2, 4, 6, 8, 10 and 12 h after the morning dose. For the other pharmacokinetic evaluations involving sparse data, blood samples were collected at limited sampling times (0, 0.5, 2 h, or 0, 0.5, 2 and 8 h). MMF was administered under fasting conditions, and unstandardized meals were given 1.0 (breakfast) and 6.0 (lunch) h later.
Plasma MPA concentrations were determined via a validated high-performance liquid chromatography (HPLC) method29. The performance of the MPA assay was monitored externally by the International MPA Proficiency Testing Programme provided by St George's Hospital Medical School, London, UK (Dr David HOLT, http://www.bioanalytics.co.uk). The lower limit of quantitation was 0.25 mg/L. Intra- and inter-day precision were <6% and 8%, respectively. Accuracy was 97.9%–101.8%. Quality control (QC) samples at three different levels (2.0, 10.0 and 40.0 mg/L) were included in each analytical sequence together with unknown samples to verify the stability of study samples during storage and accuracy and the precision of analysis. The results were accepted only when QC samples fell within ±15% of their respective nominal values.
Genotyping
Genomic DNA was extracted from EDTA-treated blood via a manual phenol-chloroform method and the DNA was stored at 4 °C until use. The polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) procedure described below was established for UGT2B7 211G>T genotyping. Oligonucleotide primer pairs used to amplify a 227 bp fragment encompassing nucleotide 211 of UGT2B7 were: sense (5′-TGCTTTAGCTCTGGGAATTGT-3′) and antisense (5′-TGCATGATGAAATTCTCCAAC-3′). PCR amplification was performed with a GeneAmp PCR system 2700 (Biocompare, CA, USA). Reactions contained DNA polymerase (1 U; MBI Fermentas, Hanover, MD, USA), 0.75 μL dNTPs (10 mmol/L), genomic DNA (50 ng), 2.0 μL MgCl2 (25 mmol/L), and reaction buffer in a total volume of 25 μL. The initial denaturation (94 °C for 2 min) was followed by 30 cycles comprising denaturation (94 °C for 30 s), primer annealing (50 °C for 30 s) and extension (72 °C for 30 s), and then a final extension (72 °C for 5 min). The resulting 227 bp PCR product was detected on 2% (w/v) ethidium bromide-stained agarose gel, and 10 μL of the PCR product was digested with Mbi I (MBI Fermentas, Hanover, MD, USA) at 37 °C for 6 h. Finally, digested PCR products were resolved by electrophoresis on agarose-ethidium bromide gels and visualized using an ultraviolet (UV) transilluminator.
UGT1A9*22 was genotyped by direct sequencing with a PCR procedure. Primer sets were sense: 5′-CTTCCCACTGCGTGCGATGTATCTTAG-3′; antisense: 5′-AGGTCAGCAGCAGACACACACATAGAG-3′. PCR was conducted as specified for UGT2B7, except that the initial denaturation step lasted for 3 min and 35 amplification cycles were performed. The amplified DNA fragments were submitted for DNA sequencing.
Data splitting
The patients enrolled in the study were randomly assigned to a model building set (population group) or to a model validation set (validation group). Seventy-nine patients were assigned to the population group to build the population pharmacokinetic model using nonlinear mixed effects modeling. This group included 61 patients with one full pharmacokinetic profile (among them, 13 patients had one or two additional pharmacokinetic evaluations during treatment from 3 or 4 sampling time-points), 1 patient with two full pharmacokinetic profiles, 1 patient with 3 full pharmacokinetic profiles, and 16 patients with limited sampling time-points for whom one to three pharmacokinetic evaluations had been performed during treatment. Thirty-nine patients were assigned to the validation group: twenty-nine patients with one full pharmacokinetic profile (among them, 6 patients had one or two additional pharmacokinetic evaluations from sparse data), 1 patient with two full pharmacokinetic profiles, and 9 patients with limited sampling time-points, for whom one to three pharmacokinetic evaluations had been performed during treatment.
The covariates screened were as follows: age, gender, body weight (BW), UGT2B7 genotype, UGT1A9 genotype, liver function tests [total bilirubin (TB), alanine transferase (ALT), aspartate transferase (AST), alkaline phosphatase (ALKP) and γ-glutamyltransferase (GGT), albumin (ALB)], blood urea nitrogen (BUN), serum creatinine (Scr), uric acid (UA), hematocrit (HCT), concurrent CsA (or tacrolimus) concentrations, and concurrent therapy with corticosteroids.
Model building
Population pharmacokinetic model-building and model-validation analyses were performed with NONMEM software (version 5.1.1, Globomax LLC, Andover, MD, USA) through Visual-NM (RDPP, Montpellier, France), a WindowsTM-based interface to NONMEM that contains graphical and statistical tools. The population characteristics of the pharmacokinetic parameters (fixed and random effects) were estimated with the subroutine ADVAN-4 or ADVAN-6 from the library of programs provided with the NONMEM-PREDPP package.
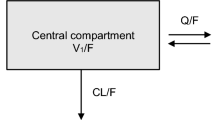
Both first-order (FO) and first-order conditional estimation (FOCE) methods were used to determine population pharmacokinetic parameters. A two-compartment model was selected based on previous knowledge of MPA pharmacokinetics in renal transplant patients30,31,32,33. Moreover, previous studies that compared first-order and zero-order input rates showed that the first-order provided the best fit30,31,33. Thus, the following structural models were tested (Figure 1): (1) a two-compartment model with a first-order input rate with a lag time (model 1), (2) a two-compartment model with a first-order input rate with no lag time (model 2), (3) a two-compartment model plus an enterohepatic recycling with no lag time (model 3). The structural model was chosen on the basis of changes in -2 log likelihood and on graphic analyses of the goodness-of-fit. Because -2 log likelihood is approximately χ2 distributed, and the addition of 1 compartment increases the degrees of freedom by a factor of 2, a change of 5.99 in -2 log likelihood was required at the 5% significance level to select the more complex model. The overall fitting was evaluated on the basis of plots of predicted versus observed concentrations, weighted residuals versus observed concentrations and individual weighted residuals versus individual predicted concentrations. Interindividual variability in pharmacokinetic parameters was modeled with an exponential model as follows:
Different models tested to fit MPA data. GI, gastrointestinal tract; 1, central compartment; 2, peripheral compartment; ka, first-order input rate; k12 and k21, intercompartmental rate constants; k10, elimination rate constant; k1B and kB1, transfer rate constants between the central compartment and bile; EHR, enterohepatic recirculation.

in which Pj is the required pharmacokinetic parameter in the jth individual and ηj is a random variable distributed with mean zero and variance of ω2η about the average value (Pmean) in the population.
Various error models were also tested. At each step of model building, diagnostic plots were analyzed for similarity and randomness along the line of identity on the observed versus predicted concentration plot, as well as randomness along the residual and weighted residual zero line on the predicted concentrations versus residuals or weighted residuals plot. The error on the concentration measurements of the individual j was best described by a combined additive and exponential model given below:

where Pj is the pharmacokinetic parameter of the subject j; tij is the time of the ith measurement; Dj is the dosing history of the subject j; ƒ is the pharmacokinetic model; ε1ij and ε2ij represent the residual departure of the model from the observations and contain contributions from intraindividual variability, assay error, and model misspecification for the dependent variable. ε1 and ε2 are assumed to be random Gaussian variables with mean zero and variances of σ2ε1 and σ2ε2.
The interoccasion variability in CL/F and V1/F was evaluated via interoccasion variability (IOV) as described by Karlsson and Sheiner34.
The predicted plasma concentrations (CIPRED) were calculated for each individual by means of the empirical Bayes estimate of pharmacokinetic parameters using the POSTHOC option in the NONMEM program.
Data analysis was performed using a 3-step approach:
-
1
The population parameters, fixed and random effects, and individual posterior estimates were calculated assuming that pharmacokinetic parameters and covariates were not mutually dependent.
-
2
Following the selection of the basic structural and statistical models, the influence of covariates was investigated. The first approach involved graphic exploration of each covariate and each of the estimated pharmacokinetic parameters. Where a relationship appeared to exist, the selected covariates were individually included in the model and tested for statistical significance. The change in the NONMEM objective function (OF) produced by the inclusion of a covariate term (asymptotically distributed as χ2 with degrees of freedom equal to the number of parameters added to the model) was used to compare alternative models. A change in objective function value (OFV) of at least 3.8 (P<0.05 with 1 degree of freedom) was required for statistical significance at the initial covariate screening stage. Finally, accepted covariates were included in the model and the population pharmacokinetic parameters were estimated. To demonstrate that the retained covariates contributed to an improved fit in the population pharmacokinetic model, each covariate was sequentially deleted from the final model (backward elimination) to confirm the statistical significance (χ2 test). If the variation in the OFV was not statistically significant, the relationship between the covariate and the pharmacokinetic parameter was ignored.
-
3
Only covariates that provided a significant change in the OFV when introduced into the model were retained for analysis. The population parameters were estimated, taking into account their relationship with the covariates. Closeness to and randomness along the line of unity on the observed versus predicted concentration plot, as well as randomness along the residual and weighted residual zero line on the predicted concentration versus residual or weighted residual plot, were considered qualitative evidence of the goodness of fit.
Model validation
Individual pharmacokinetic parameters of each patient in the validation group were calculated on the basis of the Bayesian approach using the 'post hoc' subroutine of NONMEM without an estimation step (MAXEVAL=0) and setting mean parameter values, interindividual and intraindividual variability to population values previously obtained.
The performance of estimation by the Bayesian methodology was assessed by comparing the observed concentrations (DV) to the ones estimated by the Bayesian approach and all the available concentration-time points (IPRED) by using the following criteria:
The bias or mean predictor error:

The precision or root mean square error:

In these expressions, the index i refers to the concentration number, and N is the sample size. The 95% confidence interval (CI) for bias was calculated, and the t test was used to compare the bias to 0.
Results
Patients and data collection
Data were collected retrospectively from 118 adult renal transplant recipients. The characteristics of the patients are presented in Table 1.
The mean age was 42.5±11.4 (range 18–76) years and mean body weight was 58.3±9.91 (range 36.8–94) kg at the time of the first pharmacokinetic study. A total of 1172 blood concentration–time measurements were collected. The number of MPA concentrations per patient ranged from 3 to 30 samples, with a mean (±SD) of 9.93±4.12 samples; concentrations ranged from 0.25 to 47.5 mg/L, with a mean of 5.85±6.51 mg/L. Samples were drawn from 48 to 2006 h after the beginning of treatment (Figure 2).
Observed MPA concentrations versus time after the beginning of treatment (n=1172).
Population pharmacokinetic modeling
The population group database consisted of 783 MPA concentrations from 79 patients. The basic population pharmacokinetic model (before the inclusion of covariates) was best described by a two-compartment model with first-order input rate with no lag time (model 2, Table 2). The FOCE method was used during the model-building process because it markedly improved the fit compared to the FO method: the OFV decreased from 2482 to 2370.
The model was parameterized in terms of the initial volume of distribution (V1/F=θ1), apparent clearance (CL/F=θ2), transfer rate constant from the central compartment to the tissue compartment (k12=θ3), transfer rate constant from the tissue compartment to the central compartment (k21=θ4) and absorption rate constant (ka=θ5).
During covariate analysis, four covariates were identified as being of importance. Significant correlations were found between CL/F and Scr (slope=0.022, intercept=15.1, r=0.4; P=6.27×105), CsA dose (slope=0.0048, intercept=11.4, r=0.29; P=0.012), and BW (slope=0.20, intercept=6.8, r=0.35; P=0.0065), and between V1/F and Scr (slope=0.108, intercept=25.1, r=0.23; P=0.022), CsA dose (slope=0.039, intercept=20.8, r=0.25; P=0.016), and UGT2B7 genotype (slope=6.48, intercept=17.7, r=0.46; P=3.79×106). In the model-building phase, after the inclusion of each covariate in the model, all significantly decreased the OFV by 3.8 or more when tested against the baseline model. In a forward inclusion model building step, only the cumulative inclusion of Scr and BW in the CL/F model and inclusion of Scr and UGT2B7 genotype in the V1/F model significantly decreased the OFV at each addition. Finally, in the backward elimination step, Scr did not exceed the OF cutoff value when omitted from the V1/F model, and so this covariate was removed. Thus, Scr and BW were identified as the most important factors that contributed to the variability in CL/F (Figure 3), and UGT2B7 genotype was identified as the most important factor that explained the variability in V1/F (Figure 3). The final population parameters were calculated taking into account these relationships. The inclusion of this second-stage model (model 9) significantly improved both the relationship between model-predicted and observed concentrations (the OFV decreased from the baseline model of 2370 to 2311, P<0.001, Table 2) and the plot of weighted residuals versus model-predicted concentrations (data not shown). Moreover, this model decreased interindividual variability in CL/F (from 40.6% to 34.2%) and V1/F (from 27.2% to 21.3%), and decreased the residual error (ɛ1: from 19.7% tο 15.8%; ɛ2: from 0.33 to 0.15 mg/L) compared with the baseline model.
Relationships between pharmacokinetic parameters and covariates. (A) Scatter plot of individual total clearance/F (Bayesian estimates) versus weight; (B) Scatter plot of individual total clearance/F (Bayesian estimates) versus Scr; (C) Scatter plot of initial volume of distribution versus UGT2B7 genotype.
Plots of model-predicted versus observed concentrations for the final model, based on individual and population parameter estimates, are shown in Figure 4A and 4B, respectively. Various statistical tests were carried out and demonstrated that (1) there was no significant difference when the regression line of individual predicted concentrations versus observed concentrations (slope=0.995 with 95% CI of 0.95, 1.04; intercept =-0.28 mg/L, with 95% CI of −0.62, 0.06) was compared with the reference line (slope=1 and intercept=0) (Figure 4A); (2) the bias (-0.31 mg/L with 95% CI of -0.46, 0.15) was not significantly different from zero (as shown by a t-test); and (3) the vast majority of the weighted residuals lay within 2 units of perfect agreement and were symmetrically distributed around the zero ordinate (Figure 4C).
Model performance and diagnostic plots (n=79 patients; 783 MPA concentrations, population group). (A) Model-predicted versus observed MPA concentrations obtained from the final model, based on population parameter estimates. The dotted line represents the line of identity; (B) Model-predicted versus observed MPA concentrations obtained for the final model, based on individual parameter estimates. The solid line represents the linear regression line, and the dotted line the line of identity; (C) Weighted residuals versus model-predicted MPA concentrations obtained from final model based on population parameter estimates.
Population pharmacokinetic parameters with and without covariates are presented in Table 3. Interoccasion variability was moderate, with a mean variation of 13.7%. Mean pharmacokinetic parameters in different occasions after renal transplantation are presented in Table 4. From the individual (Bayesian estimates) primary pharmacokinetic parameters, the following secondary pharmacokinetic parameters were calculated: the elimination half-life (t1/2 elim, 29.6 h; interindividual variability, 80.7%) and the area under plasma concentration versus time curve (AUC normalized to a 1 g administered dose, 62.5 mg·h/L; interindividual variability, 39.0%). Mean pharmacokinetic parameters for different UGT2B7 genotypes are presented in Table 5.
Evaluation of the Bayesian pharmacokinetic parameter prediction
Individual pharmacokinetic parameters for the patients in the validation group (389 MPA concentrations, 39 patients) were determined with the population characteristics and all the available concentration-time data. Mean pharmacokinetic parameters were as follows: CL/F=19.2 L/h (CV=33.3%), V1/F=31.8 L (CV=31.1%), k12=0.998 1/h (CV=46.4%), k21=0.0835 1/h (CV=73.7%), and ka=1.89 1/h (CV=63.3%). These parameters were similar to those computed in the population group. The regression line of individual predicted MPA concentrations (Bayesian estimation) and observed concentrations did not differ significantly from the reference line of slope=1 and intercept=0 (Figure 5). The bias (-0.27 mg/L) was not significantly different from zero (t test), and the 95% CI (-0.55, 0.012) included the zero value. The mean elimination half-life was 33.9 h (interindividual variability, 81.5%) and the AUC normalized to a 1 g administered dose was 58.2 mg·h/L (interindividual variability, 34.6%).
Individual predicted MPA concentrations versus observed concentrations in the validation group. The dotted line represents the line of identity, and the solid line the linear regression line.
Population pharmacokinetic parameters calculated from the entire group of patients
From the entire group of patients (n=118, 1172 MPA concentrations), population pharmacokinetic parameters were as follows: CL/F=18.3 L/h (interindividual variability, 32.1%), V1/F=31.3 L (interindividual variability, 22.1%), k12=0.808 1/h (interindividual variability, 41.7%), k21=0.0506 1/h (interindividual variability, 131%), ka=2.11 1/h (interindividual variability, 58.7%), t1/2 elim=33.8 h (interindividual variability, 60.9%) and AUC normalized to a 1 g administered dose=60.9 mg·h/L (interindividual variability, 35.4%). These parameters were similar to those calculated from the patients in the population group.
Discussion
The individualization of MMF treatment for the optimization of post-transplant immunosuppression has been well recognized. Monitoring the AUC rather than C0 is recommended for MPA8,35, and models for AUC estimation are needed. A number of LSSs have been published for MPA AUC estimation using multiple linear regression (MLR)28,31,34,35. However, MLR depends on timed concentrations for the prediction of AUC; sampling time is critical and deviations from the target sampling times may significantly affect the accuracy of AUC prediction, especially in models that utilize early postdose sampling times due to rapid concentration changes during the absorption and distribution phases. Additionally, the application of the LSS is restricted to the dosage regimen and patient population that was used in the MLR.
Bayesian forecasting is a useful tool for therapeutic drug monitoring based on the estimation of individual pharmacokinetic parameters subsequently used to simulate concentrations under any administration regimen. However, Bayesian models are useful only if a population database that includes patients with different characteristics is available. Population pharmacokinetic analysis is the first step in the development of a Bayesian method. It allows the estimation of mean pharmacokinetic parameters and offers the advantage of considering individual characteristics that may help in interpreting different pharmacokinetic behaviors between patients. Until now, population pharmacokinetic data were lacking for MPA in Chinese renal transplant patients. The present study is the first to describe the population pharmacokinetics of MPA in Chinese adult renal transplant patients. Different pharmacokinetic models were tested. The structural model was chosen on the basis of the changes in -2 log likelihood and qualitative assessment of diagnostic plots. A two-compartment model with a first-order input rate with no lag time was found to fit the data satisfactorily. Different covariates were examined. Scr and BW were identified as the most important factors that influenced the CL/F of MPA, and UGT2B7 genotype was the main factor that explained the interindividual variability in V1/F. Population pharmacokinetic parameters were calculated taking both the relationship between Scr, BW and CL/F and the relationship between UGT2B7 genotype and V1/F into account in the final population model (Table 3). Mean pharmacokinetic parameters were similar to those obtained from conventional pharmacokinetic studies in Chinese renal transplant patients13,36,37.
Although the enterohepatic recycling (EHR) of MPAG to MPA has been reported, this is responsible for the secondary rise in MPA concentration that typically occurs approximately 6–12 h after the oral administration of MMF38. However, in the present study, the inclusion of EHR in the model did not improve the goodness of fit: the OFV increased from 2370 to 2806 when EHR was considered in the model. The secondary concentration peaks seen in most of our patients were relatively small. It appears that EHR did not influence MPA pharmacokinetics to a large extent in the renal transplant population we studied. Therefore, EHR was not included in the pharmacokinetic model.
To date, several population pharmacokinetic analyses for MPA in renal transplant recipients have been published. One study was performed in children30, and the other studies were carried out in adult renal transplant patients31,32,33,39,40,41 at different post-renal transplant periods. The main population pharmacokinetic parameters were: CL/F ranged from 11.9 to 34.9 L/h; V1/F ranged from 5 to 98 L; and absorption rate constant (ka) ranged from 0.63 to 4.1 1/h. CL/F showed significant time-dependency. A higher value of 34.9 L/h was reported in one study performed 1 week after renal transplantation39. Lower values of 27.1, 15.7 and 14.1 L/h were reported in studies performed within one month31, more than six months32 and one year41 after renal transplantation, respectively. Pharmacokinetic evaluations for most of the patients in this study were performed within one month. The population estimate of CL/F was 18.3 L/h, which was lower than that reported in the study performed in the same time period after renal transplantation31, indicating a slower clearance in Chinese renal transplant patients. When MMF is given at a fixed dose of 1 or 1.5 g twice daily in accordance with the dosage recommended by the manufacturer, adverse effects (eg, gastrointestinal and hematological, etc) and infections are very common in Chinese renal transplant patients42,43; slow clearance and consequent accumulation are probably the veritable reasons.
A large variability in MPA pharmacokinetics was previously reported in many studies. The interpatient variabilities were 20%–51% for CL/F, 35%–91% for V1/F, and 44%–111% for ka30,31,32,33. In the present study, the interpatient variability for CL/F, V1/F and ka were 34.2%, 21.3% and 51.3%, respectively. Following transplantation, patients undergo marked changes in physiological functions associated with the transplanted organs. Drug absorption, distribution, and elimination undergo a time-dependent transition from abnormal changes associated with organ failure to the normal state. In addition, the genetic polymorphism in uridine diphosphate glucuronosyltransferases (UGTs) or transporters (multi-drug resistance protein 2, MRP-2), which are involved in the processes such as glucuronidation and active biliary secretion of MPA, may contribute to the wide interindividual variability in MPA pharmacokinetics. Hypoalbuminemia, co-medications and renal dysfunction are very common in renal transplant patients. All these different factors may contribute to large inter- and intra-individual variability in MPA pharmacokinetics.
Time-dependent clearance of MPA was reported in a previous study in renal transplant recipients44. The mean CL decreased from 35 L/h (CV=44%) in the first week after transplantation to 17 L/h (CV=38%) after 6 months. Creatinine clearance, albumin concentration, hemoglobin and CsA predose concentration explained 19%, 12%, 4% and 3% of the within-patient variability in MPA CL/F, respectively. In the present study, the pharmacokinetic evaluations of most of our patients were performed within one month after transplantation. Some patients had two pharmacokinetic evaluations performed during this period. Only a few patients had additional pharmacokinetic evaluations performed at a later period after six months. MPA pharmacokinetics in different occasions after transplantation were evaluated, the results showed that they were quite similar (Table 4). The interoccasion variability was 13.7%.
A statistically significant correlation has been reported between MPA CL/F and ALB concentration: MPA CL/F decreased significantly with increasing ALB concentration33,39. A cutoff value of 31 g/L for serum ALB concentration was previously identified to be a good predictor of altered free MPA percentage in renal transplant patients; below this cutoff value, MPA free fraction was considered to be significantly elevated45. MPA pharmacokinetics may be presumed to change significantly with the alteration of the MPA free fraction. No significant correlation was observed between MPA clearance and ALB concentration in the present study. The approximately normal ALB concentration in most of our patients may explain such difference; only 29 of 118 patients had an ALB concentration lower than 31 g/L.
Significant correlations between MPA pharmacokinetic parameters and creatinine clearance were identified in a previous study in renal transplant patients33, in which both MPA clearance and V1 were significantly correlated with creatinine clearance. MPA clearance decreased with creatinine clearance, and a cutoff value of creatinine clearance of 25 mL/min was reported. Renal transplant patients with creatinine clearance <25 mL/min showed a significantly higher mean MPA clearance on day 3, 7 and 21 after transplantation compared with patients with a creatinine clearance of >25 mL/min. In the present study, significant correlations between MPA CL/F and V1/F and Scr level were also observed. MPA CL/F increased with Scr level. A fall in Scr from >500 to 200 μmol/L (ie, an increase in creatinine CL (CLCR) from <10 to 25 L/h) decreased MPA clearance from 30 to 20 L/h, whereas a modest decrease in MPA clearance from 20 to 17 L/h was observed when Scr decreased from 200 to 120 μmol/L (ie, CLCR from 40 to 20 L/h). These results were in accordance with those reported by van Hest et al33. However, another study in stable patients with moderate impairment of renal function more than 6 months after transplantation reported the absence of the influence of renal function on MPA CL/F32. These conflicting results may be ascribed to the renal function of the studied populations. In the early days after transplantation, especially the first six months, the patients are unstable and renal dysfunction is more common. Acidosis and uremia are associated with impaired renal function and will decrease MPA binding to albumin46. Moreover, accumulation of the glucuronide metabolite of MPA (MPAG) during renal impairment will displace MPA from its albumin binding sites47. As MPA is thought to be a restrictively cleared drug, the resulting increase in MPA free fraction will lead to an increase in the amount of MPA available for glucuronidation and hence to a higher MPA CL/F46,48,49.
In the present study, a significant correlation between the CL/F of MPA and BW (P=0.0065) was found: CL/F increased with increasing BW. The inclusion of the relationship between CL/F and BW significantly improved the fit of the basic model (the OFV decreased from the baseline model of 2370 to 2359, P=0.001). Kagaya et al50 found similar results; a lower BW was independently predictive for a higher dose-adjusted AUC0-12 of MPA. Yamada et al51 also found that patients with a lower BW tended to have a higher MPA AUC:MMF dose ratio than patients with a higher BW. Le Guellec et al32 previously reported a positive correlation between the CL/F of MPA and BW in renal transplant patients. However, the consideration of BW only reduced between-subject variability in CL/F by 6.6%, suggesting that the administration of MMF on a per kilogram basis would be of limited value32.
Pharmacokinetic interactions with frequently used comedications have also been described for MPA. CsA has been reported to interfere with MPA clearance: it inhibits MPA enterohepatic recirculation and thereby reduces MPA daily exposure52. A positive correlation between the daily CsA dose and MPA clearance was reported in a previous study33. In the present study, we found a positive correlation between CsA dose and CL/F of MPA (slope=0.0048, intercept=11.4, r=0.29, P=0.01) and a positive correlation between CsA dose and V1/F of MPA (slope=0.039, intercept=20.8, r=0.25, P=0.016) during the initial covariate analysis, but the statistical significance was not established when CsA dose was included in the final model. This result indicates that the modification of MMF dosage is not necessary during CsA dosage adjustment. Corticosteroid therapy was not found to be an important covariate in this study. Higher doses of corticosteroids have been thought to induce UGT activity and increase the apparent clearance of MPA53. Patients in this study were usually given methylprednisolone sodium succinate 500 mg intravenously during surgery and the dosage was then progressively tapered and one week later maintained at a low oral dose. These doses may be too low for the induction effect to be significant.
Concerning the influence of age and gender on the pharmacokinetics of MPA, Tang et al54 reported that age did not significantly affect the pharmacokinetics or pharmacodynamics of MPA. However, Yoo et al55 reported that MPA exposure was associated with developmental changes in children, and demonstrated that small children are at a significantly greater risk of low MPA trough levels than adolescents, highlighting the need for pharmacokinetic monitoring of MPA. The influence of patient gender on the pharmacokinetics of MPA has been evaluated in three previous population pharmacokinetic studies in renal transplant patients. Two of them found no significant influence32,39. An 11% increase in MPA clearance in males was reported in the third study33. In the present study, neither the age nor gender of patients was found to be correlated to the pharmacokinetic parameters of MPA, suggesting that it would not be necessary to consider the age and gender of a patient when MMF is administered in renal transplant patients. It is important to note, however, that no pediatric renal transplant recipients were included in our study.
Processes such as glucuronidation and active biliary secretion of MPA are likely to be under genetic control, and genetic differences may contribute to pharmacokinetic variations. UGTs metabolize MPA via glucuronidation in the gastrointestinal tract, liver and kidney56. UGT 1A9 and 2B7 are believed to be the major isoforms involved in MPA glucuronidation, possibly because of their high hepatic and renal expression56. Genetic polymorphism in these isoforms may contribute to pharmacokinetic variations. Thus, UGT2B7 and UGT1A9*22 gene polymorphisms were studied. Kuypers et al57reported that the -275T>A and -2152C>T single-nucleotide polymorphisms of the UGT1A9 gene promoter are associated with significantly lower MPA concentrations in Caucasian renal transplant recipients after the oral administration of MMF. The allele frequencies of UGT1A9 -275T>A and -2152C>T in Caucasian renal recipients are reported to be 16.8% and 12.6%, respectively57. However, these polymorphisms are not found in Asian populations and are not clinically important for MPA disposition in Asians58,59. The base insertion of thymidine in a promoter region of UGT1A9 resulting in A(T)10AT was reported to increase reporter gene expression in HepG2 cells. The allele was termed UGT1A9*22. In the present study, we first reported no influence of UGT1A9*22 gene polymorphisms [UGT1A9 (T)9, (T)9/(T)10 and (T)10] on MPA pharmacokinetics in Chinese renal transplant patients. This result indicated that UGT1A9*22 genotypes do not contribute to interindividual differences in MPA pharmacokinetics. Takeshi Hirota et al found a new SNP(G211T) in the coding region of the UGT2B7 gene. At position 211 (G211T), Ala71 is replaced by Ser, which results in a change from a lipophilic residue to a hydrophilic residue60. It was demonstrated that the opioid binding site in UGT2B7 is within the first 119 amino-terminal amino acids (N-terminal half of the protein)61. Polymorphisms in UGT2B7 showed significant association with MPA AUC in previously reported studies62,63,64. In our study, the UGT2B7 211G>T genotype was found to be an important factor that contributes to the variability in MPA pharmacokinetics. A significantly higher MPA exposure was found in patients with the UGT2B7 211GT genotype than in those with the UGT2B7 211GG and UGT2B7 211TT genotypes.
The high-performance liquid chromatography (HPLC) method used in this study29 is highly specific for MPA and has low imprecision across the analytical range (coefficient of variation <8%). In comparison, the enzyme-multiplied immunoassay technique (EMIT) contributed to a large residual variability reported by Le Guellec et al32. Larger measurement errors may be caused by the EMIT assay due to its cross-reaction with acyl-MPA glucuronide, the concentrations of which vary over the administration interval65. The residual random error in our model may result from intraindividual variability in pharmacokinetic parameters. The rebound of MPA plasma concentration due to enterohepatic recycling of MPA is probably an important reason for the differences between individually predicted and observed concentrations. Sampling errors and patient compliance errors may also be involved in view of the retrospective nature of this study. Such small variability ensures the usefulness of these population pharmacokinetic data for individualizing dosage predictions of MPA in renal transplant patients.
In conclusion, we report the population pharmacokinetics of MPA in Chinese adult renal transplant patients. This study included a large number of patients and samples and examined many different covariates, some of which had previously been identified as important factors that influenced MPA pharmacokinetics. The Bayesian approach developed in this study accurately predicts concentrations of MPA in plasma, and this was demonstrated in patients in the validation group. The patient group included in this study is representative of “real-life” Chinese renal transplant recipients. The dosage of MMF given to different patients (and during the course of each individual's therapy) varied considerably. Data collected in the current study resembled data that are normally available for renal transplant patients. Thus, findings from this study should be applicable to Chinese renal transplant patients. However, a prospective evaluation of the clinical utility of these results needs to be undertaken to assess dosage prediction.
Author contribution
Zi-cheng YU, Pei-jun ZHOU, Da XU, and Xiang-hui WANG designed the study; Bressolle FRANÇOISE performed population pharmacokinetic data analysis; Wei-xia ZHANG performed the genotype detection; Bing CHEN participated in MPA concentration determination; Zi-cheng YU and Bressolle FRANÇOISE wrote the manuscript; all authors discussed the results and contributed to the revision of the final manuscript.
References
Allison AG, Eugui EM . Mechanisms of action of mycophenolate mofetil in preventing acute and chronic allograft rejection. Transplantation 2005; 80: 181S–90S.
Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation 1996; 61: 1029–37.
Van Gelder T, Hilbrands LB, Vanrenterghem Y, Weimar W, de Fijter JW, Squifllet JP, et al. A randomized double-blind, multicenter plasma concentration controlled study of the safety and efficacy of oral mycophenolate mofetil for the prevention of acute rejection after kidney transplantation. Transplantation 1999; 68: 261–6.
Tsai MK, Lee CY, Hu RH, Lee PH . Conversion to combined therapy with sirolimus and mycophenolate mofetil improved renal function in stable renal transplant recipients. J Formos Med Assoc 2007; 106: 372–9.
Flechner SM, Goldfarb D, Solez K, Modlin CS, Mastroianni B, Savas K, et al. Kidney transplantation with sirolimus and mycophenolate mofetil-based immunosuppression: 5-year results of a randomized prospective trial compared to calcineurin inhibitor drugs. Transplantation 2007; 83: 883–92.
Michel M, Jacques M . Djamila CE, Martine DM, Josiane K, Raf S, et al. Correlation of mycophenolic acid pharmacokinetic parameters with side effects in kidney transplant patients treated with mycophenolate mofetil. Clin Chem 2001; 47: 88–94.
Hale MD, Nicholls AJ, Bullingham RE, Hené R, Hoitsma A, Squifllet JP, et al. The pharmacokinetic–pharmacodynamic relationship for mycophenolate mofetil in renal transplantation. Clin Pharmacol Ther 1998; 64: 672–83.
Kuypers DR, De Jonge H, Naesens M, de Loor H, Halewijck E, Dekens M, et al. Current target ranges of mycophenolic acid exposure and drug-related adverse events: a 5-year, open-label, prospective, clinical follow-up study in renal allograft recipients. Clin Ther 2008; 30: 673–83.
Mourad M, Malaise J, Chaib Eddour D, De Meyer M, König J, Schepers R, et al. Pharmacokinetic basis of efficient and safe use of low mycophenolate mofetil (MMF, 1 g/day) in combination with tacrolimus in kidney transplant patients. Clin Chem 2001; 47: 1241–8.
de Winter BC, Mathot RA, Sombogaard F, Vulto AG, van Gelder T . Nonlinear relationship between mycophenolate mofetil dose and mycophenolic acid exposure: implications for therapeutic drug monitoring. Clin J Am Soc Nephrol 2011; 6: 656–63.
DeNofrio D, Loh E, Kao A, Korecka M, Pickering FW, Craig KA, et al. Mycophenolic acid concentrations are associated with cardiac allograft rejection. J Heart Lung Transplant 2000; 19: 1071–6.
van Hest RM, Mathot RA, Vulto AG, Lizermans JN, van Gelder T . Within-patient variability of mycophenolic acid exposure: therapeutic drug monitoring from a clinical point of view. Ther Drug Monit 2006; 28: 31–4.
Yu ZC, Zhou PJ, Xu D, Wang XH, Chen HZ . Investigation on pharmacokinetics of mycophenolic acid in Chinese adult renal transplant patients. Br J Clin Pharmacol 2006; 62: 446–52.
Filler G, Alvarez-Elías AC, McIntyre C, Medeiros M . The compelling case for therapeutic drug monitoring of mycophenolate mofetil therapy. Pediatr Nephrol 2017; 32: 21–9.
van Hest RM, Hesselink DA, Vulto AG, Methot RA, van Gelder T . Individualization of mycophenolate mofetil dose in renal transplant recipients. Expert Opin Pharmacother 2006; 7: 361–76.
Kuypers DR, Le Meur Y, Cantarovich M, Tredger MJ, Tett SE, Cattaneo D, et al. Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation. Clin J Am Soc Nephrol 2010; 5: 341–58.
Brunet M, Martorell J, Oppenheimer F, Vilardell J, Millán O, Carrillo M, et al. Pharmacokinetics and pharmacodynamics of mycophenolic acid in stable renal transplant recipients treated with low doses of mycophenolate mofetil. Transplant Int 2000; 13: S301–5.
Filler G, Mai I . Limited sampling strategy for mycophenolic acid area under the curve. Ther Drug Monit 2000; 22: 169–73.
Johnson AG, Rigby RJ, Taylor PJ, Jones CE, Allen J, Franzen K, et al. The kinetics of mycophenolic acid and its glucuronide metabolite in adult kidney transplant recipients. Clin Pharmacol Ther 1999; 66: 492–500.
Pawinski T, Hale M, Korecka M, Fitzsimmons WE, Shaw LM . Limited sampling strategy for the estimation of mycophenolic acid area under the curve in adult renal transplant patients treated with concomitant tacrolimus. Clin Chem 2002; 48: 1497–504.
Schutz E, Armstrong VW, Shipkova M, Weber L, Niedmann PD, Lammersdorf T, et al. Limited sampling strategy for the determination of mycophenolic acid area under the curve in pediatric kidney recipients. German Study Group on Mycophenolate Mofetil Therapy in Pediatric Renal Transplant Recipients. Transplant Proc 1998; 30: 1182–4.
Shaw LM, Korecka M, DeNofrio D, Brayman KL . Pharmacokinetic, pharmacodynamic, and outcome investigations as the basis for mycophenolic acid therapeutic drug monitoring in renal and heart transplant patients. Clin Biochem 2001; 34: 17–22.
Baraldo M, Isola M, Feruglio MT, Francesconi A, Franceschi L, Tursi V, et al. Therapeutic mycophenolic acid monitoring by means of limited sampling strategy in orthotopic heart transplant patients. Transplant Proc 2005; 37: 2240–3.
Li J, Liu Y, Huang J, Fu Q, Chen E, Liu L, et al. Evaluation of mycophenolic acid exposure using a limited sampling strategy in renal transplant recipients. Am J Nephrol 2013; 37: 534–40.
Cai W, Ye C, Sun X, Qin K, Qin Y, Zhao D, et al. Limited sampling strategy for predicting area under the concentration-time curve for mycophenolic Acid in Chinese adults receiving mycophenolate mofetil and tacrolimus early after renal transplantation. Ther Drug Monit 2015; 37: 304–10.
Tanigawara Y, Yano I, Kawakatsu K, Nishimura K, Yasuhara M, Hori R . Predictive performance of the Bayesian analysis: effects of blood sampling time, population parameters, and pharmacostatistical model. J Pharmacokinet Biopharm 1994; 22: 59–71.
Proost JH . Adaptive control of drug dosage regimens using maximum a posteriori probability Bayesian fitting. Int J Clin Pharmacol Ther 1995; 33: 531–6.
Thomson AH, Whiting B . Bayesian parameter estimation and population pharmacokinetics. Clin Pharmacokinet 1992; 22: 447–67.
Yu ZC, Cai WM, Xu D, Wang XH . HPLC determination of mycophenolate mofetil and its active metabolite mycophenolic acid in human plasma. Chin J Pharm Anal 2005; 25: 381–4.
Payen S, Zhang D, Maisin A, Popon M, Bensman A, Bouissou F, et al. Population pharmacokinetics of mycophenolic acid in kidney transplant pediatric and adolescent patients. Ther Drug Monit 2005; 27: 378–88.
Shum B, Duffull SB, Taylor PJ, Tett SE . Population pharmacokinetic analysis of mycophenolic acid in renal transplant recipients following oral administration of mycophenolate mofetil. Br J Clin Pharmacol 2003; 56: 188–97.
Le Guellec C, Bourgoin H, Buchler M, Le Meur Y, Lebranchu Y, Marquet P, et al. Population pharmacokinetics and Bayesian estimation of mycophenolic acid concentrations in stable renal transplant patients. Clin Pharmacokinet 2004; 43: 253–66.
van Hest RM, van Gelder T, Vulto AG, Mathot RA . Population pharmacokinetics of mycophenolic acid in renal transplant recipients. Clin Pharmacokinet 2005; 44: 1083–96.
Karlsson MO, Scheiner LB . The importance of modelling interoccasion variability in population pharmacokinetic analyses. J Pharmacokinet Biopharm 1993; 21: 735–50.
Shaw LM, Holt DW, Oellerich M, Meiser B, van Gelder T . Current issues in therapeutic drug monitoring of mycophenolic acid: report of a roundtable discussion. Ther Drug Monit 2001; 23: 305–15.
Lu XY, Huang HF, Sheng-Tu JZ, Liu J . Pharmacokinetics of mycophenolic acid in Chinese kidney transplant patients. J Zhejiang Univ Sci B 2005; 6: 885–91.
Liang MZ, Lu YP, Nan F, Li YP . Pharmacokinetics of mycophenolic acid and its glucuronide after a single and multiple oral dose of mycophenolate mofetil in Chinese renal transplantation recipients. Transplant Proc 2006; 38: 2044–7.
Bullingham RES, Nicholls AJ, Kamm BR . Clinical pharmacokinetics of mycophenolate mofetil. Clin Pharmacokinet 1998; 34: 429–55.
Staatz CE, Duffull SB, Kiberd B, Fraser AD, Tett SE . Population pharmacokinetics of mycophenolic acid during the first week after renal transplantation. Eur J Clin Pharmacol 2005; 61: 507–16.
Premaud A, Debord J, Rousseau A, Le Meur Y, Toupance O, Lebranchu Y, et al. A double absorption phase model adequately describes mycophenolic acid plasma profiles in de novo renal transplant recipients given oral mycophenolate mofetil. Clin Pharmacokinet 2005; 44: 837–47.
Cremers S, Schoemaker R, Scholten E, den Hartigh J, König-Quartel J, van Kan E, et al. Characterizing the role of enterohepatic recycling in the interactions between mofetil and calcineurin inhibitors in renal transplant patients by pharmacokinetic modelling. Br J Clin Pharmacol 2005; 60: 249–56.
Wang YR, Wang XF, Liu YL, Zhu L . Clinical analysis of adverse reactions caused by mycophenolate mofetil. Central South Pharmacy 2004; 2: 368–70.
Wang KJ, Zhang HT, Li YP, Lu YP, Wei Q, Li H, et al. Safety of mycophenolate mofetil versus azathioprin in renal transplantation: A systematic review. Chin J Evidence-based Med 2005; 5: 365–74.
van Hest RM, van Gelder T, Bouw R, Goggin T, Gordon R, Mamelok RD, et al. Time-dependent clearance of mycophenolic acid in renal transplant recipients. Br J Clin Pharmacol 2007; 63: 741–52.
Atcheson BA, Taylor PJ, Kirkpatrick CM, Duffull SB, Mudge DW, Pillans PI, et al. Free mycophenolic acid should be monitored in renal transplant recipients with hypoalbuminemia. Ther Drug Monit 2004; 26: 284–6.
Shaw LM, Korecka M, Aradhye S, Grossman R, Bayer L, Innes C, et al. Mycophenolic acid area under the curve values in African American and Caucasian renal transplant patients are comparable. J Clin Pharmacol 2000; 40: 624–33.
Nowak I, Shaw LM . Mycophenolic acid binding to serum albumin: characterization and relation to pharmacodynamics. Clin Chem 1995; 41: 1011–7.
Kaplan B, Meier-Kriesche HU, Friedman G, Mulgaonkar S, Gruber S, Korecka M, et al. The effect of renal insufficiency on mycophenolic acid protein binding. J Clin Pharmacol 1999; 39: 715–20.
Shipkova M, Strassburg CP, Braun F, Streit F, Gröne HJ, Armstrong VW, et al. Glucuronide and glucoside conjugation of mycophenolic acid by human liver, kidney and intestinal microsomes. Br J Pharmacol 2001; 132: 1027–34.
Kagaya H, Niioka T, Saito M, Inoue T, Numakura K, Habuchi T, et al. Effect of hepatic drug transporter polymorphisms on the pharmacokinetics of mycophenolic acid in patients with severe renal dysfunction before renal transplantation. Xenobiotica 2017; 47: 916–22.
Yamada S, Shiohira H, Uehara H, Hokama N, Saitou S, Ooshiro Y . Implications of clinical mycophenolate mofetil dose according to individual body weight in Japanese renal transplant recipients. Transplant Proc 2016; 48: 35–41.
Cattaneo D, Merlini S, Zenoni S, Baldelli S, Gotti E, Remuzzi G, et al. Influence of co-medication with sirolimus or cyclosporine on mycophenolic acid pharmacokinetics in kidney transplantation. Am J Transplant 2005; 5: 2937–44.
Cattaneo D, Perico N, Gaspari F, Gotti E, Remuzzi G . Glucocorticoids interfere with mycophenolate mofetil bioavailability in kidney transplantation. Kidney Int 2002; 62: 1060–7.
Tang JT, de Winter BC, Hesselink DA, Sombogaard F, Wang LL, van Gelder T . The pharmacokinetics and pharmacodynamics of mycophenolate mofetil in younger and elderly renal transplant recipients. Br J Clin Pharmacol 2017; 83: 812–22.
Yoo EC, Alvarez-Elías AC, Todorova EK, Filler G . Developmental changes of MPA exposure in children. Pediatr Nephrol 2016; 31: 975–82.
Picard N, Ratanasavanh D, Premaud A, Le Meur Y, Marquet P . Identification of the UDP-glucuronosyltrans -ferase isoforms involved in mycophenolic acid phase II metabolism. Drug Metab Dispos 2005; 33: 139–46.
Kuypers DR, Naesens M, Vermeire S, Vanrenterghem Y . The impact of uridine diphosphate-glucuronosyl transferase 1A9 (UGT1A9) gene promoter region single-nucleotide polymorphisms T-275A and C-2152T on early mycophenolic acid dose-interval exposure in de novo renal allograft recipients. Clin Pharmacol Ther 2005; 78: 351–61.
Saeki M, Saito Y, Jinno H, Sai K, Ozawa S, Kurose K, et al. Haplotype structures of the UGT1A gene complex in a Japanese population. Pharmacogenomics J 2006; 6: 63–75.
Kagaya H, Inoue K, Miura M, Satoh S, Saito M, Tada H, et al. Influence of UGT1A8 and UGT2B7 genetic polymorphisms on mycophenolic acid pharmacokinetics in Japanese renal transplant recipients. Eur J Clin Pharmacol 2007; 63: 279–88.
Hirota T, Ieiri I, Takane H, Sano H, Kawamoto K, Aono H, et al. Sequence variability and candidate gene analysis in two cancer patients with complex clinical outcomes during morphine therapy. Drug Metab Dispos 2003; 31: 677–80.
Coffman BL, Kearney WR, Green MD, Lowery RG, Tephly TR . Analysis of opioid binding to UDP-glucurosyltransferase 2B7 fusion proteins using nuclear magnetic resonance spectroscopy. Mol Pharmacol 2001; 59: 1464–9.
Xie XC, Li J, Wang HY, Li HL, Liu J, Fu Q, et al. Associations of UDP-glucuronosy-ltransferases polymorphisms with mycophenolate mofetil pharmacokinetics in Chinese renal transplant patients. Acta Pharmacol Sin 2015; 36: 644–50.
Pithukpakorn M, Tiwawanwong T, Lalerd Y, Assawamakin A, Premasathian N, Tasanarong A, et al. Mycophenolic acid AUC in Thai kidney transplant recipients receiving low dose mycophenolate and its association with UGT2B7 polymorphisms. Pharmgenomics Pers Med 2014; 7: 379–85.
Guo D, Pang LF, Han Y, Yang H, Wang G, Tan ZR, et al. Polymorphisms of UGT1A9 and UGT2B7 influence the pharmacokinetics of mycophenolic acid after a single oral dose in healthy Chinese volunteers. Eur J Clin Pharmacol 2013; 69: 843–9.
Vogl M, Weigel G, Seebacher G, Griesmacher A, Laufer G, Müller MM . Evaluation of the EMIT mycophenolic acid assay from Dade Behring. Ther Drug Monit 1999; 21: 638–43.
Acknowledgements
We thank Prof Térol A and Prof Bataille B for advice and support. This work was supported by the National Natural Science Foundation of China (No 30500626).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, Zc., Zhou, Pj., Wang, Xh. et al. Population pharmacokinetics and Bayesian estimation of mycophenolic acid concentrations in Chinese adult renal transplant recipients. Acta Pharmacol Sin 38, 1566–1579 (2017). https://doi.org/10.1038/aps.2017.115
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/aps.2017.115
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
Pharmacokinetic comparability between two populations using nonlinear mixed effect models: a Monte Carlo study
Journal of Pharmacokinetics and Pharmacodynamics (2023)
-
Clinical Evidence on the Purported Pharmacokinetic Interactions between Corticosteroids and Mycophenolic Acid
Clinical Pharmacokinetics (2023)
-
The Impact of Genetic Polymorphisms on the Pharmacokinetics and Pharmacodynamics of Mycophenolic Acid: Systematic Review and Meta-analysis
Clinical Pharmacokinetics (2021)
-
Model based development of tacrolimus dosing algorithm considering CYP3A5 genotypes and mycophenolate mofetil drug interaction in stable kidney transplant recipients
Scientific Reports (2019)
-
Population Pharmacokinetics of Mycophenolic Acid Co-Administered with Tacrolimus in Corticosteroid-Free Adult Kidney Transplant Patients
Clinical Pharmacokinetics (2019)