Abstract
Metal-based nanoparticles offer a one-pot solution for pollution mitigation due to the wide range of pollutants removed using multiple mechanisms such as catalytic oxidation, reduction, photothermal degradation/transformation and magnetic adsorption. Herein, we give an insight into the effect of key factors such as synthetic method, electronic properties of metals and nature of the pollutants and their dispersion media, on the efficiency of metal-based nanoparticles in pollution mitigation. Silver nanoparticles are mostly biosynthesized and applied in the removal of pathogenic bacteria, where the removal efficiency is enhanced by the closeness in the chemical resemblance between the biological corona of extracts used in the synthesis of the nanoparticles and that of the bacteria colony biofilm or cell membrane/wall makeup. On the other hand, chemical and physical methods are used to synthesize most transition metal-based nanoparticles for versatile applications in curbing various biological and chemical pollutants. In general, pollutant removal efficiency increases with an increase in the concentration of the metal nanoparticles and the use of multiple metals, the availability of ligand hetero atoms and the stability of products formed by the degradation or transformation of chemical pollutants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A clean environment is crucial for the sustainability of both aquatic and terrestrial ecosystems, however, the proportion of water for consumption, and uncontaminated soils and air is declining due to the continuous influx of pollutants not only from anthropogenic activities such as agriculture, mining, manufacturing and chemical industries but also from natural sources such as volcanic eruptions, rock withering and pathogenic microbes from sewage. Heavy metals in water are anticipated to be carcinogenic and cause hormonal complications [1] and skeletal/bone-related diseases [2, 3]. On the other hand, chemical pollutants with slow decay rates, including pharmaceuticals and their metabolites, industrial dyes and polycyclic aromatic hydrocarbons and other persistent organic pollutants, have been reported in industrial effluents, lakes and aquatic organisms, especially fish [4, 5], and unintended ingestion can lead to acute and chronic detrimental health effects [6, 7]. It is worth noting that the imminent danger comes from pathogenic bacteria that cause water-borne illnesses [8, 9].
The choice of water purification technique largely depends on the nature of pollutants, with physical methods such as reverse osmosis [10], filtration [11], and the widely used adsorption employing activated carbon [12, 13] having a greater advantage because of zero chemical use during the actual purification process, which minimizes secondary pollution. One major disadvantage of these physical methods is the inability to destroy the pollutants which often remain in the same state during cleaning or disposal of the filter gadgets and thus the release of pollutants to a new environment. This poses a challenge from persistent organic pollutants and rapidly multiplying disease-causing microbes necessitating methodologies that not only capture but also modify/destroy/kill pollutants.
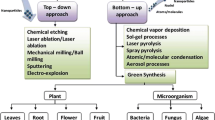
Metal-based nanoparticles (MbNPs) are a promising class of pollutant detectors/sensors [14,15,16,17] and water purifiers [18, 19] attributed to the large surface area and the versatile combat mode on both chemical pollutants and disease-causing pathogens (Fig. 1). Metals that absorb the end of the visible radiation near the infrared region have been employed to kill bacteria by heat generated at the metal surface by a photothermal process after electron excitation and relaxation [20] to extremely high temperatures with the highest temperature of 200 °C recorded by Khantamat et al. using functionalized gold nanoshells [21]. In addition, MbNPs have been employed to overcome bacterial resistance, which mostly arises from the formation of biofilms [22,23,24] by altering functional groups of the bacteria’s peptidoglycan cell wall making the microbes more susceptible to environmental stress and external magnetic fields [25,26,27], oxidative stress [28,29,30] and destruction of cells by the interaction of the metals with phosphates and sulfide bonds of DNA and protein, respectively, in the cytoplasm which alters the structure of DNA/enzymes [31, 32]. Similarly, the complete degradation of rhodamine B (RhB) dye was attributed to the generation of superoxide radicals by iron(III) oxide nanoparticles according to research findings by Haimiao et al. [33].
The chemical functional groups used to reduce metal ions and subsequently stabilize MNPs during synthesis of MbNPs determine whether pollutant mitigation proceeds by magnetics attraction, electrostatic interactions or chemical coordination with the functional groups of the pollutant that can result in change of their electronic structure causing the decomposition of chemical pollutants; for example, the degradation of methylene blue (MB), tetracycline, oxytetracycline and ciprofloxacin by black titanium (IV) oxide nanoparticles reported by Yu et al. [34]. Magnetic NPs made of iron and its oxides remove toxic metal ions by magnetic forces, which increase with decreasing nanoparticle size [35]. MbNPs also act as catalysts in pollutant mitigation; for example, gold nanoparticles have been reported to mediate the catalytic reduction of organic pollutants using sodium borohydride as the reducing agent [36].
Important to mention is that the method of preparation of MbNPs determines their distribution, stability and electronic properties and therefore affects the contact surface area and mode of interaction with inorganic and organic pollutants, hence dictating their efficiency in pollutant mitigation. Consequently, some limitations arise, for instance, the leaching of supported nanoparticles or residual chemical compounds, which may be toxic and have detrimental effects on non-target environment and living organisms and therefore MbNPs should be used with extreme precaution and regulatory framework/guidelines should be set in case of large-scale usage.
2 Properties of nanometals based on the method of preparation
The stability of MbNPs largely depends on the method of synthesis and the dispersion media/support system used to reduce the metal ions and to prevent aggregation of the NPs, both of which dictate the physical/chemical attributes such as size, shape, active surface area and electronic properties which directly affect the efficiency of pollutant adsorption/modification/destruction by MbNPs. Methods of preparation of MbNPs reported mostly involve green synthesis using bio extracts [37, 38], chemical synthesis [39] and physical doping/embedment on solid supports [34, 40].
The chemistry of the metals used does not change; however, the size and shape of nanoparticles determine the active surface area and hence the efficiency of MbNPs in the process involved in pollutant mitigation. The aggregation of the nanoparticles is minimal for presynthesized nanoparticles embedded on solid supports, as is evident from very small-sized particles but the size generally increases due to aggregation in methodologies that reduce metal ions to nanometals. Generally, the size varies with chemical functional groups used in the order, synthetic chemicals < biological corona < natural/artificial polymers (Fig. 2).
In recent works, the biosynthesis of metal-based nanoparticles has been achieved using extracts of microorganisms and plant parts (Table 1). Silver nanoparticles synthesized from leaf extracts are most common and are mostly used to combat pathogenic bacteria.
Different sizes and morphologies of silver nanoparticles (AgNPs) were achieved by bacterial synthesis using Pseudomonas putida KT2440 and Escherichia coli K12 MG1655, where spherical, triangular and hexagonal particles with sizes in the range of 15–40 nm were obtained from the former, and relatively large AgNPs (30–70 nm) for the latter (Table 1, entries 1 and 2) [41]. Both kinds of AgNPs were able to eliminate the target pathogenic P. aeruginosa PAO1 and E. coli UTI 89 colonies with minimum bactericidal concentrations (MBCs) of 1 μg/mL and 8 μg/mL, respectively, which was attributed to the similarity between the biological corona of the pathogenic cellular envelope/biofilm and the biochemical constituents of the corresponding harmless bacteria used to synthesize the AgNPs. The similar chemical environment surrounding the AgNPs facilitated penetration through the pathogenic biofilms and subsequent elimination of the target microbes. In related works, spherical AgNPs with sizes ranging between 5–70 nm (average 26 nm) were synthesized using leaf and fungal extracts from Tahiti lime (Citrus latifolia tan) and Aspergillus flavus, respectively, and showed a minimum inhibitory concentration (MIC) of 4–128 μg/mL against multidrug-resistant P. aeruginosa strains (Table 1, entry 3) [42]. Similarly, AgNPs synthesized using culture extracts of Shewanella sp. ARY1 with an average size of 38 nm were active against E. coli and Klebsiella pneumoniae with an MBC and MIC of 32 μg/mL and 8–16 μg/mL, respectively (Table 1, entry 4), a clear manifestation of the importance of the resemblance of chemical functional groups of the nanoparticles and pathogenic bacteria as observed from the MBC of 8 μg/mL using the same E. coli corona compared to a fourfold for nanoparticles synthesized using a different bacterial extract from Shewanella sp. ARY1 (Table 1, entries 2 and 4).
Interestingly, similar-sized biosynthesized nanoparticles are obtained using extracts from the same plant extract irrespective of the plant part used, as seen from zinc oxide nanoparticles (ZnONPs) synthesized from leaf and fruit extracts of Ziziphus jujuba, which had sizes approximately 20 nm [50, 51]. On the contrary, Fe2O3NPs synthesized from the leaf extract of Daphne merezeum were relatively smaller (9.2 nm) [52] than the 16.99 nm and 13.13–24.93 nm Fe2O3NPs synthesized using extracts from Peltophorum pterocarpum [53] and Purpureocillium lilacinum [54], respectively, with different biological coronas which suggests that the size of the nanoparticles is dependent on the biochemical composition of the extracts, which varies among living organisms.
The importance of the closeness of the biological corona can also be observed from the MBC of 8 μg/mL using the same E. coli corona compared to fourfold for nanoparticles synthesised from extracts with similar corona composition from a different bacterial extract (Shewanella sp. ARY1). The importance of the closeness of the biological corona can also be observed from the MBC of 1 μg/mL vs MIC of 4–128 μg/mL using AgNPs synthesized from extracts of the same species (P. putida) and the diverse leaf/fungal extracts against resistant P. aeruginosa (Table 1, entries 1 and 3). The similarity between the functional groups retained on the AgNPs and the biological corona of the pathogens can be observed from the Fourier transform infrared (FTIR) spectra in Fig. 3, with common peaks between 3750 and 2000 cm−1 corresponding to functional groups such as hydroxyl, amine, and carbonyl groups of the sugars and proteins making up the cell walls and membranes of bacteria. The peaks in this region are present in the FTIR spectra of E. coli (Fig. 3A) and AgNPs synthesized by E. coli extract (Fig. 3B) [41]. Important to note, is the similarity in functional groups present in the AgNPs synthesized by E. coli and Shewanella sp. ARY1 extracts as seen in the FTIR spectra in Fig. 3B [41] and Fig. 3C [43], respectively. The existance of common functional groups, for example, the peaks at 3735 cm−1, 2250 cm−1, and several in the region bwtween 1000–2000 cm−1 in nanoparticles synthesized using extracts of different bacteria species not only suggests the resemblance of the biological corona among bacteria but also an indication that metal nanoparticles synthesized by bioextracts may be effective against species which bare close resemblance.
It is important to note, that chemical resemblance to pathogens can also be achieved by chemical synthesis. In work reported by Soudabeh et al. [55], very close resemblance of the FTIR spectra of poly-amidoamine modified titanium oxide nanoparticles (PAMAM/TiO2) (Fig. 4) and E. coli extract (Fig. 3A) could be among the contributors for the complete removal of the pathogen.
FTIR patterns of PAMAM/TiO2 nanohybrid [55]
Similarly, iron nanoparticles (FeNPs) synthesized using cow and goat milk had almost identical FTIR spectra because of the chemical resemblance of animal milk. Worthy to mention is the similar elemental compositions, except for the presence of sulfur in the goat milk-synthesized iron nanoparticles (GM-FeNPs) (Fig. 5A, B) [56]. The slightly greater proportion of Fe in the GM-FeNPs can be attributed to the relatively greater proportion of ligand atoms, including oxygen and phosphorous, and the presence of sulfur, which coordinates more MNPs (Fig. 5C, D). The GM-FeNPs exhibited a faster rate of degradation of methyl orange, which was attributed to a greater potential difference, likely due to the greater proportion of Fe in the GM-FeNPs.
FTIR (A and B) and EDS (C and D) spectra of the FeNPs synthesized from cow and goat milk, respectively [56]
It worth to tell that new functional groups can be formed by redox reactions between the biological corona and the metal ions, subtle chemical reactions of chemical additives or decomposition under extreme temperature and pH conditions during the synthesis of MbNPs. For example, the disappearance of prominent function groups in Surtheriandia frutescens extract and the subsequent introduction of new chemical groups in the biosynthesized nickel oxide nanoparticles using the extract reported by Motene et al. [44].
In comparisons to biosynthesized MbNPs which use mostly silver, physical and chemical methods have been used to synthesize different metal nanoparticles to mitigate various pollutants such as pathogenic microbes, hazardous inorganic and organic compounds as summarized in Table 2. The size of nanoparticles synthesized by physical/chemical method show dependency on the surface charge, crystallinity and size of the support system. For instance, all MbNPs synthesized by anchorage to polymeric support have large sizes (> 50 nm) as reported using silica gels (53–456 nm) (Table 2, entries 3 and 4), chitosan/polyurethane (Table 2, entry 10) and agarose (> 100 nm) (Table 2, entry 13) compared to < 15 nm with non-polymeric supports such as covalent organic frameworks (COF), microgels and simple inorganic and ligand supports (Table 2, entries 1, 5, 6 and 8). The ability to create crystalline phases either by the support systems, for example, the use of COF or the synthetic method used such as solvothermal and hydrothermal synthesis gives small-sized MbNPs (Table 2, entries 7, 9 and 16) which can be attributed to the orderly arrangement of crystalline phases that minimizes aggregation. Despite the subtle correlation between size and efficiency of pollutant removal by MbNPs, small-sized nanoparticles generally have a higher edge over bulky ones due to possession of superior optical and electronic properties. The relative low removal (44%) of chromium(VI) ions by reductions using gold NPs (Table 2, entry 7) is likely due to oxidation of the Au NPs by the pollutant to Au ions which are dispersed and lost in the solution. This stresses out the limitation of naked NPs during pollutant mitigation. Matrix properties such as pH also play a great role in pollutant removal. For example, the enhanced removal of S. aureus and E. coli from 65 to 100% was observed when pH increased from 5 to 7 as reported by Soudabeh et al. [55].
3 Dependency of pollutant removal efficiency on the electronic properties of the metal and nature of chemical pollutant
The performance of MbNPs in the degradation of organic dyes is highly dependent on the nature of the metal ion used, as manifested by the different degrees of degradation of methylene blue by different MbNPs (Fig. 6). The efficiency of the MbNPs correlates with the transition metal chemistry, where the lowest decomposition (85%) by ZnONPs [51] is likely due to the relatively stable Zn2+ having a stable 3d10 configuration, while the highest decomposition (94%) achieved using CuONPs [71] among the monometallic NPs is attributed to the unstable 3d9 configuration of Cu2+ anticipated to easily attain a stable 3d10 by oxidizing the dye molecules. The stable and relatively less stable 3d5 and 3d8 configurations, respectively of the metal ions in the NiO and Fe2O3 NPs, respectively, agree with the observed decomposition of 89–90%, which lies between those obtained using the stable and unstable ions of ZnO and CuO NPs, respectively. The electronic properties are further manifested by the nearly complete decomposition of methylene blue by bimetallic ZnFe2O4 NPs, likely due to the introduction of another metal [72]. However, compared with bimetallic NPs, trimetallic ZnO-ZnFe2O4 NPs performed poorly probably due to the high proportion of stable Zn2+ in the NPs [73]. The combination of more than one metal has also been used to kill pathogenic bacteria in the work reported by Zhanhua et al., where yolk-shell multifunctional nanoparticles made of Ag and Fe2O3 exhibited superparamagnetism at room temperature, which enabled the capture of 97% of bacteria using 64 μg/mL (107 CFU/mL of E. coli ER2566 cells) and the near-complete removal (≥ 99%) using double the concentration of the NPs [74].
Considering the chemistry of pollutants and media and their interactions with MbNPs not only guides the selection of metals and their most suitable oxidation states/forms but also help to predict the best class of target pollutants which maximizes the removal efficiency. For example, the aqueous environment (water) is a weak field ligand system giving rise to high spin Fe(II) and Fe(III) mixture in Fe2O3 NPs which induces paramagnetism, therefore allowing efficient magnetic adsorption of metal ions (Table 2, entry 4), which would otherwise be impossible in a medium with strong field ligands. Furthermore, in most applications, AgNPs and AuNPs remove pollutants by catalytic reduction because both the naturally existing and oxidized forms of silver and gold are stable due to their stable electronic configurations: 4d105S1/5d106S1 and 4d10/5d10, respectively. The two classes of NPs have been employed in the reduction of harmful Cr(VI) to harmless Cr(III) by Au NPs and nitroaromatic pollutants by AgNPs.
The ability to form resonance structures or stable intermediates/products during the chemical process that destroys the pollutant by degrading or transforming it to harmless compounds is key in the removal of organic compounds, and this process is usually monitored by ultraviolet/visible (UV/Vis) spectroscopy and a change in colour or the disappearance of a prominent peak is interpreted as successful removal of the pollutant. For instance, the excellent degradation of rhodamine B compared to that of tetracycline (98% vs 84%) using Au/C3N4 NPs (Table 2, entry 7) and methylene blue (83% vs 68%) by oxidation using Ag/Bi2WO3 NPs (Table 2, entry 9) and the catalytic reduction of 4-nitrophenol versus MB (95% vs 82%) using Cu and Ni NPs (Table 2, entry 13) was attributed to sequential loss of the ethyl groups of RhB by photocatalytic oxidation to N,N,N-triethyl-rhodamine, N,N-diethyl-rhodamine, N-ethyl-rhodamine and finally Rh-110, which have been monitored by UV/Vis spectroscopy and absorb at wavelengths of 539 nm, 522 nm, 510 nm and 498 nm, respectively (Fig. 7) as reported by Khanam et al. [64] and previous reports [75, 76]. The relatively low removal efficiency of MB, a close analogue of RhB, is likely due to the difficulty in losing the less bulky methyl carbonium ions, which are less stable than the ethyl carbonium ions.
UV/Vis spectra of the degradation of RhB by the catalytic oxidation of RhB to Rh-110 using Ag/Bi2WO6 NPs [64]
4 Conclusions
Metal-based nanoparticles remove a wide range of pollutants, from pathogenic bacteria to complex organic chemicals. Physically doped nanoparticles maintain their smallest size, whereas metal nanoparticles synthesized by chemical reduction and embedment on solid supports suffers from particle aggregation manifested by relatively large nanoparticles. Pathogenic microbes are efficiently removed by gold and silver NPs, and the close chemical resemblance of the biological corona/chemical functional groups of MbNPs and the pathogens enhances the removal of the latter. Generally, any transition metal can be used, and its electronic properties determine the mode of action during pollutant mitigation. In addition, the medium containing the pollutant and the nature of the pollutant also affect the removal efficiency. A weak field ligand environment promotes paramagnetism enhancing the removal of metal pollutants, while the formation of stable products increases the possibility of pollutant degradation. Enhanced pollutant removal is also achieved by use of more than one metal, metal ions with unstable d-configuration, higher metal loading and the presence of ligand atoms in chemical functional groups used to stabilize the nanoparticles. Therefore, for practical applications, the nature of MbNPs, target pollutant and their dispersion matrix should be considered. For instance, support systems/methodologies which introduce crystallinity will have versatile applications allowing use with both inert and harsh media/pollutants. In addition, for the mitigation of bio-pollutants, functional group resemblance of the chemical/bio-extract and pathogen should be put in consideration in order maximize removal efficiency.
Data availability
No datasets were generated or analysed during the current study.
References
Cebi A, Kaya Y, Gungor H, Demir H, Yoruk IH, Soylemez N, Gunes Y, Tuncer M. Trace elements, heavy metals and vitamin levels in patients with coronary artery disease. Int J Med Sci. 2011;8:456–60. https://doi.org/10.7150/ijms.8.456.
Rodríguez J, Mandalunis PM. A review of metal exposure and its effects on bone health. J Toxicol. 2018;2018:4854152. https://doi.org/10.1155/2018/4854152.
Fukushima M, Ishizaki A, Sakamoto M, Hayashi E. On distribution of heavy metals in rice field soil in the “itai-itai” disease epidemic district. Nippon Eiseigaku Zasshi Japanese J Hyg. 1970;24(5–6):526–35. https://doi.org/10.1265/jjh.24.526.
Lan SM, Amaeze NH, Obanya HE, Okoroafor CU. Occurrence of selected pharmaceuticals in industrial wastewater, receiving waters and fish. African J Aquat Sci. 2019;44(4):401–8. https://doi.org/10.2989/16085914.2019.1680339.
Nantaba F, Wasswa J, Kylin H, Palm W-U, Bouwman H, Kümmerer K. Occurrence, distribution, and ecotoxicological risk assessment of selected pharmaceutical compounds in water from lake victoria Uganda. Chemosphere. 2020. https://doi.org/10.1016/j.chemosphere.2019.124642.
Gawkrodger DJ. Occupational skin cancers. Occup Med. 2004;54(7):458–63. https://doi.org/10.1093/occmed/kqh098.
Hsu P-C, Chen I-Y, Pan C-H, Wu K-Y, Pan M-H, Chen J-R, Chen C-J, Chang-Chien G-P, Hsu C-H, Liu C-S, et al. Sperm DNA damage correlates with polycyclic aromatic hydrocarbons biomarker in coke-oven workers. Int Arch Occup Environ Health. 2006;79(5):349–56. https://doi.org/10.1007/s00420-005-0066-3.
Cabral JPS. Water microbiology bacterial pathogens and water. Int J Environ Res Public Health. 2010. https://doi.org/10.3390/ijerph7103657.
Pandey PK, Kass PH, Soupir ML, Biswas S, Singh VP. Contamination of water resources by pathogenic bacteria. AMB Express. 2014;4(1):51. https://doi.org/10.1186/s13568-014-0051-x.
Yang Z, Zhou Y, Feng Z, Rui X, Zhang T, Zhang Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers. 2019. https://doi.org/10.3390/polym11081252.
Teow YH, Mohammad AW. New generation nanomaterials for water desalination a review. Desalination. 2019;451:2–17. https://doi.org/10.1016/j.desal.2017.11.041.
Karume I, Bbumba S, Kigozi M, Nabatanzi A, Mukasa IZT, Yiga S. One-pot removal of pharmaceuticals and toxic heavy metals from water using xerogel-immobilized quartz/banana peels-activated carbon. Green Chem Lett Rev. 2023;16(1):2238726. https://doi.org/10.1080/17518253.2023.2238726.
Karume I, Bbumba S, Tewolde S, Mukasa IZT, Ntale M. Impact of carbonization conditions and adsorbate nature on the performance of activated carbon in water treatment. BMC Chem. 2023;17(1):162. https://doi.org/10.1186/s13065-023-01091-1.
Cho HH, Jung DH, Heo JH, Lee CY, Jeong SY, Lee JH. Gold nanoparticles as exquisite colorimetric transducers for water pollutant detection. ACS Appl Mater Interfaces. 2023;15(16):19785–806. https://doi.org/10.1021/acsami.3c00627.
Mocan T, Matea CT, Pop T, Mosteanu O, Buzoianu AD, Puia C, Iancu C, Mocan L. Development of nanoparticle-based optical sensors for pathogenic bacterial detection. J Nanobiotechnol. 2017;15(1):25. https://doi.org/10.1186/s12951-017-0260-y.
Verma MS, Rogowski JL, Jones L, Gu FX. Colorimetric biosensing of pathogens using gold nanoparticles. Biotechnol Adv. 2015;33:666–80. https://doi.org/10.1016/j.biotechadv.2015.03.003.
Ibrahim NH, Taha GM, Hagaggi NSA, Moghazy MA. Green synthesis of silver nanoparticles and its environmental sensor ability to some heavy metals. BMC Chem. 2024;18(1):7. https://doi.org/10.1186/s13065-023-01105-y.
Hlongwane GN, Sekoai PT, Meyyappan M, Moothi K. Simultaneous removal of pollutants from water using nanoparticles: a shift from single pollutant control to multiple pollutant control. Sci Total Environ. 2019;656:808–33. https://doi.org/10.1016/j.scitotenv.2018.11.257.
Soni R, Pal AK, Tripathi P, Lal JA, Kesari K, Tripathi V. An overview of nanoscale materials on the removal of wastewater contaminants. Appl Water Sci. 2020;10(8):189. https://doi.org/10.1007/s13201-020-01275-3.
Borzenkov M, Pallavicini P, Taglietti A, D’Alfonso L, Collini M, Chirico G. Photothermally active nanoparticles as a promising tool for eliminating bacteria and biofilms. Beilstein J Nanotechnol. 2020;11:1134–46. https://doi.org/10.3762/bjnano.11.98.
Khantamat O, Li C-H, Yu F, Jamison AC, Shih W-C, Cai C, Lee TR. Gold nanoshell-decorated silicone surfaces for the near-infrared (nir) photothermal destruction of the pathogenic bacterium E. Faecalis ACS Appl Mater Interfaces. 2015;7(7):3981–93. https://doi.org/10.1021/am506516r.
Wu K, Li H, Cui X, Feng R, Chen W, Jiang Y, Tang C, Wang Y, Wang Y, Shen X, et al. Mutagenesis and resistance development of bacteria challenged by silver nanoparticles. Antimicrob Agents Chemother. 2022;66(10):e00628-e722. https://doi.org/10.1128/aac.00628-22.
Romero D, Aguilar C, Losick R, Kolter R. Amyloid fibers provide structural integrity to bacillus subtilis biofilms. Proc Natl Acad Sci U S A. 2010;107(5):2230–4. https://doi.org/10.1073/pnas.0910560107.
Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomed. 2017;12:1227–49. https://doi.org/10.2147/IJN.S121956.
Benoit DSW, Sims KRJ, Fraser D. Nanoparticles for oral biofilm treatments. ACS Nano. 2019;13(5):4869–75. https://doi.org/10.1021/acsnano.9b02816.
Nguyen T-K, Lam SJ, Ho KKK, Kumar N, Qiao GG, Egan S, Boyer C, Wong EHH. Rational design of single-chain polymeric nanoparticles that kill planktonic and biofilm bacteria. ACS Infect Dis. 2017;3(3):237–48. https://doi.org/10.1021/acsinfecdis.6b00203.
Liu Y, Naha PC, Hwang G, Kim D, Huang Y, Simon-Soro A, Jung H-I, Ren Z, Li Y, Gubara S, et al. Topical ferumoxytol nanoparticles disrupt biofilms and prevent tooth decay in vivo via intrinsic catalytic activity. Nat Commun. 2018;9(1):2920. https://doi.org/10.1038/s41467-018-05342-x.
Kim JS, Kuk E, Yu KN, Kim J-H, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang C-Y, et al. Antimicrobial effects of silver nanoparticles. Nanomed Nanotechnol Biol Med. 2007;3(1):95–101. https://doi.org/10.1016/j.nano.2006.12.001.
Gupta D, Singh A, Khan AU. Nanoparticles as efflux pump and biofilm inhibitor to rejuvenate bactericidal effect of conventional antibiotics. Nanoscale Res Lett. 2017;12(1):454. https://doi.org/10.1186/s11671-017-2222-6.
Hwang ET, Lee JH, Chae YJ, Kim YS, Kim BC, Sang B-I, Gu MB. Analysis of the toxic mode of action of silver nanoparticles using stress-specific bioluminescent bacteria. Small. 2008;4(6):746–50. https://doi.org/10.1002/smll.200700954.
Li WR, Xie XB, Shi QS, Zeng HY, You-Sheng OU-Yang, Yi-Ben Chen. Antibacterial activity and mechanism of silver nanoparticles on escherichia coli. Appl Microbiol Biotechnol. 2010;85(4):1115–22. https://doi.org/10.1007/s00253-009-2159-5.
García-Contreras R, Argueta-Figueroa L, Mejía-Rubalcava C, Jiménez-Martínez R, Cuevas-Guajardo S, Sánchez-Reyna PA, Mendieta-Zeron H. Perspectives for the use of silver nanoparticles in dental practice. Int Dent J. 2011;61(6):297–301. https://doi.org/10.1111/j.1875-595X.2011.00072.x.
Yu H, Fu J, Zhu X, Zhao Z, Sui X, Sun S, He X, Zhang Y, Ye W. Tribocatalytic degradation of organic pollutants using fe2o3 nanoparticles. ACS Appl Nano Mater. 2023;6(15):14364–73. https://doi.org/10.1021/acsanm.3c02360.
Fang Y, Li Y, Zhou F, Gu P, Liu J, Chen D, Li N, Xu Q, Lu J. An efficient photocatalyst based on black tio2 nanoparticles and porous carbon with high surface area: degradation of antibiotics and organic pollutants in water. Chempluschem. 2019;84(5):474–80. https://doi.org/10.1002/cplu.201900103.
Caruntu D, Caruntu G, Chen Y, O’Connor CJ, Goloverda G, Kolesnichenko VL. Synthesis of variable-sized nanocrystals of Fe3o4 with high surface reactivity. Chem Mater. 2004;16(25):5527–34. https://doi.org/10.1021/cm0487977.
Qu Y, Li X, Lian S, Dai C, Jv Z, Zhao B, Zhou H. Biosynthesis of gold nanoparticles using fungus trichoderma Sp WL-Go and their catalysis in degradation of aromatic pollutants. IET Nanobiotechnol. 2019;13(1):12–7. https://doi.org/10.1049/iet-nbt.2018.5177.
Gole A, John D, Krishnamoorthy K, Wagh NS, Lakkakula J, Khan MS, Odeibat HAM, Tarique M, Islam MR. Role of phytonanotechnology in the removal of water contamination. J Nanomater. 2022;2022:7957007. https://doi.org/10.1155/2022/7957007.
Santhosh PB, Genova J, Chamati H. Green synthesis of gold nanoparticles: an eco-friendly approach. Chemistry (Easton). 2022;4(2):345–69. https://doi.org/10.3390/chemistry4020026.
Jamkhande PG, Ghule NW, Bamer AH, Kalaskar MG. Metal nanoparticles synthesis: an overview on methods of preparation, advantages and disadvantages, and applications. J Drug Deliv Sci Technol. 2019. https://doi.org/10.1016/j.jddst.2019.101174.
Fanta FT, Dubale AA, Bebizuh DF, Atlabachew M. Copper doped zeolite composite for antimicrobial activity and heavy metal removal from waste water. BMC Chem. 2019;13(1):44. https://doi.org/10.1186/s13065-019-0563-1.
Singh P, Mijakovic I. Antibacterial effect of silver nanoparticles is stronger if the production host and the targeted pathogen are closely related. Biomedicines. 2022. https://doi.org/10.3390/biomedicines10030628.
Campo-Beleño C, Villamizar-Gallardo RA, López-Jácome LE, González EE, Muñoz-Carranza S, Franco B, Morales-Espinosa R, Coria-Jimenez R, Franco-Cendejas R, Hernández-Durán M, et al. Biologically synthesized silver nanoparticles as potent antibacterial effective against multidrug-resistant pseudomonas aeruginosa. Lett Appl Microbiol. 2022;75(3):680–8. https://doi.org/10.1111/lam.13759.
Mondal AH, Yadav D, Mitra S. Biosynthesis of silver nanoparticles using culture supernatant of shewanella Sp. ARY1 and their antibacterial activity. Int J Nanomed. 2020;15:8295–310. https://doi.org/10.2147/IJN.S274535.
Motene K, Mahlaule-Glory LM, Ngoepe NM, Mathipa MM, Hintsho-Mbita NC. Photocatalytic degradation of dyes and removal of bacteria using biosynthesised flowerlike Nio nanoparticles. Int J Environ Anal Chem. 2023;103(5):1107–22. https://doi.org/10.1080/03067319.2020.1869730.
Khan R, Hoque SM, Hossain KFB, Siddique AB, Uddin K, Rahman M. Green synthesis of silver nanoparticles using ipomoea aquatica leaf extract and its cytotoxicity and antibacterial activity assay. Green Chem Lett Rev. 2020;13(4):303–15. https://doi.org/10.1080/17518253.2020.1839573.
Verma V, Singh M, Pal Singh P, Singh J, Rawat M. Highly stable Au/Ag core-shell nanoparticles prepared via novel green approach for the abatement of nitro pollutants. Micro Nano Lett. 2020. https://doi.org/10.1049/mnl.2020.0275.
Moustafa MT. Removal of pathogenic bacteria from wastewater using silver nanoparticles synthesized by two fungal species. Water Sci. 2017;31(2):164–76. https://doi.org/10.1016/j.wsj.2017.11.001.
Singh J, Kumar V, Singh Jolly S, Kim K-H, Rawat M, Kukkar D, Tsang YF. Biogenic synthesis of silver nanoparticles and its photocatalytic applications for removal of organic pollutants in water. J Ind Eng Chem. 2019;80:247–57. https://doi.org/10.1016/j.jiec.2019.08.002.
Shad S, Belinga-Desaunay-Nault MFA, Sohail NB, Lynch I. Removal of contaminants from canal water using microwave synthesized zero valent iron nanoparticles. Environ Sci Water Res Technol. 2020;6(11):3057–65. https://doi.org/10.1039/D0EW00157K.
Alharthi MN, Ismail I, Bellucci S, Salam MA. Green synthesis of zinc oxide nanoparticles by ziziphus jujuba leaves extract: environmental application, kinetic and thermodynamic studies. J Phys Chem Solids. 2021. https://doi.org/10.1016/j.jpcs.2021.110237.
Golmohammadi M, Honarmand M, Ghanbari S. A Green approach to synthesis of ZnO nanoparticles using jujube fruit extract and their application in photocatalytic degradation of organic dyes. Spectrochim Acta Part A Mol Biomol Spectrosc. 2020. https://doi.org/10.1016/j.saa.2019.117961.
Beheshtkhoo N, Kouhbanani MAJ, Savardashtaki A, Amani AM, Taghizadeh S. Green synthesis of iron oxide nanoparticles by aqueous leaf extract of daphne mezereum as a novel dye removing material. Appl Phys A. 2018;124(5):363. https://doi.org/10.1007/s00339-018-1782-3.
Anchan S, Pai S, Sridevi H, Varadavenkatesan T, Vinayagam R, Selvaraj R. Biogenic synthesis of ferric oxide nanoparticles using the leaf extract of peltophorum pterocarpum and their catalytic dye degradation potential. Biocatal Agric Biotechnol. 2019. https://doi.org/10.1016/j.bcab.2019.101251.
Hammad EN, Salem SS, Mohamed AA, El-Dougdoug W. Environmental impacts of ecofriendly iron oxide nanoparticles on dyes removal and antibacterial activity. Appl Biochem Biotechnol. 2022;194(12):6053–67. https://doi.org/10.1007/s12010-022-04105-1.
Matboo SA, Nazari S, Niapour A, Niri MV, Asgari E, Mokhtari SA. Antibacterial effect of TiO2 modified with poly-amidoamine dendrimer – g3 on s aureus and e coli in aqueous solutions. Water Sci Technol. 2022;85(2):605–16. https://doi.org/10.2166/wst.2022.007.
Isha Gautam TG, Fernando H. Degradation of the dye methyl orange using cow and goat milk iron nanoparticles. Green Chem Lett Rev. 2023;16(1):2174818. https://doi.org/10.1080/17518253.2023.2174818.
Islam MT, Dominguez A, Alvarado-Tenorio B, Bernal RA, Montes MO, Noveron JC. Sucrose-mediated fast synthesis of zinc oxide nanoparticles for the photocatalytic degradation of organic pollutants in water. ACS Omega. 2019;4(4):6560–72. https://doi.org/10.1021/acsomega.9b00023.
Liu J, Chen H, Shi X, Nawar S, Werner JG, Huang G, Ye M, Weitz DA, Solovev AA, Mei Y. Hydrogel microcapsules with photocatalytic nanoparticles for removal of organic pollutants. Environ Sci Nano. 2020;7(2):656–64. https://doi.org/10.1039/C9EN01108K.
Shishehbore MR, Afkhami A, Bagheri H. Salicylic acid functionalized silica-coated magnetite nanoparticles for solid phase extraction and preconcentration of some heavy metal ions from various real samples. Chem Cent J. 2011;5(1):41. https://doi.org/10.1186/1752-153X-5-41.
Gong T, Zhou Y, Sun L, Liang W, Yang J, Shuang S, Dong C. Effective adsorption of phenolic pollutants from water using β-cyclodextrin polymer functionalized Fe3o4 magnetic nanoparticles. RSC Adv. 2016;6(84):80955–63. https://doi.org/10.1039/C6RA16383A.
Agarwal S, Phukan P, Sarma D, Deori K. Versatile precursor-dependent copper sulfide nanoparticles as a multifunctional catalyst for the photocatalytic removal of water pollutants and the synthesis of aromatic aldehydes and NH-triazoles. Nanoscale Adv. 2021;3(13):3954–66. https://doi.org/10.1039/D1NA00239B.
Chang Y, Liu Z, Shen X, Zhu B, Macharia DK, Chen Z, Zhang L. Synthesis of Au nanoparticle-decorated carbon nitride nanorods with plasmon-enhanced photoabsorption and photocatalytic activity for removing various pollutants from water. J Hazard Mater. 2018;344:1188–97. https://doi.org/10.1016/j.jhazmat.2017.10.040.
Chatterjee A, Kar P, Wulferding D, Lemmens P, Pal SK. Flower-Like BiOI microspheres decorated with plasmonic gold nanoparticles for dual detoxification of organic and inorganic water pollutants. ACS Appl Nano Mater. 2020;3(3):2733–44. https://doi.org/10.1021/acsanm.0c00090.
Khanam S, Rout SK. Enhanced photocatalytic oxidation of rhb and MB using plasmonic performance of Ag deposited on Bi2WO6. Chemistry (Easton). 2022;4(2):272–96. https://doi.org/10.3390/chemistry4020022.
Sasidharan AP, Meera V, Raphael VP. Coliform removal efficacy of polyurethane foam impregnated with chitosan nanoparticles and silver/silver oxide nanoparticles. Water Supply. 2022;22(5):5675–87. https://doi.org/10.2166/ws.2022.186.
Zahid S, Alzahrani AK, Kizilbash N, Ambreen J, Ajmal M, Farooqi ZH, Siddiq M. Preparation of stimuli responsive microgel with silver nanoparticles for biosensing and catalytic reduction of water pollutants. RSC Adv. 2022;12(51):33215–28. https://doi.org/10.1039/D2RA05475B.
Keerthana M, Malini TP, Kamaraj P, Vivekanand PA, Arulnangai R, Kumar SJS, Harikumar S, Arumugam N, Almansour AI, Perumal K. Efficient photocatalytic degradation of water pollutant brufen using lutetium doped cerium oxide nanoparticles synthesized by chemical precipitation method. J Taiwan Inst Chem Eng. 2023. https://doi.org/10.1016/j.jtice.2023.105118.
Kamal T, Asiri AM, Ali N. Catalytic reduction of 4-nitrophenol and methylene blue pollutants in water by copper and nickel nanoparticles decorated polymer sponges. Spectrochim Acta Part A Mol Biomol Spectrosc. 2021. https://doi.org/10.1016/j.saa.2021.120019.
Wang Y, Liu J, Wang P, Werth CJ, Strathmann TJ. Palladium nanoparticles encapsulated in core-shell silica: a structured hydrogenation catalyst with enhanced activity for reduction of oxyanion water pollutants. ACS Catal. 2014;4(10):3551–9. https://doi.org/10.1021/cs500971r.
Wang R-L, Li D-P, Wang L-J, Zhang X, Zhou Z-Y, Mu J-L, Su Z-M. The preparation of new covalent organic framework embedded with silver nanoparticles and its applications in degradation of organic pollutants from waste water. Dalt Trans. 2019;48(3):1051–9. https://doi.org/10.1039/C8DT04458A.
Rafique M, Shaikh AJ, Rasheed R, Tahir MB, Gillani SSA, Usman A, Imran M, Zakir A, Khan ZUH, Rabbani F. Aquatic biodegradation of methylene blue by copper oxide nanoparticles synthesized from azadirachta indica leaves extract. J Inorg Organomet Polym Mater. 2018;28(6):2455–62. https://doi.org/10.1007/s10904-018-0921-9.
Krishnan S, Murugesan S, Vasanthakumar V, Priyadharsan A, Alsawalha Murad, Alomayri Thamer, Yuan Baoling. Facile green synthesis of ZnFe2O4/RGO nanohybrids and evaluation of its photocatalytic degradation of organic pollutant photo antibacterial and cytotoxicity activities. Colloids Surfaces A Physicochem Eng Asp. 2021. https://doi.org/10.1016/j.colsurfa.2020.125835.
Sahoo SK, Panigrahi GK, Sahoo A, Pradhan AK, Dalbehera A. Bio-hydrothermal synthesis of ZnO–ZnFe2O4 nanoparticles using psidium guajava leaf extract: role in waste water remediation and plant immunity. J Clean Prod. 2021. https://doi.org/10.1016/j.jclepro.2021.128522.
Wei Z, Zhou Z, Yang M, Lin C, Zhao Z, Huang D, Chen Z, Gao J. Multifunctional Ag@Fe2O3 yolk-shell nanoparticles for simultaneous capture{,} Kill{,} and removal of pathogen. J Mater Chem. 2011;21(41):16344–8. https://doi.org/10.1039/C1JM13691G.
Liang H, Liu S, Zhang H, Wang X, Wang J. New insight into the selective photocatalytic oxidation of rhb through a strategy of modulating radical generation. RSC Adv. 2018;8(24):13625–34. https://doi.org/10.1039/C8RA01810C.
Liang H, Jia Z, Zhang H, Wang X, Wang J. Photocatalysis oxidation activity regulation of Ag/TiO2 composites evaluated by the selective oxidation of rhodamine B. Appl Surf Sci. 2017;422:1–10. https://doi.org/10.1016/j.apsusc.2017.05.211.
Acknowledgements
The authors acknowledge the facilitation by Makerere University to access the literature used in writing this review.
Funding
The authors did not receive funding for writing this review.
Author information
Authors and Affiliations
Contributions
I.K., M.K., and A.N. wrote the main manuscript. S.B. gathered all the research articles used, populated the data in tables and generated figures. M.M.A. and H.K.N. correlated the findings and did proofreading and extensive language editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Karume, I., Kigozi, M., Nabatanzi, A. et al. Impact of synthetic method and metal type on the efficiency of metal-based nanoparticles against pathogens and chemical pollutants. Discov. Chem. 1, 16 (2024). https://doi.org/10.1007/s44371-024-00020-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44371-024-00020-y