Abstract
Introduction
Massive hemorrhage protocols are widely used to facilitate the administration of blood components to bleeding trauma patients. Delays in this process are associated with worse patient outcomes. We used in situ simulation as a novel and iterative quality improvement technique to reduce the mean time between massive hemorrhage protocol activation and blood administration during actual trauma resuscitations.
Methods
We completed monthly, risk-informed unannounced in situ trauma simulations at a Canadian Level 1 trauma centre. We identified three major latent safety threats: (1) massive hemorrhage protocol activation; (2) transport of blood components; and (3) situational awareness of team members. Process improvements for each latent safety threats were tested and implemented during subsequent in situ simulation sessions. We evaluated the effect of this simulation-based intervention on the care of patients before, during and after the intervention. Demographic, clinical and massive hemorrhage protocol data were collected. The primary outcome was mean time between massive hemorrhage protocol activation and blood administration during actual trauma resuscitations as analyzed using a two-sample t test.
Results
Each group was similar in demographic and injury characteristics. The time from massive hemorrhage protocol activation to blood administration decreased from 11.6 min pre-intervention to 9.1 min post-intervention. This represented a significant reduction (2.5 min, 95% confidence interval, 0.03–5.08) following the in situ simulation-based quality improvement intervention.
Conclusions
A comprehensive, in situ simulation-based quality improvement project was associated with a significant reduction in the mean time between massive hemorrhage protocol activation and blood administration among injured patients. In situ simulation represents a novel approach to the identification and mitigation of latent safety threats during massive hemorrhage protocol activation.
Résumé
Introduction
Les protocoles d’hémorragie massive sont largement utilisés pour faciliter l’administration de composants sanguins aux patients souffrant de traumatismes hémorragiques. Les retards dans ce processus sont associés à de pires résultats pour les patients. Nous avons utilisé la simulation in situ comme une technique novatrice et itérative d'amélioration de la qualité pour réduire le temps moyen entre l'activation du protocole d'hémorragie massive et l'administration de sang lors des réanimations de traumatismes réels.
Les méthodes
Nous avons effectué des simulations mensuelles de traumatismes in situ, sans préavis et en tenant compte des risques, dans un centre de traumatologie de niveau 1 au Canada. Nous avons identifié trois grandes menaces latentes pour la sécurité : 1) l'activation du protocole d'hémorragie massive ; 2) le transport de composants sanguins ; et 3) la connaissance de la situation des membres de l'équipe. Des améliorations de processus pour chaque menace latente à la sécurité ont été testées et mises en œuvre lors de séances de simulation in situ subséquentes. Nous avons évalué l'effet de cette intervention basée sur la simulation sur la prise en charge des patients avant, pendant et après l'intervention. Des données démographiques, cliniques et de protocole d'hémorragie massive ont été recueillies. Le critère de jugement principal était le temps moyen entre l'activation du protocole d'hémorragie massive et l'administration de sang pendant les réanimations traumatiques réelles, tel qu'analysé à l'aide d'un test t à deux échantillons.
Résultats
Chaque groupe était similaire en termes de caractéristiques démographiques et de blessures. Le temps entre l'activation du protocole d'hémorragie massive et l'administration de sang est passé de 11,6 minutes avant l'intervention à 9,1 minutes après l'intervention. Cela a représenté une réduction significative (2,5 minutes, intervalle de confiance de 95%, 0,03 à 5,08) suite à l'intervention d'amélioration de la qualité basée sur la simulation in situ.
Conclusions
Un projet exhaustif d'amélioration de la qualité basé sur une simulation in situ a été associé à une réduction significative du temps moyen entre l'activation du protocole d'hémorragie massive et l'administration de sang chez les patients blessés. La simulation in situ représente une nouvelle approche pour l'identification et l'atténuation des menaces latentes pour la sécurité lors de l'activation du protocole d'hémorragie massive.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
What is known about the topic? |
The timely administration of blood is linked closely to the survival of bleeding trauma patients. |
What did this study ask? |
Can in situ simulation identify delays and improve the timely administration of blood for patients requiring massive transfusions? |
What did this study find? |
In situ simulation was associated with a 21% relative reduction in the time between massive hemorrhage protocol activation and blood component administration. |
Why does this matter to clinicians? |
Simulation as a technique to improve time to blood component administration may positively impact survival among bleeding patients. |
Introduction
Hemorrhage remains the leading cause of preventable death after trauma [1]. Damage control resuscitation is now the preferred strategy to manage hemorrhaging trauma patients as it results in improved clinical outcomes and patient survival [2]. This approach prioritizes early, ratio-based, blood component administration and prompt definitive hemostasis. Many of these patients require a massive transfusion, typically defined as > 10 units of packed red blood cells (PRBCs) in 24 h [3]. Massive hemorrhage protocols facilitate the transport and administration of large volumes of blood components resulting in improved survival and reductions in multisystem organ failure, blood component wastage and transfusion-related complications [4–7]. A key metric of massive hemorrhage protocol performance is the time between protocol activation and blood component administration [8]. Delays to blood component administration are associated with worse patient outcomes, highlighting the importance of optimizing the blood delivery process [9].
A massive hemorrhage protocol is a multi-step process with a coordinated effort among a multi-disciplinary team of transfusion medicine, core laboratory, logistical, and clinical staff [3]. Its inherent complexity increases the potential for errors or delays that may lead to a negative impact on patient outcomes. The evaluation of system-based safety threats and barriers to efficient blood administration following massive hemorrhage protocol activation is a crucial step in the care of bleeding trauma patients.
In situ simulation is simulation that occurs within the actual clinical workspace. It provides a unique opportunity to diagnose gaps in system-based and process issues [10]. These hazards, labelled as latent safety threats, represent “system-based threats to patient safety that can materialize at any time and are previously unrecognized by healthcare providers” [11]. The real-life applicability of in situ simulation allows simulation facilitators to recreate high-stakes situations and thereby predictably expose potential latent safety threats [12, 13].
We used in situ simulation as a novel, prospective, quality improvement method to identify opportunities and change our processes to reduce the time between massive hemorrhage protocol activation and blood component administration. We evaluated the effect of this simulation-based intervention on the care of patients before and after its implementation. Specifically, we compared the mean time between massive hemorrhage protocol activation and blood component administration before and after an in situ simulation-based quality improvement intervention.
Methods
Setting and patients
We conducted this study at a Canadian Level 1 trauma center with approximately 80,000 emergency department (ED) visits and 1100 trauma team activations, annually. This study was reviewed and approved by the St Michaels Hospital Research Ethics Board (REB # 16-304).
All trauma patients were prospectively included in our local trauma registry. Data collection and management were overseen by a dedicated trauma research nurse. We performed a retrospective analysis of trauma patients requiring massive hemorrhage protocol activations from Jan 2014 to Dec 2017 identified in our trauma registry. We divided this study into three phases: (1) pre-intervention (January 2014–July 2015), (2) intervention (August 2015–July 2016) and (3) post-intervention (Aug 2016 to July 2017), which represented approximately 12 months before, during and after the completion of the in situ simulation-based quality improvement intervention, respectively. Given the in situ simulation intervention spanned 12 months, we planned to compare with one year before and one year after, expecting an approximately equal number of massive hemorrhage protocol activations for each time interval. We extended the pre-intervention phase to 18 months due to a limited number of massive hemorrhage protocol activations identified.
Inclusion and exclusion criteria
All trauma patients > 18 years of age during the study period who required massive hemorrhage protocol and received their first blood component in the trauma bay were eligible for inclusion. We excluded patients who were declared dead before blood component administration and who received their first blood component after leaving the trauma bay (e.g., operating room). All charts were reviewed by one author (AG) to determine study eligibility. A second physician (AP) independently reviewed 10% of all charts and with complete agreement, none were deemed ineligible. To further ensure we captured all eligible patients, we reviewed all massive hemorrhage protocol trauma activations from our institutional transfusion medicine database and no additional patients were missed.
Summary of massive hemorrhage protocol before in situ simulation-based quality improvement interventions
-
1.
The massive hemorrhage protocol was activated at the discretion of the trauma lead physician who communicated this to the trauma team.
-
2.
A trauma team nurse made two calls: first to notify the transfusion medicine laboratory of the need for massive hemorrhage protocol, and second to hospital locating to request a porter for blood component transport.
-
3.
The porter arrived in the trauma bay to gather requisition forms with patient identifiers and brought this to the transfusion medicine laboratory which released the first cooler of blood components.
-
4.
The porter transported the blood components to the trauma bay, where two nurses checked the blood components before administration.
-
5.
Blood components continued to be released in a 1:1:2 ratio (fresh frozen plasma units:dose of platelets:red blood cell units) until the most responsible physician terminated the protocol.
In situ simulation-based quality improvement intervention
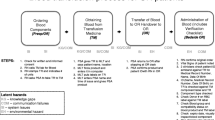
In July 2015 we began the TRUST (Trauma Resuscitation Using in Situ simulation for Training) study, and a full description of the protocol is published elsewhere [14]. The study involved 12 monthly, risk-informed unannounced in situ simulation scenarios for the on-call trauma team. After the first two simulation scenarios requiring massive hemorrhage protocol activations, we identified three high-risk latent safety threats related to the protocol. We reviewed the process supported by video evidence through a human factors perspective and developed process improvement strategies that we tested using simulation before implementation with patients (Table 1). A total of eight in situ simulation sessions required massive hemorrhage protocol activation though it was a primary objective in only four sessions. We notified ED and hospital personnel involved in the massive hemorrhage protocol (including study participants) of identified latent safety threats and subsequent protocol changes via team meetings, intra-departmental huddles, educational sessions and email notifications. We devised these strategies in keeping with the hierarchy of intervention effectiveness (Fig. 1), which suggests that interventions focused on system-level changes, in contrast to those reliant on individuals’, are more likely to result in change [15]. A potentially more convenient solution would be a blood component containing fridge but this was not feasible within our institution throughout the study period.
Data collection/analysis
We used a standardized data collection form to extract demographic, clinical and time metric data for all patients who met inclusion criteria. We defined the primary outcome as the time between massive hemorrhage protocol activation and blood component administration and compared these findings before and after an in situ simulation-based quality improvement initiative. We selected this outcome as it is listed as a key performance indicator by the American College of Surgeons trauma transfusion quality improvement program [9]. One study author (AG) manually reviewed all of the nursing documentation for both massive hemorrhage protocol activation and blood administration times. In cases where documentation was lacking, we used data from the transfusion medicine paper registry. When neither the nursing chart nor transfusion medicine data indicated the time of protocol activation, we used the time of patient arrival in the trauma bay. A senior author (AP) reviewed all charts which contained discrepancies in documentation between the transfusion medicine database and trauma registry. Together both reviewers came to a consensus regarding patient inclusion in the study and time measures.
Descriptive statistics were performed. Univariate tests of interests were compared across groups using an ANOVA or Kruskal Wallace test as appropriate for continuous data and Chi-square tests (or Fisher exact tests as necessary) for categorical data. A two-sample t test with pooled variance was used to analyze the primary outcome defined as the time between massive hemorrhage protocol activation and blood component administration with a comparison of the pre-intervention and post-intervention period. To compare the proportion of cases that met recommendations that blood components be available within 10 min between study phases, odds ratios were used [9, 16]. A two-sided significance level of 0.05 was used to assess statistical significance. Statistical analysis was performed using Excel 2015 (Microsoft Corp., Redmond, WA) and R software (https://www.R-project.org). The run chart was generated following typical processes and evaluated for run chart rules according to accepted principles [17, 18].
Results
Simulation outcomes
To ensure our process improvement strategies worked as intended, we tested, using in situ simulation, our updated massive hemorrhage protocol integrated with the process improvement strategies listed in Table 1. We observed a mean decrease in time between protocol activation and blood component administration from 20.5 to 10.25 min (difference of 10.25 min, 95% CI 3.1–17.4). We updated our original institutional massive hemorrhage protocol to include these changes and we integrated it into clinical care in August 2016.
Clinical outcomes
We reviewed 185 patient records for eligibility during the 3-year study period, and 145 patients met inclusion criteria (Fig. 2). Massive hemorrhage protocol activation times were missing from the nursing documentation in 12 pre-intervention, 28 intervention and 15 post-intervention charts. We used data from our blood bank in 7 pre-intervention, 10 intervention and 7 post-intervention charts while patient arrival time was used as massive hemorrhage protocol activation time in 5, 18 and 8 cases, respectively.
We excluded patients if they first received blood in the operating room (massive hemorrhage protocol OR, n = 6), the intensive care unit (massive hemorrhage protocol ICU, n = 1), or in diagnostic imaging (massive hemorrhage protocol CT, n = 2). We excluded patients if the time of blood administration was not documented (n = 17) including 2 from the pre-intervention, 10 from the intervention and 5 from the post-intervention groups. Additionally, we excluded patients when the massive hemorrhage protocol was activated but that clinical documentation explicitly stated that blood was never administered (n = 14). Each group was similar in demographic data, trauma characteristics and injury severity score (Table 2). Two exceptions were the number of nurses in the trauma bay and the post-trauma bay disposition.
The primary outcome, the mean time between massive hemorrhage protocol activation and blood component administration, decreased from 11.6 min pre-intervention to 9.1 min post-intervention. This represents a significant relative reduction of 21% (2.5 min, 95% CI, 0.03–5.08, p = 0.047) sustained over one year following an in situ simulation-based quality improvement intervention. During the intervention, the mean time between massive hemorrhage protocol activation and blood component administration was 10.4 min. A run chart (Fig. 3) illustrates the time to blood component administration throughout the study period. The median time decreases from 10 min in the pre-intervention to 9.5 min in the intervention and to 8 min in the post-intervention phase. Rules are met in each of the study phases: one shift above median (i.e. longer time) in the pre-intervention phase; one shift above and one shift below the median as well as a trend going up (i.e. increased time) in the intervention phase; and one shift below the median in the post-intervention phase.
There was no difference in the proportion of patients who received blood components in less than 10 min from the time of protocol activation between the post-intervention and pre-intervention (OR 1.26, 0.55–2.85, p = 0.59), intervention and pre-intervention (OR 0.54, 0.23–1.28, p = 0.16) or post-intervention and intervention (OR 0.68, 0.31–1.53, p = 0.36).
Discussion
We applied a novel in situ simulation-based quality improvement process to improve blood component administration for bleeding trauma patients resulting in a sustained 21% (2.5 min) mean reduction in time between massive hemorrhage protocol activation and blood component administration. While a reduction of 2.5 min may not seem substantial, one study found a 5% increased odds of death associated with every minute delay to blood product delivery [9]. Extrapolating these findings to our study would suggest that our initiative could have been associated with a reduction of > 10% odds of death for patients requiring blood components. Optimizing this complex process is essential and directly impacts patient outcomes.
Medical errors are frequently attributed to breakdowns in the coordination of staff availability, team interaction, equipment design, inter-departmental coordination, and task complexity [19, 20]. Many of these elements exist within a massive hemorrhage protocol and as such, efforts to closely scrutinize each step is essential [21]. In situ simulation, a workplace-based simulation technique, is increasingly recognized as a means to identify latent safety threats [22–25] and that regular in situ simulation is associated with improved cardiac arrest survival [26–28]. While these data sets are observational and limit causal conclusions, it likely represents a signal that in situ simulation as a technique can impact patient outcomes. The application of in situ simulation to improve trauma care, however, includes only a few studies linking in situ simulation training with improved trauma team performance [29–31].
In our study, we used in situ simulation to identify massive hemorrhage protocol-related latent safety threats and to pilot improvement efforts before clinical implementation. This can be described as “crash testing” the system, akin to how car manufacturer’s test a vehicle’s response in a collision and modify designs in a controlled environment [11]. Using a combination of direct observation and participant feedback, we identified latent safety threats and inefficiencies that existed within our institution’s massive hemorrhage protocol. For example, while nurses had informally described the challenges with making two phone calls to activate the protocol, our study highlighted through direct observation how task overloaded they were during a high-stakes trauma resuscitation. This provided the necessary evidence to our institution’s administrative team to initiate process changes. Following the hierarchy of intervention effectiveness, we made modifications that followed principles of automation and standardization over education to increase the likelihood of success. For example, shifting to a one-call protocol activation process eliminated the possibility of neglecting to call both transfusion medicine laboratory and our hospital’s switchboard.
Beyond using in situ simulation to simply identify latent safety threats, we tested proposed changes including the one-call process before it was adopted as a formal policy. This is incredibly important because we discovered a technological glitch with our trauma bay phone not having the ability to be forwarded. While it was easily fixed by our IT team, it highlighted that even the best-intentioned changes warrant dedicated testing as they may suffer from unintended consequences when actioned into clinical workflows.
The importance of efficient interventions for the bleeding trauma patient cannot be understated, with guidelines now recommending that red blood cells be available within 10 min of massive hemorrhage protocol activation, in part, based on the demonstrated link between time to blood administration and clinical outcomes [9, 16]. Traditional quality improvement efforts are excellent at improving processes; however, identifying potential targets for improvement can be elusive. In situ simulation can be used to precisely reveal troublesome elements and pilot quality improvement interventions before patients are impacted to ensure changes function as intended. Based on our experience using in situ simulation to identify and inform massive hemorrhage protocol changes and the positive impact on patient-oriented outcomes, we advocate that in situ simulation be used as a standard process for trauma centers support the optimization of their massive hemorrhage protocol. Additionally, in situ simulation allowed us to effectively engage multiple specialties together and work towards a shared goal.
The process of blood component administration had substantial variability throughout all stages of the project as shown in the run chart, highlighting the inherent challenges related to efforts to improve the massive hemorrhage protocol process during high-stakes resuscitations. While some run chart rules were met in all phases of the project, detailed review of the circumstances surrounding each cluster by the team failed to uncover contributing factors temporally associated with these. We continue to monitor blood delivery metrics through our trauma registry and blood bank to identify further improvement opportunities.
Beyond the level of individual institutions, accreditation bodies may seek to consider the role for in situ simulation for massive hemorrhage protocol evaluation. One might imagine a future where trauma centers are required to regularly conduct in situ simulation sessions to evaluate their massive hemorrhage protocol, make improvements to the process and train their teams using this simulation technique. Future studies can be used to explore in situ simulation for improving other systems processes in the trauma bay or to evaluate in situ simulation in other clinical settings, such as the OR or ICU.
Limitations
This study has several limitations. First, we prospectively identified massive hemorrhage protocol-based latent safety threats leading to modifications in our protocol. Our evaluation of the impact of these changes, however, used a retrospective chart review methodology. As a result, we cannot establish a causal relationship between our in situ simulation-based quality improvement intervention and the improved time to blood administration for trauma patients. However, our changes did translate in time-based improvements during our subsequent in situ simulation sessions, suggesting some effect. Furthermore, the only changes made to our institution’s massive hemorrhage protocol during this period resulted from issues identified during our study, increasingly the likelihood that our interventions are linked to our chart review findings. Second, as a single-center study it is uncertain whether these findings are generalizable to other trauma centers. However, it is the application of in situ simulation to uncover and improve local latent safety threats that is most important rather than our specific findings. In situ simulation does improve other time-dependent processes, supporting its use as a key technique for optimizing massive hemorrhage protocols [22]. Finally, we relied on clinical documentation to measure the time to blood administration. Inherently these records are often made during complex and time-pressured trauma resuscitations and may not be accurate. We did cross reference the clinical notes with our blood bank records to ensure further accuracy of our results which remained uniform throughout the study period.
Conclusions
We observed a 21% reduction (2.5 min) in time-to-blood administration following an in situ simulation-based quality improvement process to identify and mitigate latent safety threats. This study represents a novel application of in situ simulation that may positively impact outcomes for bleeding trauma patients. Our study identified important opportunities for improvement related to the activation process, the transportation of blood components and the prioritization of a massive hemorrhage protocol among a multi-disciplinary team. Further research is required to establish a causal relationship between in situ simulation-based quality improvement initiatives and patient outcomes.
References
Spinella PC. Zero preventable deaths after traumatic injury: an achievable goal. J Trauma Acute Care Surg. 2017;82(6S Suppl 1):S2–8.
Cannon JW, Khan MA, Raja AS, Cohen MJ, Como JJ, Cotton BA, et al. Damage control resuscitation in patients with severe traumatic hemorrhage: A practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2017;82(3):605–17.
Pham HP, Shaz BH. Update on massive transfusion. Br J Anaesth. 2013;111(Suppl 1):i71-82.
van der Meij JE, Geeraedts LMG Jr, Kamphuis SJM, Kumar N, Greenfield T, Tweeddale G, et al. Ten-year evolution of a massive transfusion protocol in a level 1 trauma centre: have outcomes improved? ANZ J Surg 2019;89:1470–1474. https://doi.org/10.1111/ans.15416
Cotton BA, Au BK, Nunez TC, Gunter OL, Robertson AM, Young PP. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J Trauma 2009;66(1):41–8.
Riskin DJ, Tsai TC, Riskin L, Hernandez-Boussard T, Purtill M, Maggio PM, et al. Massive transfusion protocols: the role of aggressive resuscitation versus product ratio in mortality reduction. J Am Coll Surg. 2009;209(2):198–205.
Dente CJ, Shaz BH, Nicholas JM, Harris RS, Wyrzykowski AD, Patel S, et al. Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma after institution of a massive transfusion protocol in a civilian level I trauma center. J Trauma. 2009;66(6):1616–24.
Committee on Trauma ACoS. ACS TQIP Massive transfusion in trauma guidelines2014. 2019. https://www.facs.org/-/media/files/quality-programs/trauma/tqip/transfusion_guildelines.ashx?la=en.
Meyer DE, Vincent LE, Fox EE, O’Keeffe T, Inaba K, Bulger E, et al. Every minute counts: time to delivery of initial massive transfusion cooler and its impact on mortality. J Trauma Acute Care Surg. 2017;83(1):19–24.
Guise JM, Mladenovic J. In situ simulation: identification of systems issues. Semin Perinatol. 2013;37(3):161–5.
Patterson MD, Geis GL, Falcone RA, LeMaster T, Wears RL. In situ simulation: detection of safety threats and teamwork training in a high risk emergency department. BMJ Qual Saf. 2013;22(6):468–77.
Blike GT, Christoffersen K, Cravero JP, Andeweg SK, Jensen J. A method for measuring system safety and latent errors associated with pediatric procedural sedation. Anesth Analg. 2005;101(1):48–58.
Wheeler DS, Geis G, Mack EH, LeMaster T, Patterson MD. High-reliability emergency response teams in the hospital: improving quality and safety using in situ simulation training. BMJ Qual Saf. 2013;22(6):507–14.
Fan M, Petrosoniak A, Pinkney S, Hicks C, White K, Almeida AP, et al. Study protocol for a framework analysis using video review to identify latent safety threats: trauma resuscitation using in situ simulation team training (TRUST). BMJ Open. 2016;6(11):e013683.
Cafazzo JA, St-Cyr O. From discovery to design: the evolution of human factors in healthcare. Healthc Q. 2012;15:24–9.
Callum JL, Yeh CH, Petrosoniak A, McVey MJ, Cope S, Thompson T, et al. A regional massive hemorrhage protocol developed through a modified Delphi technique. CMAJ Open. 2019;7(3):E546–61.
Perla RJ, Provost LP, Murray SK. The run chart: a simple analytical tool for learning from variation in healthcare processes. BMJ Qual Saf. 2011;20(1):46–51.
Chartier LB, Vaillancourt S, Cheng AHY, Stang AS. Quality improvement primer part 3: evaluating and sustaining a quality improvement project in the emergency department. CJEM. 2019;21(2):261–8.
Chua WC, D’Amours SK, Sugrue M, Caldwell E, Brown K. Performance and consistency of care in admitted trauma patients: our next great opportunity in trauma care? ANZ J Surg. 2009;79(6):443–8.
Gruen RL, Jurkovich GJ, McIntyre LK, Foy HM, Maier RV. Patterns of errors contributing to trauma mortality: lessons learned from 2,594 deaths. Ann Surg. 2006;244(3):371–80.
Nunez TC, Young PP, Holcomb JB, Cotton BA. Creation, implementation, and maturation of a massive transfusion protocol for the exsanguinating trauma patient. J Trauma. 2010;68(6):1498–505.
Ajmi SC, Advani R, Fjetland L, Kurz KD, Lindner T, Qvindesland SA, et al. Reducing door-to-needle times in stroke thrombolysis to 13 min through protocol revision and simulation training: a quality improvement project in a Norwegian stroke centre. BMJ Qual Saf. 2019;28(11):939–48.
Amiel I, Simon D, Merin O, Ziv A. Mobile in situ simulation as a tool for evaluation and improvement of trauma treatment in the emergency department. J Surg Educ. 2016;73(1):121–8.
Chan S, Babcock L, Geis G, Frey M, Robinson V, Kerrey B. In situ simulation to mitigate threats to participation in a multicenter clinical trial in high-acuity, low-frequency setting. Simul Healthc. 2019;14(1):1–9.
Lutgendorf MA, Spalding C, Drake E, Spence D, Heaton JO, Morocco KV. Multidisciplinary in situ simulation-based training as a postpartum hemorrhage quality improvement project. Mil Med. 2017;182(3):e1762–6.
Andreatta P, Saxton E, Thompson M, Annich G. Simulation-based mock codes significantly correlate with improved pediatric patient cardiopulmonary arrest survival rates. Pediatr Crit Care Med. 2011;12(1):33–8.
Theilen U, Leonard P, Jones P, Ardill R, Weitz J, Agrawal D, et al. Regular in situ simulation training of paediatric medical emergency team improves hospital response to deteriorating patients. Resuscitation. 2013;84(2):218–22.
Josey K, Smith ML, Kayani AS, Young G, Kasperski MD, Farrer P, et al. Hospitals with more-active participation in conducting standardized in-situ mock codes have improved survival after in-hospital cardiopulmonary arrest. Resuscitation. 2018;133:47–52.
Bradley NL, Innes K, Dakin C, Sawka A, Lakha N, Hameed SM. Multidisciplinary in-situ simulation to evaluate a rare but high-risk process at a level 1 trauma centre: the “Mega-Sim” approach. Can J Surg. 2018;61(5):357–60.
Knobel A, Overheu D, Gruessing M, Juergensen I, Struewer J. Regular, in-situ, team-based training in trauma resuscitation with video debriefing enhances confidence and clinical efficiency. BMC Med Educ. 2018;18(1):127.
Long AM, Lefebvre CM, Masneri DA, Mowery NT, Chang MC, Johnson JE, et al. The golden opportunity: multidisciplinary simulation training improves trauma team efficiency. J Surg Educ. 2019;76(4):1116–21.
Acknowledgements
We wish to thank the staff at the Allan Waters Family Simulation Centre for making each simulation possible. We also wish to thank Amanda McFarlan, the TRUST study team, the emergency department staff and the trauma program staff at St. Michael’s Hospital.
Funding
This study was funding by the St. Michael’s Hospital AFP innovation grant, Sim-One and the Royal College Medical Education Research Grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors affirm that they have no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gray, A., Chartier, L.B., Pavenski, K. et al. The clock is ticking: using in situ simulation to improve time to blood administration for bleeding trauma patients. Can J Emerg Med 23, 54–62 (2021). https://doi.org/10.1007/s43678-020-00011-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43678-020-00011-9