Abstract
The concept of circular bioeconomy is embraced now-a-days as an integrated approach for sustainable production of renewable materials having minimum energy demand, maximum utility, and recycling capacity, minimizing the production waste with complete degradation. This bio-circularity needs cutting-edge technologies that have the potential to promote economic growth and are responsible for solving various environmental complications. Among various technologies, metagenomics is proving its mettle that can lead to sustainability. The identification and characterization of diverse microbial communities present in various environments such as water, soil, and waste streams help in sustainable resource utilization. The power of the unseen majority is enormous and unfolds the genetic information and functions of the microbes that can be applied in various avenues of combating societal problems. The discovery of novel catalysts and genes responsible for waste utilization and their valorization leads to the closed-loop system in which biological resources are reused and recycled. The production of biofuels, bioplastics, and other value-added biobased chemicals from various waste materials is aligning with the principles of circular bioeconomy. Moreover, optimization of microbial consortia for specific applications by understanding the interactions between different microorganisms and their metabolic functions by metagenomics is facilitating the efficient utilization of bioresources for the production of desired bioproducts. Therefore, the present review highlighted the synergy between the development of metagenomics and circular bioeconomy transforming the role of metagenomics in the development of circular bioeconomy by facilitating various paradigms of societal needs such as bioenergy, health, agriculture, and environment and has the potential to fulfill sustainable development goals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The world today is facing huge challenges including rising demand of natural resources, food, raw materials, climate change, pollution, energy and increase in population that is imparting cataclysmic impact on global economy. In order to maintain the sustainable economic growth, the utilization of renewable feedstocks through biological means is providing suitable alternative that can reduce the reliance on fossil-based economy. This led to the transition towards linear model of economic growth to circular biobased economy [1]. The landmark publication of EU Circular Economy Action Plan published in 2015 is gateway for embracing the concepts and approaches of circular economy where the resources can be re-incorporated back into the system or reutilize with minimizing detrimental externalities and encouraging regeneration of value-added products. Though circular bioeconomy is a combinatorial aspect that is taken by academics, policy makers, and industrial community together to achieve sustainability but innovations led by technology is the main stakeholder for the development of circular bioeconomy. Since the dawn of civilization, microbes are coevolved with humanized activities, and they are serving for the entire mankind through various products. Though the microbial technologies in both qualitative and quantitative aspects have been broadened over the time but the quantum has been heralded by gene revolution since 1970s and further metagenomics revolutionize the intensified exploration of microbial diversity, creation of novel metabolic routes and metabolism, novel analytical instrumentation and procedures, miniaturization [2]. These developments enhanced the panorama of microbiome applications in diverse fields. By keeping in view that metagenomics comprises high end technologies of next generation sequencing, it’s quite anticipated that this will be serving pathbreaking technologies for achieving sustainable goals of any nation [3]. The potential of biobased economy has been realized and its circularity has been proven in various fields of societal needs though the explicit definition of circular bioeconomy is still under development [4]. Thus, metagenomics will be considered as one of the key tools for untapping the natural potential of microbes from diverse environments and holds promising prospects for combating various problems prevalent in the world. Thus, this article advocates the correlation of metagenomics that can be helpful for reaching the goals of circular bioeconomy. Simultaneously it also posits current challenges for its implementation and prospects for the comprehensive utilization at global context by emphasizing synergy with sustainable developmental goals laid down by United Nations.
Concept of Circular Economy: A Transition Towards Circular Bioeconomy
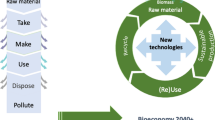
It is well written in the literature “we received this world as an inheritance from past generations, but also as a loan from future generations” [4]. Industrialization and prosperity in living ecosystems has been envisaged from a take-make-use-dispose linear economic system since industrial revolutions but astonishingly overwhelming use of natural resources and mismanagement of disposals and exhaustive anthropogenic activities leads to impeding challenges of global climatic changes. These changes substantially lead to a cataclysmic impact on not only human life but every living ecosystem residing in land, water, and air. It is sacrosanct to say that we should have a sense of sensitivity and perseverance for our “mother nature” to remain sustainable. Therefore, in the past decade, the realization of a sustainable economy has been visualized and among that continuum, the emergence of a circular bioeconomy came into existence. There is hundreds of definitions of circular bioeconomy based on different scientific opinions as per geographical locations and their requirements rather it is considered as an “essentially contested concept,” that describes the common consensus on goals and concepts but disparity in formulation of common definition [5]. As per the simple thought of the line, the concept of circular economy embraces the “4R” strategy i.e., reduce, reuse, recycle, and recover [6]. Technically two definitions of circular economy have been postulated based on the Ellen MacArthur Foundation (EMAF) (2019) and the non-EMAF-based definitions formed by other researchers. As per EMAF, the design of circular bioeconomy is a framework of restorative and regenerative design in which focus is laid on three major principles including minimization of depletion of resources, preventing the loss of natural resources, and encouragement of regenerative practices [7, 8]. The line of thought of non-EMAF is based on the reduction of the societal production-consumption system through cascading the flow of materials and energy into circular and renewable forms. As a circular economy is majorly focused on the reduction of dependency on natural resources therefore it is complemented by a bioeconomy that involves economic growth through biological processes, products for materials, energy, and chemicals. The intersection of circular economy and bioeconomy is called circular bioeconomy [4, 9]. Thus, circular bioeconomy conceptualizes the framework of the production of renewable resources from land water, and air and facilitates their conversion into various biobased products with the development of new industries and jobs that can boost the economic growth of any nation [10]. The bibliometric analysis depicted in Fig. 1 is giving conceivable evidence for the rapid increment of the concepts of circular bioeconomy and its correlation with metagenomics and sustainability parameters.
There is strong skepticism and debate on the relationship between circular economy versus bioeconomy on various points such as are these concepts are separate but reinforcing. Are both concepts integrated or partially antagonistic? Is bioeconomy acting as a precondition to achieving circular bioeconomy, are circular bioeconomy is considered a transition from fossil-based economy to biomass-based economy [1, 4, 9, 11]? Infact, there is a line of confusion and questions about the sustainability of circular bioeconomy. The incremental demand for bioenergy leads to competition for land use, water consumption, and food production which affects the social sustainability regimes [12]. More recently, it has been observed that bio-based materials posit deleterious environmental impacts in many facets such as acidification, eutrophication, ozone depletion water consumption. Thus, technology and innovation play a pivotal role in achieving sustainability and now-adays they are considered as intertwined. Metagenomics is serving as an important platform to unravel natures mysteries that can be helpful for the sustainable production of energy, food and feed and medicines and also facilitates the conservation of biodiversity by recycling the biomass into various value-added product [13]. It is a roadmap to achieve the “cradle to cradle” concept of bioeconomy that can been made circular more promisingly bio-circular in nature [1]. The timeline diagram of metagenomics and bioeconomy has been shown in Figs. 2 and 3 that is showing close synergy in their development. We can predict by visualizing this diagrams that the as the next generation technology progress the economic transition can also take place from linear model to circular. Though the road to achieve circular bioeconomy is still arduous but the future will be certainly hold tremendous potential for the growth of a circular bioeconomy.
Why Circular Bioeconomy Should be Promoted?
Currently, the most pressing challenge of the century is sustainability and circular bioeconomy is an evolving concept that will be serving as a most dynamic, socioeconomic, and eco-friendly way to achieve it. Despite the disparate interpretations, controversies, and debates, circular bioeconomy can be considered as the working action for tomorrow’s generation therefore the implementation of the concept should be started today. There are lots of opportunities and driving forces in the current environmental scenario that can proficiently promote this concept. The vision of utilizing renewable resources and the potential of making wealth from various biomass waste, novel portfolios of bioproducts with eco-friendly and recyclable properties, establishment of new industries and generation of new employment opportunities and business models, creation of innovation and research centric environment for technological developments, proving platform for making new sustainable governmental policies and regulations for its implementation, supports the local economical wealth for value generation makes this concept for covering holistic parameters such as social, economic, political and technological for developing sustainability [14]. Thus, whatever seems to be overambitious aspect in current scenario, will be a promising solution for futuristic world.
Avenues of Metagenomics for Making the Roadmap of Circular Bioeconomy
Metagenomics for Sustainable Environment
Metagenomics is advancing rapidly in the direction of attaining environmental sustainability. The pollution of land, are water and air posit severe threats to the human health and surrounding ecosystems [13]. Microbial bioremediation of various pollutants has been successfully realized for combating the land pollution [15]. The canopy of microbes from land to sea and extreme environments provides excellent opportunity of finding novel microbes and genes with broader applicability, higher degradation efficiency and stable expression in different environments. Metagenomics aids in finding out the microbes for ammonium oxidation, phosphorus accumulation that helps in removal of biological nitrogen and phosphorus from various wastes [16]. The novel genes have been discovered that can have a broader specificity of degrading polycyclic aromatic hydrocarbons, pesticides, petroleum hydrocarbons lead to a novel paradigm in microbial bioremediation. The identification of microbial electrochemically active cells (MECs) opens a promising path for harnessing electrical energy from organic waste [17]. The identification of novel proteolytic enzymes from compost for degradation of pesticides, benzoate degrading microbe from Black Sea sediment, cold adapted microbes for biodegradation of oil spill in Deep water Horizon, bio-stimulation of uranium remediation in US DOE Rifle site in Colorado as well as Oak Ridge Field Research Center (ORFRC) site in Tennessee represented as a notable exemplar of contribution of metagenomics in the removal of diverse pollutants from the environment [18, 19].
More recently, another major burden in environment has to be cope with deadly and unavoidable plastic pollution. It has been predicted that in 2050, 12,000 million metric tons of plastic wastes would be accumulated in environment and landfills [20]. Biodegradation of plastics through microbes viz. fungi, bacteria and other extremophiles become has gained immense attention now-a-days due to higher metabolic capabilities as potential degraders of diverse plastic polymers and is considered as an eco-friendly measure for combating this threat. However, the biochemical mechanism of plastic degradation remains elusive due to the limitation of a culture-dependent approach for microbial exploration [21]. Metagenomics has tremendous potential for deciphering the microbial community for plastic degradation in various environmental samples and also opens up the route for finding novel genes and enzymes for plastic degradation [22]. The growing continuum of various enzymes and microbes leads to the development of a separate open-access database for the Plastics-Active Enzymes Database (PAZy) [23]. The database comprises experimentally verified enzymes with approximately 3000 homologs for PET degradation through profile Hidden Markov model (HMM) and 2000 homologs for PU degrading enzymes from BLAST searches [23]. Leaf-branch compost cutinase (LCC) enzyme isolated from compost metagenome has shown significant PET degrading activity [24]. More recently, metagenomes from 108 marine and 25 terrestrial sources retrieved from the Integrated Microbial Genome database IMG have been analyzed and 349 putative PET hydrolases were predicted and out of these two thermostable enzymes were partially characterized [25]. The microbial community analysis based on amino acid similarities reached a common consensus that major phyla corresponding to the production of PET hydrolases are Bacteroidetes, Actinobacteria, Proteobacteria and within Proteobacteria, Deltaproteobacteria, Betaproteobacteria and Gammaproteobacteria were the most abundant in investigated metagenomes [26]. This, the research is thriving in this area and more potential plastic degrading enzymes can be retrieved from metagenomics that can be used for plastic degradation and also paving the way for valorization of plastic wastes for contributing towards circular bioeconomy.
Apart from that, the release of methane in the climate from landfills, oil, and gas industries contributes 22% of its release which has a profound effect on global warming [27, 28]. Research studies speculated methane oxidation is the most promising way to mitigate the emission of methane and a variety of microbes from both aerobic and anaerobic physiology have the potential to oxidize the methane [29]. Recently metagenomics application has been envisaged to isolate and characterize the archaea Methanoperedens nitroreducens that catalyze the nitrate-driven anaerobic oxidation of methane (nitrate-AOM) [30]. Apart from that high level of methane has been released from ruminants, biogas plants, insect guts, rice paddies, and leaks in gas pipelines. Extensive metagenomic studies have been pursued to assess the association between rumen microbiome and host genetics through Bayesian gene networks [31]. The analysis of microbial diversity gave valuable insights into the structural and functional aspects of ruminants and their association with host and methane emission [32]. In addition to that, metagenomic analysis can be part of surveillance projects for CO2 monitoring [33]. Research studies evidenced that the fluctuation in geochemical parameters in the environment has been strongly linked with the community structure of prokaryotes, as they have a high ability to fix CO2 into organic carbon [34]. The past decade has seen tremendous developments in metagenomic community analysis of various sediment sites such as Håkon Mosby mud volcano whale falls hydrothermal vents methane seeps [35]. The community analysis of Johansen formation revealed the abundance of six Nitrosopumilus maritimus SCM1, a CO2 fixing strains and genes for CO2 fixation pathways [34]. This community has been in more abundance in the raised level of CO2 therefore acting as a good indicator for CO2 monitoring.
Metagenomics for Sustainable Agriculture and Food Security
The “zero hunger” second sustainable development goal set by UN leads to the humongous efforts for advancing sustainable agriculture, improved nutrition and food security [36]. For the successful achievement of this goal, there is a dire need for widespread awareness of modern agriculture, better access of nutritious food and use of high-end research through advanced technologies. The inculcation of metagenomics is proving as a boon for sustainable agriculture and food security. The comprehensive understanding of microbial diversity in soil ecosystems for the production of growth-promoting metabolites, novel catalysts for pest resistance, chemotaxis, phosphate solubilization, nitrogen fixation, siderophore production, indole-acetic acid production provides an avenue for the development of sustainable agriculture [37]. Furthermore, the alleviation of soil contamination can be done through the identification of microbes) such as Denitrifying bacteria and Geobacter) and genes for the degradation of heavy metal ions and radionucleotides (uranium) as well as hydrocarbons, harsh chemicals that have been released through rigorous anthropogenic activities. The identification of new enzymes including laccases, carboxylesterases, trichlorophenol degrading enzymes, monooxygenases, polyaromatic hydrocarbon degrading enzymes, phenol and alkane degrading enzymes, hexadecane hydrolyzing enzymes for catalyzing the degradation of a variety of pollutants is another milestone that has been successfully achieved through metagenomics [38]. Thus, metagenomic approaches can be used as a sliver line approach for the development of innovative agricultural solutions through early diagnosis of plant pathogens, reduction in crop loss, and maintenance of soil health by preserving the nutrients and conserving diversity. There is a close synergism between food security and sustainable agro-ecosystems. The major food supply is dependent on a variety of agricultural modes across the globe. There is an urgent need to maintain the quality of nutrition of food materials that have been negatively impacted by intensive agricultural practices and processing, pollution, presence of a variety of hazardous substances such as hormones, pesticides, chemical preservatives, herbicides Bisphenol A. The early detection of food-borne pathogens mitigating the risk of food-borne outbreaks and improving public health outcomes is a part of the revolution in food security and safety diagnostics [39]. The emerging circular economy concepts corroborate with extensive urbanization and exponential population imposes the transformation of present food waste into a novel regenerative system.
Metagenomics and Bioenergy
The past two decades has been seen towards intensive efforts for the reduction of dependency of fossil fuels reserves and development of advanced technologies for the sustainable production of biofuels for combating the global energy crisis [40]. The metabolic diversity in microorganisms is imparting the incredible potential for the production of biofuels from the fermentation of diversified substrates from food crops, lignocellulosic biomass from waste and nutrient rich waste materials [41,42,43,44]. The colossal benefits have been realized from microbial derived biofuels such as bioethanol, biodiesel, biohydrogen and biomethane as a promising alternative to their petroleum counterparts. Even with prolific growth in the production of biofuels has been envisaged at global scale, still considerable technological bottlenecks are existing for the commercialization of their production process due to the unavailability of robust strains, lack of infrastructure, complex production process and scale up issues for industrial manufacturing. Therefore, metagenomics is proving as a cutting-edge technology for creating a sustainable framework for the bioenergy paradigm. The metagenomic identification of consortium of thermophilic Actinobacteria from rice straw compost depicted promising potential enzymes for lignocellulosic biomass degradation [45]. The discovery of novel cell wall degrading enzymes for the depolymerization of lignocellulosic biomass is providing cost effective platform for the development of biofuel technology [46]. The discovery of novel cellulase enzymes from metagenomically derived cells such as such as Thermoanaerobacterium, from Icelandic hot springs [47], Caldicelluloseruptor bescii from Valley of Geysers hot springs, glycosyl hydrolase and endoglucanase from marine metagenome, cold-active xylanase from fungus growing termites, lignin peroxidases, manganese peroxidases, phenol oxidases from various environmental samples, cold active lipases from intertidal flat sediments, esterases from a hot spring in Kamchatka, alcohol oxidoreductases, alcohol/aldehyde dehydrogenase, revolutionize the development of second generation biofuels. Furthermore, metagenomics also opens a pandora’s box for the inculcation of metabolic engineering tools for upscaling of biofuel technology. There are several approaches of metabolic engineering such as codon optimization, promoter engineering, directed enzyme evolution, tailoring toxic metabolite tolerance, competitive pathway deletion, consolidated pathway insertion and respiratory pathway manipulation [48]. The metabolic engineering of Saccharomyces cerviasse was performed by inserting the genes encoding for xylose isomerase (XI) isolated from soil samples from snail manure and from mammal gut microbes, Alistipes sp. HGB5 that enable the pentose sugar utilization by the cell for the enhanced productivity of bioethanol [49]. In addition to that, Cel-5 M derived from the rumen metagenome depicted broad substrate range including xylan, CMC, glucopyranoside, Avicel, filter paper and mannans [50]. Moreover, metagenomically derived Xyn-2, novel endo-1,4-xylanase enzyme from the camel rumen has broader catalytic range of hydrolysis of agricultural waste such as wheat straw and rice straw (56%), rice bran (41%) and wheat bran (100%) [50]. In addition to that, metagenomically derived Exo2b cellulase gene from pig ordure was fused with chh1 gene from Trichoderma reesei and expressed in T. reesei Rut-C30 that displayed enhanced cellulase activity towards carboxy methyl cellulase (CMC) [51]. The aforementioned developments can be helpful for setting various waste biorefineries in an efficient way and also paving the way for zero-waste discharge policy [43]. Thus, by employing this NGS technology in one of the promising endeavors for achieving the path of circular bioeconomy is a leading development towards the sustainability.
Metagenomics in Health Care
The past decade has seen continuous upsurge of utilizing metagenomics in health care sector that is contributing a gateway for strengthening the circular bioeconomy. The identification of novel drugs, pathogens, host-pathogen interactions, resistance towards antimicrobials, epidemiological evidence of infectious outbreaks transform the landscape of molecular diagnostics, therapeutics and treatment modalities. Infact, exhaustive applications of metagenomics in clinical samples bifurcate the conventional metagenomics to clinical metagenomics. The early identification of critical infectious pathogens in variety of clinical samples such as bronchoalveolar lavage [BAL] and tracheal aspirate as respiratory samples, sepsis in circulating blood and identification of encephalitis and meningitis in cerebrospinal fluid in ICU patients provides rapid therapeutic interventions [52]. The identification of less common pathogens such as Brucella melitensis in osteoarticular infections and Mycoplasma salivarium in bone and join infections (BJI) has been explored through clinical metagenomics [53,54,55]. Moreover, the identification of novel and unexpected virus in various diseases helps in tailoring the antiviral therapies. Research studies speculated convincing evidence of antiviral treatments by through metagenomic explorations. The ribavirin has been given to lung transplant recipient after the diagnosis of hepatitis E virus-associated meningoencephalitis [56], acyclovir treatment after the diagnosis of virus in herpes zoster laryngitis [57], interferon combination with ribavirin in astrovirus meningoencephalitis [58]. Apart from that, the identification of drug resistance-associated mutations (RASs) in target genes in the host lead to evaluation of drug efficacy profiles in various viral ailments [53]. The drug failures in case of viral hepatitis (NS3, NS5A, and NS5B genes) and HIV (reverse transcriptase, protease, and integrase genes) and cytomegalovirus (UL97, UL54, and UL56) leads to the metagenomic sequencing of various target genes respectively for the assessment of anti-viral treatments [59,60,61]. The role of metagenomics in various avenues of health has been depicted in Fig. 4.
Similarly clinical metagenomics has been immensely beneficial for evaluating the colonization of invasive fungal infection in aspergillosis and Pneumocystis jirovecii pneumonia for appropriate treatment as well as detection of various sensitive parasitic infections such ad Ascaris lumbricoides, Schistosoma mansoni and Giardia intestinalis [53]. Table 1 summarized the list of bioactive molecules that has been derived from various environmental samples through metagenomic approach and shown their health manifestations. In addition to that, the rising threat of antimicrobial resistance is pressing challenge for today therefore direct detection of antimicrobial resistance genes and their products through metagenomics paved the way for public health surveillance systems for identifying antibiotic resistant pathogens from the hospitals, community and corresponding environment. The development of Comprehensive Antibiotic Resistance Gene Database (CARD) [62] as well as bioinformatics pipeline such as the ID-seq pipeline [63] has been beneficial for identification of antimicrobial resistance gene and assessment of taxonomy. Moreover, the discovery of various antibacterial bioactive molecules such as strepchazolins, lobhoporin, lynamycins, borrelidin, Krifamycin B, ananstreps and spiroindimycins isolated from marine bacteria and divamides as anti-viral molecules have been identified from tunicates [64]. Similarly, somalimycin as an anti-inflammatory compound, antimycin as cytotoxic molecules, saccharomonopyrones as an antioxidative molecule, bonnevillamides as an antiatherosclerotic compound, paulomycin G as an antitumorigenic compound, xiamenmycin and calcium-dependent antibiotic such as malacidins have been discovered through metagenomic approaches [38, 65]. Despite of its exorbitant applications, there is slow adoption of metagenomics has been visualized in clinical laboratories. Thus, the comprehensive explorations of in-depth microbiome studies for the prevention of death, novel therapeutic modalities and early detection of disease outbreaks are directly contributing the circular bioeconomy.
Synergy of Metagenomics with Circular Bioeconomy: The Way Forward for Achieving Sustainable Developmental Goals’ (SDGs)
The rising unsustainability of our planet Earth, humans, and associated ecosystems due to the deterioration of the biosphere and quality of life posits serious concerns at the global level. The trajectories of events such as extreme climatic change, loss of biodiversity, deterioration of water and food quality, urbanization and desertification, rising health concerns and costs, poverty, hunger, regional conflicts and stigma have been responsible for such a catastrophic condition. It is the tiresome need to establish human stewardship on the earth that’s why lots of efforts have been directed towards sustainable development [66]. To overcome such disparities and reorient the biosphere towards a sustainable track, in 2015, the United Nations formulated seventeen SDGs that have been wooed across the international level and covered holistic development of the biosphere with all ecosystems [66]. Thus, a systemic approach must be steered for attaining these goals. It is widely anticipated that science, technology and innovation are playing pivotal role in advancing SDGs. As the microbes are the predominant form of life and ubiquitously present from simple to extreme environments therefore metagenomics unraveling the unseen majority has tremendous potential to tackle various societal challenges that further helps in fulfillment of various SDGs that opens the route for attaining circular economy. The diversity in the applications of microbes and associated microbiome has seem potential for achieving the eleven goals of SDGs. An overview of metagenomics circular economy and SDGs has been represented in Fig. 5. The exploration of microbiomes in generating wealth of knowledge regarding the plant and crop microbiome interactions, nutrient recycling, disease identification and regulation, stress management, enhanced farm productivity is laying significant impact on plant, animal and human health. There is a growing continuum of microbial diversity and their application in development of novel therapeutic modalities, disease diagnosis and surveillance, improved nutritional quality, enhanced productivity of food materials that has potential to contribute in achieving SDGs 2, 3. The microbial diversity explored through metagenomics have tremendous potential to remove the organic and inorganic pollutants from the environment, reduced greenhouse emissions, purification of waste water and bio-mineralization that directly contributes to SDGs 6, 13, 14. The microbial diversity represents a productive platform for the development of bioenergy paradigms including bioethanol, biobutanol, biomethane and biohydrogen that also delineates the fossil based economy to biobased economy [66]. The waste valorization through microbes and generation of value-added products, novel catalysts, platform and specialty chemicals, bioactive substances, bioelectricity and biomaterials significantly drives the industrialization and economic growth that substantially contributes in SDGS 7, 8, 9, 11,12, 15. Thus, metagenomics represents an important innovation centric scientific discipline to finding the treasure trove for novel biotechnological developments that covers all the sectors of bioeconomic growth and also has potential to maintain bio-circularity to achieve the ambitious, emerging concept of circular bioeconomy.
Current Challenges and Future Perspectives
The popular adage is well worth considering in the current scenario only “Change is constant” so it is high time to conserve our earth through restorative and regenerative systems. Prosperity and well-being cannot be sustainable with continuous exploitation of the natural resources therefore the economic shift from linear model to circular bioeconomic mode requires extensive transformations. We have seen the synergy of advanced biotechnological development like metagenomics with circular bioeconomy in previous sections, but both have different sets of challenges. For circular bioeconomy perspective, there are technological, social and legal challenges that need to be highlighted for more comprehensive understanding. The concept is still in nascent stage and there is lack of awareness of this concept among common people that leads to the apathetic attitude towards the commercialization and acquiring skills for developing bioproducts from various waste. The price competitiveness with linear products and insufficient value generation, unveiling market demand for bio-based products, feeble willingness to purchase the bioproducts, lack of adequate technology and incentives for upscaling as well as lack of policies and regulations to design and development of ecofriendly bioproduct, issue of availability of raw materials and their uniform composition and consistent product portfolios from various waste biorefineries are the major bottlenecks for the circular bioeconomy. Parallelly, metagenomics has also certain technological challenges such as collection of contamination free environmental samples, their homogenization and storage and extraction methods. Till date, there is diversity in metagenomic analysis in software’s and there is no “gold standard” software for comprehensive analysis as well as limited information on bacterial, viral and eukaryotic genome annotation further blocks the complete coverage of microbial diversity. The lack of standard data formats, assembly validation methods and requirement of huge computational power and storage data is another limitation from metagenomic perspectives. Thus, more extensive algorithms and pipelines for more apt analysis need to be developed. The discussion clearly stated that, both concepts have its own challenges but cannot be overlooked for the current context and future economy is thriving for circular bioeconomy. Thus, efforts can be directed for alleviating these bottlenecks for the effective implementation of circular bioeconomy with advanced technological inculcations. There is a dire need of increasing the education and awareness about bioeconomy, more emphatically on circular economy and their benefits and contribution towards sustainability. There should be proper governmental guidelines and policies for the adoption of CBE practices and incentivize the CBE frameworks and infrastructure. There should be promotion of collaborations of multidisciplinary teams for effective execution of CBE process. There should be more investment in innovation and research and need to establish the biofoundry for the development of various prototypes in cost effective manner that can be scalable at commercial level. In future stringent rules should be formulated for using fossil-based resources and subsidized prices and taxes on waste derived value-added products. The acquisition of flexibility of adopting global goals such SDGs and the positive externalities of promoting circular and renewable alternatives in contrast to non-renewable ones. The standardization and validation for environmental assessment of circular biobased products should be done for the wider implications. There should be strict framework for the technology-readiness level of circular products that can help in reducing the logistics challenges of the products. A prospective roadmap of role of metagenomics in circular bioeconomy has been depicted in Fig. 6. Thus, circular bioeconomy needs extensive transformation from linear products to circular products that leads to the development of new business model, employment opportunities, technological advancements and economic growth.
Conclusion
The fast urbanization and exponential population necessitate the complete transformation of global economy from waste systems into regenerative, restorative system that needs circularity concept. Therefore, the circular economy should corroborate with three major dimensions of sustainability including environment protection, social well-being and economic upliftment. Bio-based generation of wealth is one of the major armamentariums to achieve these dimensions. High end technologies such as metagenomics endeavors favors the transformation of waste to wealth economy. The unravelling of genetic intricacies of microbial communities from various ecosystems sch as soil, water, varied waste streams provide sustainable pathway for the efficient utilization of biological resources and their value addition. The discovery of novel enzymes, metabolic pathways and microbial interactions through metagenomics paving the way for tailored solutions that can be well applied for strengthening the circular bioeconomy. Metagenomics information helps in the engineering of microbial consortia for desired applications for converting waste into more valuable resources and biobased processes will drive ecological and economic prosperity. Though metagenomics is still flourishing, and circular bioeconomy also needs deeper transformation therefore it is conceivable to envision that there is close synergy between technology and sustainable development thus “A promising tête-à-tête of circular bioeconomy with metagenomics” is certainly on horizon.
Data Availability
Not applicable.
References
Pandey M, Singhal B (2021) Metagenomics: adding new dimensions in bioeconomy. Biomass Convers Biorefin 13:7461–7480. https://doi.org/10.1007/s13399-021-01585-9
Berini F, Casciello C, Marcone GL, Marinelli F (2017) Metagenomics: novel enzymes from non-culturable microbes. FEMS Microbiol Lett 364:fnx211. https://doi.org/10.1093/femsle/fnx211
Zhang L, Chen F, Zeng Z, Xu M, Sun F, Yang L, Bi X, Lin Y, Gao Y, Hao H, Yi W, Li M, Xie Y (2021) Advances in Metagenomics and its application in Enviromental Microorganinsm. Front Microbiol 12:3847. https://doi.org/10.3389/fmicb.2021.766364
Tan ECD, Lamers P (2021) Circular bioeconomy Concepts—A perspective. Front Sustain 2:701509. https://doi.org/10.3389/frsus.2021.701509
Korhonen J, NUrr C, Feldmann A, Birkie SE (2018) Circular economy as an essentially contested concept. J Clean Prod 175:544–552. https://doi.org/10.1016/j.jclepro.2017.12.111
Lieder M, Rashid A (2016) Towards circular economy implementation: a comprehensive review in context of manufacturing industry. J Clean Prod 115:36–51. https://doi.org/10.1016/j.jclepro.2015.12.042
Eberhardt LCM, Birkved M, Birgisdottir H (2022) Building design and construction strategies for a circular economy. Archit Eng Des Manag 18:93–113. https://doi.org/10.1080/17452007.2020.1781588
Hobson K, Holmes H, Welch D, Wheeler K, Wieser H (2021) Consumption work in the circular economy. J Clean Prod 321:128969. https://doi.org/10.1016/j.jclepro.2021.128969
D’Amato D, Veijonaho S, Toppinen A (2020) Towards sustainability? Forest-based circular bioeconomy business models in Finnish SMEs. Policy Econ 110:101848. https://doi.org/10.1016/j.forpol.2018.12.004
Leipold S, Petit-Boix A (2018) The circular economy and the bio-based sector-perspectives of European and German stakeholders. J Clean Prod 201:1125–1137. https://doi.org/10.1016/j.jclepro.2018.08.019
Pfau SF, Hagens JE, Dankbaar B, Smits AJM (2014) Visions of sustainability in bioeconomy research. Sustainability 6:1222–1249. https://doi.org/10.3390/su6031222
Sagar AD, Kartha S (2007) Bioenergy and sustainable development? Annu Rev Environ Resour 32:131–167. https://doi.org/10.1146/annurev.energy.32.062706.132042
Nwachukwu BC, Babalola OO (2022) Metagenomics: a tool for exploring key microbiome with the potentials for improving sustainable agriculture. Front Sustain Food Syst 6:886987. https://doi.org/10.3389/fsufs.2022.886987
Lewandowski I (2018) Bioeconomy: shaping the transition to a sustainable, biobased economy. Springer nature
Bharagava RN, Purchase D, Saxena G, Mulla SI (2019) Applications of metagenomics in microbial bioremediation of pollutants: from genomics to environmental cleanup. In: Dash S, Dash HR (eds) Microbial diversity in the genomic era, 1st edn. Elsevier, United States, pp 459–477
Wang Y, Li D, Song X, Cao X, Xu Z, Huang W, Wang Y, Wang Z, Sand W (2022) Intensifying anoxic ammonium removal by manganese ores and granular active carbon fillings in constructed wetland-microbial fuel cells: Metagenomics reveals functional genes and microbial mechanisms. Bioresour Technol 352:127144. https://doi.org/10.1016/j.biortech.2022.127114
Kadier A, Al-Shorgani N, Jadhav DA, Sonawane JM, Singh A, Kalil MS, Hasan HA, Alabbosh KF (2020) Microbial Electrolysis Cell (MEC) an innovative Waste to Bioenergy and Value‐added By‐product technology. In: Wang A, Liu W, Zhang W, Kai W (eds) Bioelectrosynthesis: principles and technologies for Value‐added products, 1st edn. Wiley‐VCH Verlag GmbH & Co. KGaA, New Jersey, pp 95–128
Chang Y (2005) In Situ Biostimulation of Uranium Reducing Microorganisms at the Old Rifle UMTRA Site. Dissertation, The University of Tennessee
Techtmann SM, Hazen TC (2016) Metagenomic applications in environmental monitoring and bioremediation. J Ind Microbiol Biotechnol 43:1345–1354. https://doi.org/10.1007/s10295-016-1809-8
Jung YS, Sampath V, Prunicki M, Aguilera J, Allen H, LaBeaud D, Veidis E, Barry M, Erny B, Patel L, Akdis C, Akdis M, Nadeau K (2022) Characterization and regulation of microplastic pollution for protecting planetary and human health. Environ Pollut 315:120442. https://doi.org/10.1016/j.envpol.2022.120442
Chen CC, Dai L, Ma L, Guo RT (2020) Enzymatic degradation of plant biomass and synthetic polymers. Nat Rev Chem 4:114–126. https://doi.org/10.1038/s41570-020-0163-6
Thakur K, Chownk M, Kumar V, Purohit A, Vashisht A, Kumar V, Yadav SK (2020) Bioprospecting potential of microbial communities in solid waste landfills for novel enzymes through metagenomic approach. World J Microbiol Biotechnol 36:34. https://doi.org/10.1007/s11274-020-02812-7
Buchholz PCF, Feuerriegel G, Zhang H, Perez-Garcia P, Nover LL, Chow J, Streit WR, Pleiss J (2022) Plastics degradation by hydrolytic enzymes: the plastics-active enzymes database—PAZy. Proteins: Struct Funct 90:1443–1456. https://doi.org/10.1002/prot.26325
Sulaiman S, Yamato S, Kanaya E, Kim JJ, Koga Y, Takano K, Kanaya S (2012) Isolation of a novel cutinase homolog with polyethylene terephthalate-degrading activity from leaf-branch compost by using a metagenomic approach. Appl Environ Microbiol 78:1556–1562. https://doi.org/10.1128/AEM.06725-11
Wedin NP (2020) Plastic Biotransformation Technologies: Development of a Novel Environmental QPCR Assay for Polyethylene Terephthalate Hydrolase, and Isolation/Characterization of Polyethylene Degrading Fungi and Bacteria from Environmental Samples. Dissertation, University of Minnesota
Azaroual SE, Kasmi Y, Aasfar A, Arroussi HE, Zeroual Y, Kadiri YE, Zrhidri A, Elfahime E, Sefiani A, Kadmiri IM (2022) Investigation of bacterial diversity using 16S rRNA sequencing and prediction of its functionalities in Moroccan phosphate mine ecosystem. Sci Rep 12:3741. https://doi.org/10.1038/s41598-022-07765-5
Molnár A (2018) Leaking away the future: the role of methane emission and natural gas supply chains in global warming. Energy Policy Stud 1:52–59
Lelieveld J, Crutzen PJ, Dentener FJ (1998) Changing concentration, lifetime and climate forcing of atmospheric methane. Tellus B: Chem Phys Meteorol 50:128–150. https://doi.org/10.1034/j.1600-0889.1998.t01-1-00002.x
Li H, Chang F, Li Z, Cui F (2022) The role of Extracellular Polymeric substances in the toxicity response of anaerobic granule sludge to different metal oxide nanoparticles. Int J Environ Res Public Health 19:5371. https://doi.org/10.3390/ijerph19095371
Arshad A, Speth DR, De Graaf RM, Op den Camp HJM, Jetten MSM, Welte CU (2015) A metagenomics-based metabolic model of nitrate-dependent anaerobic oxidation of methane by methanoperedens-like archaea. Front Microbiol 6:1423. https://doi.org/10.3389/fmicb.2015.01423
Zhang Q, Difford G, Sahana G, Løvendahl P, Lassen J, Lund MS, Guldbrandtsen B, Janss L (2020) Bayesian modeling reveals host genetics associated with rumen microbiota jointly influence methane emission in dairy cows. ISME J 14:2019–2033. https://doi.org/10.1038/s41396-020-0663-x
Cardinale S, Kadarmideen HN (2022) Host genome–metagenome analyses using Combinatorial Network methods reveal key metagenomic and Host Genetic Features for Methane Emission and feed efficiency in cattle. Front Genet 13:81. https://doi.org/10.3389/fgene.2022.795717
Håvelsrud OE, Haverkamp THA, Kristensen T, Jakobsen KS, Rike AG (2013) Metagenomics in CO2 monitoring. Energy Procedia 37:4215–4233. https://doi.org/10.1016/j.egypro.2013.06.324
Håvelsrud OE, Haverkamp THA, Kristensen T, Jakobsen KS, Rike AG (2012) Metagenomic and geochemical characterization of pockmarked sediments overlaying the Troll petroleum reservoir in the North Sea. BMC Microbiol 12:1–18. https://doi.org/10.1186/1471-2180-12-203
Knittel K, Boetius A (2009) Anaerobic oxidation of methane: progress with an unknown process. Annu Rev Microbiol logy 63:311–334. https://doi.org/10.1146/annurev.micro.61.080706.093130
Arora NK, Mishra I (2022) Current scenario and future directions for sustainable development goal 2: a roadmap to zero hunger. Environ Sustain 5:129–133. https://doi.org/10.1007/s42398-022-00235-8
Iquebal MA, Jagannadham J, Jaiswal S, Prabha R, Rai A, Kumar D (2022) Potential use of microbial community genomes in various dimensions of agriculture productivity and its management: a review. Front Microbiol 13:708335. https://doi.org/10.3389/fmicb.2022.708335
Datta S, Rajnish KN, Samuel MS, Pugazlendhi A, Selvarajan E (2020) Metagenomic applications in microbial diversity, bioremediation, pollution monitoring, enzyme and drug discovery. A review. Environ Chem Lett 18:1229–1241. https://doi.org/10.1007/s10311-020-01010-z
Billington C, Kingsbury JM, Rivas L (2022) Metagenomics Approaches for Improving Food Safety: a review. J Food Prot 85:448–464. https://doi.org/10.4315/JFP-21-301
Sartaj K, Patel A, Matsakas L, Prasad R (2022) Unravelling Metagenomics Approach for Microbial Biofuel Production. https://doi.org/10.3390/genes13111942. Genes 13:1942
Qaiser H, Kaleem A, Abdullah R, Iqtedar M, Hoessli DC (2021) Overview of Lignocellulolytic Enzyme Systems with Special Reference to Valorization of Lignocellulosic Biomass. Protein Pept Lett 28:1349–1364. https://doi.org/10.2174/0929866528666211105110643
Kucharska K, Hołowacz I, Konopacka-Łyskawa D, Rybarczyk P, Kamiński M (2018) Key issues in modeling and optimization of lignocellulosic biomass fermentative conversion to gaseous biofuels. Renew Energy 129:384–408. https://doi.org/10.1016/j.renene.2018.06.018
Ferreira JA, Agnihotri S, Taherzadeh MJ (2019) Waste biorefinery. In: Taherzadeh MJ, Bolton K, Wong J, Pandey A (eds) Sustainable resource recovery and zero waste approaches. Elsevier, United States, pp 35–52. https://doi.org/10.1016/B978-0-444-64200-4.00003-7
Majidian P, Tabatabaei M, Zeinolabedini M, Naghshbandi MP, Chisti Y (2018) Metabolic engineering of microorganisms for biofuel production. Renew Sustain Energy Rev Vo 82:3863–3885. https://doi.org/10.1016/j.rser.2017.10.085
Wang C, Dong D, Wang H, Müller K, Qin Y, Wang H, Wu W (2016) Metagenomic analysis of microbial consortia enriched from compost: new insights into the role of Actinobacteria in lignocellulose decomposition. Biotechnol Biofuels 9:1–17. https://doi.org/10.1186/s13068-016-0440-2
Curran LMCLK, Sale KL, Simmons BA (2022) Review of advances in the development of laccases for the valorization of lignin to enable the production of lignocellulosic biofuels and bioproducts. Biotechnol Adv 54:107809. https://doi.org/10.1016/j.biotechadv.2021.107809
Dimitra Z, Kissas D, Sayer C, Gudbergsdottir SR, Ladoukakis E, Isupov MN, Chatziioannou A et al (2016) Discovery and characterization of a thermostable and highly halotolerant GH5 cellulase from an Icelandic hot spring isolate. PLoS ONE 11:e0146454. https://doi.org/10.1371/journal.pone.0146454
Nevoigt E (2008) Progress in metabolic engineering of Saccharomyces cerevisiae. Microbiol Mol Biol Rev 72:379–412. https://doi.org/10.1128/mmbr.00025-07
Peng B, Huang S, Liu T, Geng A (2015) Bacterial xylose isomerases from the mammal gut Bacteroidetes cluster function in Saccharomyces cerevisiae for effective xylose fermentation. Microb Cell Fact 14:1–14. https://doi.org/10.1186/s12934-015-0253-1
Patel M, Patel HM, Dave S (2020) Determination of bioethanol production potential from lignocellulosic biomass using novel Cel-5m isolated from cow rumen metagenome. Int J Biol Macromol 153:1099–1106. https://doi.org/10.1016/j.ijbiomac.2019.10.240
Alei G, Zou G, Yan X, Wang Q, Zhang J, Liu F, Zhu B, Zhou Z (2012) Expression and characterization of a novel metagenome-derived cellulase Exo2b and its application to improve cellulase activity in Trichoderma reesei. Appl Microbiol Biotechnol 96:951–962. https://doi.org/10.1007/s00253-012-3873-y
Neyton LPA, Langelier CR, Calfee CS (2023) Metagenomic sequencing in the ICU for Precision diagnosis of critical infectious illnesses. Crit Care 27:90. https://doi.org/10.1186/s13054-023-04365-1
D’Humières, Salmona M, Delliere S et al (2021) The potential role of clinical metagenomics in infectious diseases. Therapeutic Perspect Drugs 81:1453–1466. https://doi.org/10.1007/s40265-021-01572-4
Zhao M, Tang K, Liu F, Zhou W, Fan J, Yan G, Qin S, Pang Y (2020) Metagenomic next-generation sequencing improves diagnosis of osteoarticular infections from abscess specimens: a multicenter retrospective study. Front Microbiol 11:2034. https://doi.org/10.3389/fmicb.2020.02034
Thoendel M, Jeraldo P, Greenwood-Quaintance KE, Chia N, Abdel MP, Steckelberg JM, Osmon DR, Patel R (2007) A novel prosthetic joint infection pathogen, Mycoplasma salivarium, identified by metagenomic shotgun sequencing. Clin Infect Dis 65:332–335. https://doi.org/10.1093/cid/cix296
Murkey JA, Chew KW, Carlson M, Shannon CL, Sirohi D, Sample HA, Wilson MR et al (2017) Hepatitis E virus–associated meningoencephalitis in a lung transplant recipient diagnosed by clinical metagenomic sequencing. Open Forum Infect Dis 4:ofx121. https://doi.org/10.1093/ofid/ofx121
Hagiya H, Yoshida H, Shimizu M, Motooka D, Nakamura S, Iida T, Yamamoto N, Akeda Y, Tomono K (2016) Herpes zoster laryngitis in a patient treated with fingolimod. J Infect Chemother 22:830–832. https://doi.org/10.1016/j.jiac.2016.07.011
Frémond ML, Perot P, Muth E, Cros G, Dumarest M, Mahlaoui N, Seilhean D et al (2015) Next-generation sequencing for diagnosis and tailored therapy: a case report of astrovirus-associated progressive encephalitis. J Pediatr Infect Dis Soc 4:e53–e57. https://doi.org/10.1093/jpids/piv040
Fourati S, Rodriguez C, Hézode C, Soulier A, Ruiz I, Poiteau L, Chevaliez S, Pawlotsky JM (2019) Frequent antiviral treatment failures in patients infected with hepatitis C virus genotype 4, subtype 4r. Hepatology 69:513–523. https://doi.org/10.1002/hep.30225
Wylie KM, Wylie TN, Buller R, Herter B, Cannella MT, Storch GA (2018) Detection of viruses in clinical samples by use of metagenomic sequencing and targeted sequence capture. J Clin Microbiol 56:e01123–e01118. https://doi.org/10.1128/jcm.01123-18
Lewandowski K, Xu Y, Pullan ST, Lumley SF, Foster D, Sanderson N, Vaughan A et al (2019) Metagenomic nanopore sequencing of influenza virus direct from clinical respiratory samples. J clin Microbiol 58:e00963–e00919. https://doi.org/10.1128/JCM.00963-19
Alcock BP, Raphenya AR, Lau TTY, Tsang KK, Bouchard M, Edalatmand A, Huynh W et al (2020) CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids res 48:D517–D525. https://doi.org/10.1093/nar/gkz935
Gemler BT, Mukherjee C, Howland C, Fullerton PA, Spurbeck RR, Catlin LA, Smith A, Minard-Smith AT, Bartling C (2023) UltraSEQ, a Universal Bioinformatic platform for information-based clinical metagenomics and Beyond. Microbiol Spectr 11:e0416022. https://doi.org/10.1128/spectrum.04160-22
Yi M, Lin S, Zhang B, Jin H, Ding L (2020) Antiviral potential of natural products from marine microbes. Eur J Med Chem 207:112790. https://doi.org/10.1016/j.ejmech.2020.112790
Hover BM, Kim SH, Katz M et al (2018) Culture-independent discovery of the malacidins as calcium-dependent antibiotics with activity against multidrug-resistant Gram-positive pathogens. Nat Microbiol 3:415–422. https://doi.org/10.1038/s41564-018-0110-1
Timmis K, de Vos WM, Ramos JL, Vlaeminck SE, Prieto A et al (2017) The contribution of microbial biotechnology to sustainable development goals. Microb Biotechnol 10:984–987. https://doi.org/10.1111/1751-7915.12818
Acknowledgements
The authors greatly acknowledge the support of Gautam Buddha University for conceptualizing and writing this manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent of Publication
The authors have given consent for the publication.
Competing Interests
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, S., Chakraborty, S., Puri, P. et al. Metagenomics- Paving the Path for Sustainable Circular Bioeconomy. Circ.Econ.Sust. 4, 1677–1696 (2024). https://doi.org/10.1007/s43615-024-00376-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43615-024-00376-4