Abstract
Reusing expired cement (EC) in cement manufacturing is an alternative way for managing such industrial waste, so this process contributes to preserving the environment and reducing the need for raw materials which creates an innovative solution in circular economy strategies as well as producing a sustainable material. The purpose of this study is to investigate the possibility of using EC as mineral additives for the manufacture of new cement. The different formulations of cements are the results of CEMI subtraction up to 35% by weight with the EC. These modified cements have passed the same tests as a characterization of a CEMII. The obtained results show that EC changes the characteristics of cementitious materials (anhydrous, paste, and mortar). The new cement produced fulfilled the requirements in terms of chemical characteristics. In addition, the decrease in strength caused by EC incorporation is in accordance with standards. It can be stated that EC plays the role of a diluting agent in the new cement. Environmentally, it may be more efficient to use EC as an ecofriendly additive material.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The cement industry generates 5% of global CO2 emissions; it is about 850 kg per ton of cement [1]. Firing and grinding are the most polluting stages with 80% and 10% respectively [2]. Because of these statistics, the percentage of clinker in cement decreased from 85% in 2003 to 77% in 2010, and it is predicted to decreasing, reaching 71% in the future [3]. The clinker substitution by adding minerals is the solution commonly used to reduce CO2 emissions [4]. The incorporation of low carbon mineral additives reduces environmental impacts, produces an environmentally friendly material, and minimizes energy consumption [5, 6]. These additives may offer other options such as improving certain characteristics of concrete including strength, durability, rheological, and transfer properties [7–11]. In addition, the variation in the percentage of addition to the cement contributes to the improvement of the finished product. These researches for modern additive products can often be a necessity to satisfy and participate in self-sufficiency in many countries [4].
When the cement is unconsumed within the recommended storage period or if it has not been properly stored, it will expire. The EC is generated by torn cement bags, large-scale storage, or moisture exposure. Moreover, EC can cause severe health problems due to its direct effect on the respiratory system particularly. It contains various types of chemicals product associated with great fineness, which causes various health complications linked to breathing difficulties [12]. The specific mineral composition, the particle size distribution, and the hydration activities already controlled compose a big advantage in its development [13]. Therefore, the reuse of EC represents a significant opportunity, as it affects the availability of raw resources, energy consumption, and CO2 emissions from cement manufacturing. In this sense, recycling of EC contributes therefore to prolonging the life cycle of cement which is a very effective way for creating an alternative solution in circular economy strategies and producing a sustainable material [14].
During the hydration process of cement, various minerals such C2S, C3S, C3A, and C4AF react with water and as result produce various compounds such as calcium silicate hydrate, portlandite, and ettringite. These products react not only with hydration water but also with each other, making the hydration reactions of cement more complex.
When atmospheric water is penetrating to cement through the bags of cement or the latter has not been properly covered, lumps are formed and more hardened with water penetration. These processes are done in many days or weeks depending on the environment in which cement is placed. Moreover, it is not easy to know visually if the cement is partially or totally hydrated, on the one hand, and the physical-mechanical tests require a significant time, on the other hand.
In the storage period, the strength of cement was reduced by 10% in the first month, while it reduced by 20% in the second month [15]. Therefore, it should be noted that loss in strength is closely related to the storage environment in which cement is placed, so the decrease in strength occurs more rapidly in inadequate curing conditions. Deng et al. [16] mentioned that after 1 week of moisture exposure, the portlandite and ettringite appeared with the formation of CSH gel. Chemically, Dubina et al. [17] and Whittaker et al. [18] state that CaO reacts when the relative humidity is lower than 10%, followed by the hemihydrate anhydrite at humidity about 34% and 58%, respectively. Similarly, cementitious minerals depend on their crystalline structure. Accordingly, the C3A reacts at a relative humidity of 80% and 55% for cubic and orthorhombic structures, respectively. For C3S and C2S, the corresponded humidity is respectively about 63% and 64%. Physically, Maltese et al. [19] state that when cement is exposed to moisture its setting time increases
In this work, we will respond to these environmental problems related to the cement industry. The decrease in clinker level is still the major concern of researchers and manufacturers in the cement sector. Adding, the treatment of waste in any form is, first of all, a question of well-being and health, before its extent. In this research, we will evaluate the effect of replacing clinker with EC at various percentages.
Materials and Formulation of Cements
Materials
Clinker provided from Lafarge Factory in M’sila, Algeria, is used to produce different cements. Its chemical composition is shown in Table 1. The clinker has a regular chemical and mineralogical composition (Table 1). Bogue’s equations show acceptable values, 65.47 for tricalcium silicate, 10.78 for dicalcium silicate C2S, 7.41 tricalcium aluminate, and 0.12 for tetracalcium alumino-ferrite. EC waste used in this study is taken from a pile of hydrated cement collected at Lafarge cement factory, M’sila, Algeria. This pile is generated during the production of cement and also from damaged cement bags. The visual aspect of EC waste is shown in Fig. 1. Its chemical composition is given in Table 1. Natural gypsum taken from Biskra region, Algeria, is used with a constant dosage (6%) for the preparation of different formulations. Its chemical composition is given in Table 1.
Cements Compositions
The chemical composition of cements produced according to the EC content is summarized in Table 2. The obtained cements satisfied the requirements of EN-197-1 [20]. The percentage of SO3 is less than 3.5%. Chemical indices, such as Michaelis hydraulic module (HM) comprised between 1.7 and 2.3 (Eq. (1)), silicic module (SM) ranged from 1.5 and 3.5 (Eq. (2)), and the Vicat Hydraulic index (VHI) is limited between 0.4 and 0.5 (Eq. (3)). These coefficients are in accordance with the specifications. As alkalis may be reacted with silica aggregates poorly crystallized, alkalis equivalent (Eq. (6)) should be limited at 0.6%, because beyond this value, the durability-related properties may be affected. Lime saturation factor (LSF) is ranged from 0.9 to 1 (Eq. (5)) which means that all lime will be consumed and there is no risk of expansion. Alumino-ferric module (AFM) was less than 1.5%, which means that the cement has low hydration heat.
Results and Discussion
Effect of EC on the Density of Cement
Impact assessment of the incorporation of EC on the specific gravity of cement is presented in Fig. 2. From the results, it can be seen that a regular decrease in the density of cement was observed with the increase of EC incorporation percentage in modified cements. It changed from 3.17 g/ml for the CEMI (control cement) to 3.01 g/ml for modified cement with 35% of replacement. This reduction can be explained by the fact that EC particles are lighter than that of CEMI which favored the deposit of cement at the bottom of the pycnometer. These results are in agreement with that observed by other researchers [21]. Moreover, the small size of EC particles in comparison with that of standard cement changed specific surface from 3800 to 4410 cm2/g for the cement contained 35 % of EC.
Effect of EC on Consistency
The effect of EC addition on the consistency of cement is shown in Fig. 3. It can be seen that the water amount required for the hydration of all anhydrous particles increased as the percentage of EC increased in the cement. This finding is translated by a linear relationship with a coefficient of determination R2 equal to 0.99. Modified cements (MC) have a greater fineness than control cement, which required more water to lubricate the surfaces of cement and infiltrate into the voids between fine grains; therefore, greater fineness required more water to hydrate; this observation is mentioned also by other researchers [22, 23].
Effect of EC on Paste Setting
Setting time is frequently used to quantify the early age stiffening of cement. There is two conventional setting times: the initial setting time which is the time between the water additions and the time in which the cement paste loses its fluidity. The final setting time marks the beginning of mechanical strength development. Many factors influence the setting time, particularly the w/c ratio, mixing temperature, clinker mineralogy, cement fineness, and the presence of admixtures and additions [24].
The effect of EC addition on cement setting was controlled with Vicat apparatus and the results are mentioned in Fig. 4. It can be noted from the results that the setting of cements regularly increases with the increase of EC content. When cement particles are mixed with water, the dissolution of anhydrous compounds begins by producing ionic constituents (Ca+2, OH−, Na+, ...) and consequently the precipitation of hydrates like ettringite, portlandite, and CSH. From the chemical point of view, the incorporation of EC and the increase in its proportion modified the consistency (W/C ratio) and consequently the setting times as indicated in Fig. 4. The production of Ca+2 depends principally on the chemical composition of cements and the content of calcium oxide CaO (Table 2). It can be observed that the incorporation of EC reduces the CaO content, thus retarded the setting time by 79 min between the CEMI and the MC35.
From a physical point of view, greater fineness created an additional surface for nucleation and hydration products development. The cement particles move off from each other, which created more spaces between hydration products. It should be mentioned that the volume of these spaces was increased with the increase of EC percentage in cement.
Heat of Hydration
The hydration of cement represents a complex set of exothermic chemical reactions. Figure 5 showed the different peaks of the heat generated during the hydration process.
The highest heat peak generated is observed for the CEMI with 332 J/g, while the lower one is that of MC35 (283 J/g). The most significant heat peak generated for modified cements is that of MC5 with 329 J/g.
The hydration of cement minerals (C2S, C3S) produces calcium hydroxide (CH) and hydrated calcium silicate (CSH) [25]. It assumed that the heat of hydration is proportional to the hydration products; the addition of EC decreased the creation of these products, which indicated that the EC played the role of a diluting agent
Shrinkage
This volumetric variation is the result of the development of capillary network pores, generated during hydration. The menisci cause a negative pressure on capillary water associated with a contraction of material when water is reduced by the bleeding phenomenon. As a result, pores become smaller and the concavity of menisci is more distinct [26], this is how shrinkage develops.
Usually, the existence of other additives besides cement reduces the volume of pores simultaneously by both physical and chemical mechanisms. The physical effect consists of fill-up the network of pores with additives because of their increased fineness, while the chemical effect consists to produce the hydrated calcium silicate gel CSH, which plays the role of pore size reduction and menisci.
The shrinkage of various cements is determined at 3, 7, and 28 days of the cure. The variations of shrinkage as a function of EC percentage are mentioned in Fig. 6. It can be marked that shrinkage increases with the aging of mortar and EC content.
The cementitious minerals that will give CSH gel are reduced by partial replacement of CEMI with EC. For the latter, the hydration reactions are finished and consequently cannot produce the CSH gel. As w/c is kept constant for all cements, the free water is increased by the increase of EC content, thus generated more voids after water evaporation, amplifying the capillary network, and as a result, the shrinkage is increased.
This observation is also indicated in the work of Atis [8] and Nath and Sarker [27]. They attributed the shrinkage increases, to the decrease of CEMI paste in volume. Despite this increase, it should be noted that the shrinkage in 28 days of curing is lower than the requirements of NF P 15-433, which is limited to 800 and 1000 μm/m for CEM I 32.5 and CEM I 42.5, respectively.
Compressive Strength
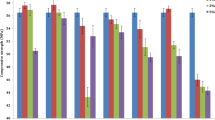
During hydration, the formation of hydrates especially ettringite, portlandite, and C-S-H gel begins. These hydrates get tangled allowing the creation of a rigid solid. Fig. 7 illustrates the evolution of compressive strength for the various standard mortars. It can be observed that compressive strength decreases as the percentage of EC increases. At 28 days of curing, compressive strength varies from 56.20 (CEMI mortar) to 42.1 MPa, when the EC is incorporated at 35%. After 90 days of hardening, the compressive strength of EC-based mortars is always lower compared to CEMI mortar. Even though the incorporation of EC makes a denser material by increasing fineness as reported above (Table 2), it should be noted that the reduction of strength might be justified by cement minerals reduction. When the C3S and C2S cement minerals hydrate, they become responsible for the development of strength in the short and long time. As for shrinkage, the physical effect cannot compete with the chemical effect in long term. The reduction of cement minerals in MCs plays the role of a diluent agent; the quantity of CSH is less in the MCs. Because of this remark, the mechanical strength of MC mortars was lower compared to CEMI mortar. This observation is also indicated in other investigations [17, 18].
Conclusion
This study was undertaken to investigate the possibility of recycling EC waste in cement manufacturing. Based on the obtained results, these conclusions can be drawn:
-
The decrease in the density of cement with the increase in the percentage of EC suggests that the EC particles are lighter than that of the CEMI.
-
The new cement required a significantly greater amount of water to moisten and then hydrate the cement. Thus, the increase in the w/c ratio, as a result of EC inclusion, prolonged the setting times. The filler effect of EC cannot compete with the decrease in cement minerals, which causes the effect of increasing the shrinkage and mechanical strength of mortars in our case.
Following these remarks, it can be declared that EC can be used as a cement addition. Its role in the modified cement will be considered as a diluent agent. In addition to that, recycling of EC help to use more efficiently the raw materials and create an ecofriendly sustainable construction material.
Data Availability
The data of this study are available on request from the corresponding author.
Code Availability
Not applicable.
References
Abdul-Wahab SA, Hassan EM, Al-Jabri KS, Yetilmezsoy K (2019) Application of zeolite/kaolin combination for replacement of partial cement clinker to manufacture environmentally sustainable cement in Oman. Environ Eng Res 24(2):246–253. https://doi.org/10.4491/EER.2018.047
Peng J, Huang L, Zhao Y, … P. C.-A. materials, & 2013, undefined. (n.d.) Modeling of carbon dioxide measurement on cement plants. Trans Tech Publ. Retrieved May 1, 2020, from https://www.scientific.net/AMR.610-613.2120
Schneider M, Romer M, Tschudin M, Concrete, H. B.-C. and, & 2011, U (2011) Sustainable cement production—present and future. Cement Concrete Res 41(7):624–650 https://www.sciencedirect.com/science/article/pii/S0008884611000950
Beddar M, Meddah A, Boubakria M, Haddad N (2014) A study of the effects of partial replacement of clinker by limestone in the cement manufacture | Badania wpływu częściowego zastąpienia klinkieru przez wapień w produkcji cementu. Cement, Wapno, Beton, 2014(3)
Meddah A (2015) Characterization of roller compacted concrete contaning rubber-tire wastes. National Polytechnic school of Algiers. Doctoral Thesis. http://catalogue2.biblio.enp.edu.dz/index.php?lvl=author_see&id=58804
Meddah A, Beddar M, Bali A (2014) Use of shredded rubber tire aggregates for roller compacted concrete pavement. J Cleaner Product 72:187–192. https://doi.org/10.1016/j.jclepro.2014.02.052
Adam N (2000) Propriétés du bétons. Eyrolles
Atis CD (2003) High-volume fly ash concrete with high strength and low drying shrinkage. J Mater Civil Eng 15(2):153–156
Chikouche MA, Ghorbel E, Bibi M (2016) The possibility of using dredging sludge in manufacturing cements : optimization of heat treatment cycle and ratio replacement. Construct Build Mater 106:330–341. https://doi.org/10.1016/j.conbuildmat.2015.12.128
Meddah A, Bensaci H, Beddar M, Bali A (2017) Study of the effects of mechanical and chemical treatment of rubber on the performance of rubberized roller-compacted concrete pavement. Innov Infrastruct Sol 2(1):17. https://doi.org/10.1007/s41062-017-0068-5
Meddah A, Laoubi H, Bederina M (2020) Effectiveness of using rubber waste as aggregates for improving thermal performance of plaster-based composites. Innov Infrastructure Sol 1–9. https://doi.org/10.1007/s41062-020-00311-0
Rahmani AH, Almatroudi A, Babiker AY, Khan AA, Alsahly MA (2018) Effect of exposure to cement dust among the workers : an evaluation of health related complications. Macedonian J Med Sci 6(6):1159–1162
Yu Y, Ge Y, Liu P (2019) Recycling of expired cement and aged supplementary cementitious materials based on close packing theory and space filling effect. J Cleaner Product 239:118064. https://doi.org/10.1016/j.jclepro.2019.118064
Nikolaou IE, Jones N, Stefanakis A (2021) Circular economy and sustainability: the past, the present and the future directions. Circular Econ Sustain 1(2):783–783. https://doi.org/10.1007/s43615-021-00054-9
Ramge P, Schmidt W, Kühne H-C (2013) Effect of the storage of cement on early properties of cementitious systems. Int Conference Advances Cement Concrete Technol Africa 1(1):339–348
Deng CS, Breen C, Yarwood J, Habesch S, Phipps J, Craster B, Maitland G (2002) Ageing of oilfield cement at high humidity: a combined FEG-ESEM and Raman microscopic investigation. J Mater Chem 12(10):3105–3112. https://doi.org/10.1039/b203127m
Dubina E, Wadsö L, Plank J (2011) A sorption balance study of water vapour sorption on anhydrous cement minerals and cement constituents. Cement Concrete Res 41(11):1196–1204. https://doi.org/10.1016/j.cemconres.2011.07.009
Whittaker M, Dubina E, Al-Mutawa F, Arkless L, Plank J, Black L (2013) The effect of prehydration on the engineering properties of CEM I Portland cement. Advances Cement Res 25(1):12–20. https://doi.org/10.1680/adcr.12.00030
Maltese C, Pistolesi C, Bravo A, Cella F, Cerulli T, Salvioni D (2007) A case history: effect of moisture on the setting behaviour of a Portland cement reacting with an alkali-free accelerator. Cement Concrete Res 37(6):856–865. https://doi.org/10.1016/j.cemconres.2007.02.020
EN-197-1 (2001) Standard: cement: composition, specifications and conformity criteria for common cements. Part 1.
Janam R, Taneia O, Sing RB (1995) Identification of expired cement by X-ray diffraction technique. J Indian Acad Forensic Sci 34(1):674–679 0378-7753/97/$17.00
Fennis SAAM, Walraven JC (2012) Using particle packing technology for sustainable concrete mixture design. Heron 57(2):73–101
Neville A (2000) Propriétés des bétons. In Edition Eyrolles
Brooks JJ, Johari MAM, Mazloom M (2000) Effect of admixtures on the setting times of high-strength concrete cement & concrete composites effect of admixtures on the setting times of high-strength. Cement & Concrete Composites 22(August):293–301. https://doi.org/10.1016/S0958-9465(00)00025-1
Mostafa NY, Brown PW (2005) Heat of hydration of high reactive pozzolans in blended cements : isothermal conduction calorimetry. Thermochimica Acta 435:162–167. https://doi.org/10.1016/j.tca.2005.05.014
Slowik V, Schmidt M, Fritzsch R (2008) Cement & concrete composites capillary pressure in fresh cement-based materials and identification of the air entry value. Cement Concrete Compos 30:557–565. https://doi.org/10.1016/j.cemconcomp.2008.03.002
Nath P, Sarker P (2011) Effect of fly ash on the durability properties of high strength concrete. Procedia Eng 14:1149–1156. https://doi.org/10.1016/j.proeng.2011.07.144
Author information
Authors and Affiliations
Contributions
Abdelaziz Meddah: conceptualization, methodology, writing-review, and editing; Mohamed Aziz Chikouche, Mohamed Yahia: resources; Moussa Deghfel: visualization and draft paper writing; Miloud Beddar: resources and supervision.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Meddah, A., Chikouche, M.A., Yahia, M. et al. The Efficiency of Recycling Expired Cement Waste in Cement Manufacturing: a Sustainable Construction Material. Circ.Econ.Sust. 2, 1213–1224 (2022). https://doi.org/10.1007/s43615-022-00161-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43615-022-00161-1