Abstract
The cementing lime mortars used in the construction of twelfth century CE Gopal Krishna temple at Western India Alandi were investigated for its mineralogical, chemical and compositional characteristics. The investigative studies were performed using particle size studies, XRF, XRD, FTIR, SEM–EDX and thermal analysis of the mortar. Though the monument is situated in Sahyadri range of Deccan basaltic trap, the mortar is marked by mixing of aggregates rich in hydrated oxides of alumina and iron. Further studies revealed natural formation of laterite capping on basaltic hillock that preferentially weathered and sediments deposited along the river basin sourced as aggregates in mortar preparation. The lime rich binder has mainly inclusion of sub-angular to sub-rounded coarse grain lateritic aggregates. The clay impurities have reduced the purity of lime as observed through thermal analysis. The data will help prepare a compatible mortar for restoration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The lime and mud mortar were main cementing materials for construction of temples and hill forts in India’s western region from the medieval period of twelfth century CE onwards. India’s western coast is famous for many ancient sea fort (Naravane, 1998) mainly constructed with cementing lime and basaltic stone blocks and functioned as centres of maritime trade (Singh, 1991; Singh & Kumar, 2019, pp. 1–14). However, for many hill forts situated at a height of 700–800 m from sea level in western India, the mud mortar was the main cementing material. This is probably due to non-availability of any lime source on hilltop, cost involved in carrying raw lime on top for slaking, and to reduce the cost of construction. However, in the plain area of Deccan trap the lime was the main building material for the ancient construction (Sen, 2001).
The composition, preparation and application techniques of lime mortar for ancient constructions varied in different regions of India and mainly dependent on the availability of raw materials, climatic conditions, intended use of the construction (Singh et al., 2014, pp. 430–434; Singh & Kumar, 2019, pp. 1–143; Singh & Singh, 2020, pp. 184–200), and period of construction (Singh & Singh, 2020, pp. 184–200). The preparation and technique of application of the lime plasters in India’s arid region is altogether different to that of central/southern part of the India and so are the aggregates found mixed in the lime works (Panda et al., 2013, pp. 837–842; Singh, 2017, pp. 237–241). Authors have observed compositional difference in the lime works of same monument depending on the period of construction and/or intendent use or function (Singh, 1991). In the northern India Gangetic plain, the aggregates used for lime preparation are mostly fine river sand and overfired brick aggregates (Singh & Kumar, 2018, pp. 482–496). The northern India Ganga plain is devoid of any hillock and it is a major source of superior clay extensively exploited for making best quality bricks from Harappan period onwards, as compared to other regions of India (Eraly, 2002). In the southern part of the India the aggregates found mixed in the plaster works were mostly sourced from the granite rich river sand (Selvaraj & Ramadoss, 2015, pp. 25–38). The western part of India is known for basaltic trap rock and aggregates used in lime works are varied grain sand mostly derived from the weathering of basaltic rock (Singh et al., 2020, pp. 110–119).
India’s western region is known for large number of rock cut caves (1st BCE to 10th CE) (Sheth et al., 2017, pp. 359–372), sea forts (Morrison, 1995, pp. 203–221), hill forts (Wescoat, 2019, pp. 175–190) and medieval ancient temples. While the mural art executed on lime/mud plasters have adequately been researched (Singh et al., 2015, pp. 156–170) not much has been done on the construction materials and technology used for the ancient fort and temples where only few research communications are available (Sathyabhama et al., 2011, pp. 1071–1075). These monuments are presently in dilapidated conditions and require adequate restoration and conservation. Moreover, the climatic conditions, human pressure, industrialisation, and environmental pollution are causing immense damage to ancient edifices. For the conservation of historical plaster, the aesthetic presentation of new repair plaster matching the ancient plaster is desired. It is important to formulate a plaster that meet the physical and chemical properties and also similar to aesthetic and visual appearance. The chemical and mineralogical analysis help to prepare compatible repair plaster matching the ancient one. In order to take up the restoration and repair of monuments of the region, there is a need to prepare compatible materials bracketing the composition of the ancient one. Due to lack of any ancient text on the preparation technology of lime works, it is necessary to analyse ancient mortars and plasters to synthesize compatible restoration lime.
The aggregates added to give strength and durability of the plaster/mortars differ in different climatic zone of India so it’s technology. The western part of India is surrounded by Deccan trap and aggregates are mostly derived from the weathered basaltic rock (Ray et al., 2007, pp. 537–551). Pure quartz grains are also found added in aggregates as quartz veins are frequently encountered in basaltic rock during natural formation (Burley et al., 2022). In the western India coastal region, the basaltic and lateritic rock rich in iron content co-exist-and the aggregates are either mixed sand grains or majorly sand grains derived due to preferential weathering of lateritic rock. To prepare compatible plasters/mortars for repair works, it is necessary to investigate the nature and amount of aggregates mixed in the lime works. This paper investigates the nature, composition and mineralogical characteristics of lime mortar used in the construction of Gopal Krishna temple at Alandi to prepare compatible restoration mortar. The investigative studies are based on grain size and shape analysis, chemical composition by X-ray fluorescence (XRF), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), Scanning electron microscope-energy dispersive X-ray analysis (SEM–EDX) and Thermal analysis (TDA-TGA) of the lime mortar samples of the temple.
2 About the Alandi temple
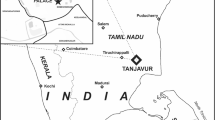
Alandi, an important Hindu pilgrimage centre near western India Pune city is situated at the confluence of Kuberganga Indrayani and Bhagirathi river. Figure 1 shows the location map of Alandi. Alandi (18°40′ N 73°53′ E) is in Khed taluka of Pune district on the left bank of river Indrayani, about 12 miles south of the Khed. Many temples such as Dyneshwar Maharaj temple, Gopal Krishna temple, Rama temple, Bhairavnath temple, Hajeri Maruti temple etc. were constructed during the period twelfth to fourteenth century CE at Alandi (Glushkova, 1997, pp. 199–213; Dindal, 2022). All the ancient temples at Alandi were found constructed using dressed basalt stone block and lime mortar as cementing material. The general view of Gopal Krishna temple, the subject of this study is shown in Fig. 2.
3 Climatic conditions around Alandi temples
The climatic condition at Alandi during rainy season is oppressive, windy and over cast. The average daily high temperature during the rainy season (June to September) is around 30 °C. Alandi also experiences extreme seasonal variation in monthly rain fall. The month with most rain is July, with an average rain fall of 315 mm. The month with least rain fall is January with an average rainfall of 2.5 mm and the average rainfall for the remaining month in rainy season is 12.5 mm. The winter season at Alandi starts from October to February every year and coldest month is January with an average low of 12 °C and high of 30 °C. The summer season starts from February to May with an average wind speed of more than 10.8 miles/h. The average daily high temperature in summer season is above 35 °C the hottest month is May with an average high of 35 °C and low at 23 °C. The windiest month of the year is July with an average wind speed of 15.1 miles/h. The season climatic variations have impacted the heritage structures and caused weathering and structural damage. There is thick vegetational growth all around the monuments Fig. 2a. The plant roots have penetrated deep in the ancient structures causing fissures and cracks Fig. 2b. Some part of the temple has collapsed due to environment impact and vegetational growth Fig. 2c. The damaged part of the temple need restoration with the compatible materials.
4 Materials and methods
Six samples of lime mortars were collected from the external walls by avoiding any external contaminations. The details of lime mortar sample collected from Gopal Krishna temple, Alandi is shown in Table 1. The surface mortars were brushed initially to remove adhering dirt, dust and atmospheric contaminants. Subsequently, a thin external layer of mortar was removed mechanically to expose the fresh inner layer. The mortar samples were scooped through pointed needle to get a representative specimen.
For the analysis of particle size distribution of lime mortar, the samples were gently broken and lightly ground in a porcelain mortar. The large aggregates (> 4.75 mm) were removed using forceps. The remaining material were gently ground so as not to break any aggregate grains. The sample was dried at 80 °C. The dried sample was dissolved in 25% warm HCl, left overnight and filtered using vacuuming. The wet residue was again dissolved in H2O2 overnight and evaporated at slow heating at around 70 °C on a hot plate for about 4 h. The aggregates were washed with de-ionized water, centrifuged twice with de-ionized water, oven dried and weighed to get the actual sand content in the sample. The insoluble residue was sieved through 4 mm, 3.55 mm, 2.36 mm, 1.40 mm, 500 μ, 425 μ, 106 μ and 75 μ sieves in the laboratory. The particle size and shape analysis was carried out at Geo-archaeological laboratory of the Deccan College, Pune.
The lime mortar samples were analysed under X-ray fluorescence spectroscopy (model Artax 200, Bruker, Germany) at National Research Laboratory for Conservation of Cultural Property, Lucknow. All the samples were washed with ethanol by rinsing to remove any contaminants before carrying out XRF analysis. The dried samples were ground to a fine powder and compressed boric acid pallets of the samples were placed in instrument holder. The instrument was operated on 50 kV voltage at 700 mA current and data collection time was 300 live seconds. At least three measurements were taken for each sample and data averaged. The analytical results were reported in the form of major oxides.
The mortar samples A to D were subjected to FTIR analysis to know the functional group present. The FTIR analysis was performed on an oven dried sample at 50 °C. The fine powdered sample was mixed with KBr and pallets were freshly prepared and used for analysis. The FTIR analysis was carried out at Centre for Advance Studies in Chemistry Department of Savitribai Phule University, Pune. The Brucker Alpha II spectrometer attached with platinum ATR with IR counterglow TM technology for continuous optimized light flux was used for analysis. The spectra were recorded at 4 cm−1 resolutions and the number of scan was 64. The KBr beam splitter was used for analysis.
In order to determine the minerals, present in the lime mortar, two samples (A and C) were analysed by X-ray diffraction technique (XRD). For this purpose, the sample were dried at 70 °C for 4 h. The mortar samples were grounded manually by employing an augite mortar. The XRD analysis was carried out by X-ray diffractometer (Model D8 advance make Bruker) on 0.2 gm of each sample and Cu kα radiation source in a 2θ range of 10–90° was considered for this analysis.
The SEM–EDX analysis was carried out at in the Department of Metallurgy, College of Engineering Pune (COEP), Technical University, Pune using Brucker make scanning electron microscope (SEM) with EDX. Plaster samples A to D were coated with the help of a brush on carbon tape and Gold sputtering on the surface of the coated sample was done for conductivity. The images were taken at different magnification on the selected area. The surface elemental composition was determined using EDX system.
Thermogravimetric analysis (TGA) was performed on Sample No. A & B to determine the weight loss that the mortar samples undergone with increase in temperature. The TGA was performed on the DS using Perkin-Elmer STA 6000 instrument under inert atmosphere (N2 gas) using a flow rate of 20 ml/min. Approximately 20 ± 1 mg of the samples were exposed up to 1000 °C from 30 °C at a heating rate of 10 °C/min.
5 Results and discussions
5.1 Grain size analysis
The particle size distribution of aggregates mixed in lime mortar is shown in Fig. 3a and b. The mortar has majority inclusion of sand size grains that make around 55–60% of the total aggregates. The silt size grains are present in proportion of 40–42% of the aggregates and clay particles of size less than 75 μ are around 2–5% in the mortar. In the sand size aggregates maximum of the aggregates grains are of particle size 4, 2.36 and 1.4 mm in the mortar. The sedimantalogical analysis was also carried out to understand the nature of aggregates sourced for the preparation of mortar. Based on the grain size and shape analysis, we aimed to understand the history of the sediments, whether they belong to in-situ location or transported long or short distance by transporting agencies like wind, water, glaciers etc. The average size of the sediments collected from the six samples of mortars clearly shows that majority of the sediments belong to coarse sand to fine silt size and their proportions are decreasing towards fine size. On the basis of size characteristics of the aggregates, it can be concluded that the sediments belong to the area where they have been transported moderate to less distance from the source region and may be categorized as partially mature sedimentary deposits (Bakshi & Singh, 2020, pp. 163–184; Dighe et al., 2021, pp. 106–116).
Figure 3b shows the shape of the aggregates mixed in this mortar. It is observed that majority of aggregate grains are sub-angular to sub-rounded in shape that give teeth to the mortar. The rounded grains are less in the mortar probably due to moderate transportation of the sediments. All the aggregate grains of varied grain size obtained from the laboratory digestion of the mortar is shown in Fig. 4.
5.2 Chemical composition of the mortar
The chemical composition of six mortar samples investigated through X-ray fluoresce (XRF) analysis is shown in Table 2.
The mortar is lime rich binder and calcium plus magnesium oxides together make around 45–50 wt% of the mortar. The magnesium percentage varies between 2.10 and 4.28 wt% indicating that Calcitic limestone having traces of magnesium was sourced for burning to prepare the lime. The SiO2 percentage varied between 14.09 and 16.25 wt% in the mortar which is very less to the ideal lime: silica ratio of 1:3 for the presentday plaster works (Genestar et al., 2006, pp. 373–379). The alumina content varies between 13.68 and 20.12 wt% which is high in proportion in the mortar. One of the important feature of the mortar is high content of iron oxide. The iron content varies between 14.32 and 18.02 wt%. The basaltic rock of the Sahyadri hills in which the temple is located generally contains iron oxide between 3 and 5% (Sukheswala & Poldervaart, 1958, pp. 1473–1494). However, the high content of iron oxide in the mortar indicates mixing of aggregates containing high iron percentage. To investigate this, the formation of surrounding hillock around the temples were studied as local sand grains are the main source of aggregates for mortar preparation.
Laterite is a reddish-brown rock which is essentially composed of hydrated iron oxide and alumina oxide. The iron oxide is present in major quantity that generally preponderates to give red colour to laterite rock. In Alandi region, the laterite rock caps the basaltic stone hillock of high altitudes (Gurav, n.d.). These trap bearing areas give rise to either a dark brown to rich red soil or black cotton soil observed in the plains all over the surrounding areas. These soils are very rich in plant nutrients as it contains lime, magnesia, iron and alkalies.
Isolated outcrops of primary laterite are observed in the source regions of Indrayani River (Sukheswala & Poldervaart, 1958, pp. 1473–1494). This excellent exposure has a solid thickness of 1.5–2 m. pisolithic crust underlain by 2.5–3 m. clay grading. Presently the laterite occurs as isolated patches, but originally it must have been continuous (Rajaguru, 1970). The laterite cap present in the source region get preferential disintegration by weathering action continuously and loose particles are transported by heavy rainwater from Sahyadri escarpment to the downstream region. Such sediments by erosion action converted into fine red aggregates, which is rich in iron. During the low energy flow, these sediments deposited along the both channel of Indrayani river. At the time of construction of cultural heritage in this region the building blocks, i.e., the basaltic stone blocks and the cementing/binding material mixed with aggregates were used. In the present context the used mortar in the temple construction shows essential amount of iron. The major iron percentage in the mortar clearly indicate that the aggregate has originated from laterite sources that caps the basaltic hillock in this region and get deposited in Indrayani River basin due to weathering. Therefore, analytical analysis of several mortar samples shows the mortar used in the construction is rich in iron content. In majority of the ancient constructions in western India, the aggregates derived from the disintegration of local basaltic stones have been used as filler. The basaltic grains give mechanical strength and durability to the plaster, however, the aggregates do not provide any hydraulicity to the plaster as they are derived of any active alumina and silica for chemical reaction with the lime. The lime plaster of Gopal Krishna temple is non-hydraulic air lime.
5.3 FTIR analysis
The FTIR analysis of lime mortar sample A to F is shown in Fig. 5. The FTIR peaks at 1740 cm−1 in the mortar sample is associated with C=O bond (Steffen et al., 2019, pp. 405–410 and intense peak at 1490 cm−1 is attributed to carbonates (Singh & Kumar, 2019, pp. 1–14; Kasem et al., 2020, pp. 515–527). The peaks at 878, 1440 and 2415 cm−1 are attributed to calcium carbonate of the mortar (Kant & Singh, 2019). The peaks between 754 and 800, 1070, 1160, 1250 to 1270 cm−1 represent Si–O–Si group in the mortar (Yan et al., 2012, pp. 1052–1057). The infrared band near 525 cm−1 suggest low intensity hematite peak (Singh & Kumar, 2018, pp. 482–496), which was also identified through XRF, XRD and SEM–EDX studies of the mortar. However, in the infrared spectroscopy only low concentration of iron oxide was detected due to more clay content along with Fe–O, Si–O peaks. Moreover, peaks near lower band of 480, 450 cm−1 are observed in the FTIR spectra denoting presence of Fe–O in the mortar (Scheidegger et al., 1993, pp. 43–65). The preferential weathering of laterite capping in the vicinity of Alandi hillock has contributed to the presence of iron oxide in the mortar. The FTIR analysis is in agreement to the chemical composition of the mortar.
5.4 XRD analysis
The representative XRD spectra of mortar samples A and C is shown in Fig. 6A and C. Due to similarities in mineralogical composition XRD data for two samples is only shown. The major peaks observed are calcite, quartz, magnetite, hematite, orthoclase and gypsum. It is observed that coat of gypsum has been applied as isolated patch recently on the outer surface of the temple wall and it has seeped inside into the mortar at some point. The traces of gypsum identified in sample A & C is due to recent contamination. Calcite is the major peak in XRD in all the samples. The mortar is marked with the presence of peaks for the quartz in major quantity. The alumino-silicates minerals are present in the mortar in the form of peaks of orthoclase. The hydrated iron and almuna oxides present in the laterite aggregates are responsible for orthoclase minerals in the mortar. The peaks of iron oxides, the magnetite and hematite are observed in the XRD spectra of the mortar. The lateritic aggregates inclusion in the mortar has contributed to the presence of iron minerals. The XRD analysis supports the FTIR and XRF data of the lime mortar.
5.5 SEM–EDX analysis
The lime mortar sample A to D were subjected to SEM–EDX analysis and the SEM photomicrograph is shown in Fig. 7A–D. Due to close morphological similarities of the lime plaster only four samples were studied using SEM. The SEM photomicrograph at the magnification 5000X (Fig. 7A) shows the blocky pore filling kaolinite (clay) separated and linked by ribbons or sheet of filamentous structure which are partially filling the micro pores. The SEM photomicrograph at the magnification of 10000X (Fig. 7B) shows quartz overgrowth partially filling a pore between detrital quartz grains. The pores are partly filled with large quartz overgrowth. In the open pores, these overgrowths grew unobstructed developing smooth, euhedral faces. In the SEM photomicrograph at the magnification of 5000X (Fig. 7C) patches of calcite are seen in between quartzes grains. The calcite appears slightly dissolved in the quartz grains. Fig. 7D shows the SEM photomicrographs of the mortar sample at a magnification 5000X. The image is marked by the presence of iron oxide in the mortar shown by the surrounding lime. The SEM analysis of the samples support the XRF, FTIR and XRD investigation of mortar.
The EDX of the mortar samples A–D is listed in Table 3. There is presence of iron oxide in all the mortar samples due to addition of laterite aggregates during mortar preparation. The amount of iron detected through EDX analysis of the plaster varied between 1.10 and 1.83 wt%. The alumina and iron presence is due to hydrated oxides of iron and aluminium present in laterite. There is traces of magnesium and the mortar is rich in calcium content.
5.6 Thermogravimetric analysis
The TGA and differential thermogravimetry (DTG) results of the lime mortar samples A and B are presented in Fig. 8A and B. As the samples were similar in composition, representative DTA/TGA of plaster were only carried out. From the figures, it can be noticed that both the mortar sample approximately loose about 16–18% weight when exposed to temperature of around 800 °C. In addition, the mortar sample show dip at around 750 °C in the DTG and there is a constant loss with a sharp dip starting from 650 °C. The probable reason for this may be evaporation of the moisture, decomposition of organic matter and dihydroxylation of hydrated oxides of alumina and iron present in the laterite aggregates mixed in the mortar. The TGA shows continuous weight loss of the mortar with a sharp dip at around 700 °C and thereafter gain in weight with increase in temperature. As the pure calcium carbonate loses weight at about 850 °C the presence of clay and traces of magnesium has lowered the melting temperature of the lime (Maniatis, 2009).
6 Conclusions
The cementing plaster used in the construction of Gopal Krishna temple at Alandi is a calcium rich non-hydraulic lime with traces of magnesium. The higher content of alumina and iron oxide in the mortar is related to preferential weathering of laterite caping on Sahyadri range of basaltic hillock during natural geological formation. The weathered laterite sediments deposited on riverbank was sourced as aggregate for mortar preparation. The mortar has inclusion of large size lateritic sand grains and fine grains are less in quantity. The analytical data will help to prepare compatible mortar in terms of durability, strength and composition needed for the major restoration of the temple architect.
Data Availability
Not applicable.
References
Bakshi, S., & Singh, M. R. (2020). A petrochemical study of Mughal plasters of Quila-I-Ark, Aurangabad with respect to technology and repair. History of Science and Technology, 10(2), 163–184.
Burley, S. D., Gould, T., Taylor, A., & Mishra, P. (2022). Syn-rift volcanism in the Barmer Basin: An intra-basin extrusive complex at the northern limit of the Deccan Volcanic Province in India. Geological Journal. https://doi.org/10.1002/gj.4659
Dighe, B., Singh, M., & Karche, T. (2021). Traditional use of organic additives (bamboo foliage, flax fibre and millet grains) in 16th century lime plaster of Solapur Fort, India. Indian Journal of Traditional Knowledge (IJTK), 20(1), 106–116.
Dindal C. (2022). Study of natural springs (Kunda’s) at Alandi: A religious textual and scientific perspective. An unpublished MA Dissertation from Deccan College Deemed University, Pune.
Eraly, A. (2002). Gem in the lotus. Penguin.
Genestar, C., Pons, C., & Más, A. (2006). Analytical characterisation of ancient mortars from the archaeological Roman city of Pollentia (Balearic Islands, Spain). Analytica Chimica Acta, 557(1–2), 373–379.
Glushkova, I. (1997). In quest of Alandi-Mahatmya. Annal of the Bhandarkar Oriental Research Institute, 78(1), 199–213.
Gurav, M. D. (n.d.). Retrieved from https://www.lulu.com/es/shop/dr-mahadev-d-gurav/a-geographical-study-of-fairs-and-festivals-in-pune-district/paperback/product-1g28878k.html
Kant, A. P., & Singh, M. R. (2019). Spectroscopic and chromatographic investigation of the wall painted surface of an 18th century Falian Temple, New Delhi. Vibrational Spectroscopy, 104, 102947.
Kasem, M. A., Yousef, I., Alrowaili, Z. A., Zedan, M., & El-Hussein, A. (2020). Investigating Egyptian archeological bone diagenesis using ATR-FTIR microspectroscopy. Journal of Radiation Research and Applied Sciences, 13(1), 515–527.
Maniatis, Y. (2009). The emergence of ceramic technology and its evolution as revealed with the use of scientific techniques. na.
Morrison, K. D. (1995). Trade, urbanism, and agricultural expansion: Buddhist monastic institutions and the state in the early historic western Deccan. World Archaeology, 27(2), 203–221.
Naravane, M. S. (1998). The maritime and coastal forts of India. Aph Publishing.
Panda, S. S., Mahapatra, P. K., Chaturvedi, R. K., & Kar, S. K. (2013). Chemical analysis of acncient mortar from excavation sites of Kondapur, Andhra Pradesh, India to understand the technology and ingredients. Current Science, 105(6), 837–842.
Rajaguru, S. N. (1970). Studies in the late Pleistocene of the Mula–Mutha. Unpublished Ph.D. Dissertation, University of Poona.
Ray, R., Sheth, H. C., & Mallik, J. (2007). Structure and emplacement of the Nandurbar-Dhule mafic dyke swarm, Deccan traps, and the tectonomagmatic evolution of flood basalts. Bulletin of Volcanology, 69, 537–551.
Sathyabhama, B., Hema, A., Smriti, H., & Mohandas, K. P. (2011). Salvankuppam coastal temple excavation and application of soil micromorphology. Current Science, 100(7), 1071–1075.
Scheidegger, A., Borkovec, M., & Sticher, H. (1993). Coating of silica sand with goethite: Preparation and analytical identification. Geoderma, 58(1–2), 43–65.
Selvaraj, T., & Ramadoss, R. (2015). Analysis and characterization of third century ancient mortars at Subramanyaswamy temple rediscovered after the 2004 tsunami near Mamalaapuram shore. India International Journal of Conservation Science, 9(1), 25–38.
Sen, G. (2001). Generation of Deccan trap magmas. Journal of Earth System Science., 110(4), 409–431.
Sheth, H., Samant, H., Patel, V., & D’Souza, J. (2017). The volcanic geoheritage of the Elephanta caves, Deccan traps, western India. Geoheritage, 9, 359–372.
Singh, M. (1991). Analysis of Golkonda fort plaster, Ist international colloquiums on role of chemistry in archaeology (pp. 81–86). BACRI.
Singh, M. R. (2017). Dolomitic plasters in Indian monuments and its characterization. Journal of Historical Archaeology & Anthropological, 1(6), 237–241.
Singh, M. R., Ganaraj, K., & Sable, P. D. (2020). Surface mediated Ca-phosphate biomineralization and characterization of the historic lime mortar, Janjira Sea Fort, India. Journal of Cultural Heritage, 44, 110–119.
Singh, M., & Kumar, S. V. (2018). Mineralogical, chemical, and thermal characterization of historic lime plasters of thirteenth–sixteenth century Daulatabad fort, India. Studies in Conservation, 63, 482–496.
Singh, M. R., & Kumar, S. V. (2019). Architectural features and charecterisation of 16th century Indian monument Farah Bagh, Ahmed Nagar, India. International Journal of Architectural Heritage, 14, 1–14.
Singh, M., Kumar, S. V., & Waghmare, S. A. (2015). Characterization of 6–11th century AD decorative lime plasters of rock cut caves of Ellora. Construction and Building Materials, 98, 156–170.
Singh, M., Waghmare, S., & Kumar, S. V. (2014). Characterization of lime plasters used in 16th century Mughal monument. Journal of Archaeological Science, 42, 430–434.
Singh, S. K., & Singh, M. (2020). The mineralogical and physical behaviour of brick aggregates in twelth century brick-lime step-well plasters of Gabdhak-ki-baoli, New Delhi. Journal of Architectural Conservation, 26(2), 184–200.
Steffen, T. T., Fontana, L. C., Hammer, P., & Becker, D. (2019). Carbon nanotube plasma functionalization: The role of carbon nanotube/maleic anhydride solid premix. Applied Surface Science, 491, 405–410.
Sukheswala, R. N., & Poldervaart, A. (1958). Deccan basalts of the Bombay area, India. Geological Society of America Bulletin, 69(12), 1473–1494.
Wescoat, J. L., Jr. (2019). Human use of landforms on the Deccan Volcanic Plateau: Formation of a geocultural region. Geomorphology, 331, 175–190.
Yan, W., Liu, D., Tan, D., Yuan, P., & Chen, M. (2012). FTIR spectroscopy study of the structure changes of palygorskite under heating. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 97, 1052–1057.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, S., Singh, M. & Sabale, P.D. Composition and characterisation of ancient lime mortar of Gopal Krishna temple, Alandi, India. Indian J Hist. Sci. 58, 271–282 (2023). https://doi.org/10.1007/s43539-023-00103-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43539-023-00103-2