Abstract
Purpose
Postoperative adhesions can be prevented by the use of bioabsorbable anti-adhesion barriers. Although the occurrence of postoperative bowel obstruction is an important concern for patients, at the time of approval of anti-adhesion barriers, its effectiveness in preventing postoperative bowel obstruction had not been evaluated. We aimed to retrospectively evaluate the incidence of bowel obstruction after colectomy in patients with colon cancer using an insurance claims database.
Methods
This retrospective cohort study analyzed the data of colon cancer patients (between 2005 and 2017 from a national insurance claims database) who underwent colectomies to compare the proportion of individuals with postoperative bowel obstruction between the barrier and no barrier groups.
Results
Of the 587 patients who met the inclusion criteria, 308 and 279 patients were identified as the barrier and no barrier groups, respectively. The incidence of postoperative bowel obstruction was significantly lower in the barrier group (log-rank test, P = 0.0483). The cumulative incidence of postoperative bowel obstruction 37 months after the initial colectomy was 6.1% and 10.9% in the barrier and no barrier groups, respectively. Moreover, consistent results were obtained in the matched cohort.
Conclusion
In colectomies for patients with colon cancer, the use of anti-adhesion barriers could significantly reduce the incidence of postoperative bowel obstruction. Evaluations using insurance claims databases could provide important information on outcomes following implementation of medical devices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Postoperative adhesions occur commonly and frequently after various abdominal surgical procedures, such as after 93–100% and 67–93% of upper and lower gastrointestinal surgeries, respectively [1]. In extreme cases, adhesions can cause postoperative bowel obstruction (PBO), resulting in prolonged hospitalization or reoperation. Approximately 60% of bowel obstructions are caused by postoperative adhesions. This poses a huge mental, physical, and economic burden on patients and their caregivers [2,3,4,5]. The mean duration of hospitalization for PBO was reported to be 16.3 and 7.0 days for surgical treatment and conservative treatment, costing £4,677.41 and £1,606.15, respectively [6].
The prevention of postoperative adhesions is the best strategy to prevent PBO, and one strategy for the prevention of postoperative adhesions is the use of a bio-absorbable anti-adhesion barrier during surgery. Currently, in Japan, four medical devices, namely Seprafilm® (a film product containing sodium hyaluronate and carboxymethylcellulose; Baxter, Chicago, IL, USA), Interceed® (a cloth-like sheet of oxidized regenerated cellulose; Johnson & Johnson, New Brunswick, NJ, USA), Ad Spray® (a spray gel of N-hydroxysuccinimidated carboxymethyl dextrin/trehalose hydrate; Terumo, Tokyo, Japan), and Tenaleaf® (a film product of gelatin derived from swine skin; Gunze, Tokyo, Japan) have been approved. Although the main ingredients of the products differ, all four products transform into a gel at the surgical site, work as adhesion barriers, and are biodegradable and absorbable.
Considering the characteristics of these medical devices, their efficacies were assessed by their preventive effects against postoperative adhesions and not against PBO in clinical trials. The primary endpoints of previous clinical trials were occurrence, area, the severity of postoperative adhesions in patients after total colectomies with ileostomies, and pelvic peritoneal adhesions in patients with rectal cancer or after uterine myomectomies [7,8,9,10]. It has been reported that Seprafilm® significantly decreased the occurrence and total area of postoperative adhesions and the reoperation rate of abdominal surgery in patients with inflammatory bowel disease. However, it showed no obvious preventive effect against the occurrence of PBO, mainly due to the low incidence of PBO [11, 12]. To clarify the effectiveness of anti-adhesion barriers against PBO, more patients may have been needed, which is not ideal for clinical trials. To the best of our knowledge, there is no medical evidence that the intra-operative use of a bioabsorbable anti-adhesion barrier prevents PBO. Although the preventive effects against postoperative adhesions were shown for the purpose of receiving approval, their preventive effects against PBO are still unknown.
The occurrence of PBO is an important concern for patients, especially those with high co-morbidity, and therefore, it is of great significance to evaluate.
In this study, we focused on patients who underwent colectomies for colon cancer because this surgical operation has a high risk of PBO [13, 14]. We used real-world data from a Japanese nationwide health insurance claims database. In Japan, a universal health insurance system has been adopted, and insurance claims data can be treated as a registry of long-term medical records. These large datasets were expected to provide a sufficient number of cases without exposing patients to the risk of being placed in the no-treatment arm in clinical trials in order to evaluate the impact of anti-adhesion barriers on PBO.
Methods
The study protocol was approved by the Ethics Committees of the Pharmaceuticals and Medical Devices Agency (approval number: R02-1, May 22, 2020) and Gifu Pharmaceutical University (approval number: 2–2, July 31, 2020). The requirement for informed consent was waived due to the untraceable and anonymous nature of the data.
Data Sources
This retrospective cohort study used an anonymized nationwide claims database constructed by the Japan Medical Data Center (JMDC) (Tokyo, Japan). JMDC has anonymized monthly billing receipts collected from hospitals, clinics, and pharmacies, providing datasets that make it possible to track individual data [15]. Data from January 2005 to December 2017 were collected, which included data from 25 health insurance associations on 1.9 million people insured under 75 years of age and their families. This constituted 1.5% of the Japanese population. Each product has been used in Japan since 1991 for Interceed®, 1997 for Seprafilm®, 2016 for Ad Spray®, and 2021 for Tenaleaf®. This means that the use of Interceed®, Seprafilm®, and Ad Spray® were included in the data of this study. In this study, the codes based on the International Classification of Diseases, 10th Edition (ICD-10), Anatomical Therapeutic Chemical, and medical device billing, medical procedure and surgery fee codes are denoted in brackets.
Study Cohort
Patients who underwent their first colectomy (K719 as surgery fee code) between January 2005 and December 2017 were included. The following patients were excluded from the analysis: those with receipt records less than 6 months prior to surgery, those with no diagnosis of colon cancer (C108-C189) prior to surgery, and those with insufficient information on the date of surgery. The selected patients were classified as follows: “group with an anti-adhesion barrier (Barrier group)” and “group without an anti-adhesion barrier (No barrier group),” according to the use of anti-adhesion barriers (730,840,000, 710,011,008, 710,011,009 as medical device billing code).
Outcome Measures
The primary outcome measure was the occurrence of PBO. PBO was defined as long tube insertion for ileus (140,007,010 as procedure fee code) or bowel obstruction surgery (150,180,210, 150,180,350, 150,180,650, 150,180,750, 150,180,850, 150,180,950, 150,271,550, 150,299,350 as procedure fee code). Baseline demographics included age, sex, follow-up period, previous intra-abdominal surgery, simultaneous surgeries, pre-/postoperative radiotherapy, history of diabetes, and postoperative chemotherapy. Radiotherapy was defined as the implementation of radiotherapy (M001-M005), and a history of diabetes mellitus (DM) was defined as the presence of prescribed DM medications (A10). Patients with type 1 and type 2 DM were not distinguished in this study. Considering that postoperative chemotherapy is recommended for patients with high-risk stage II and III cancer [16], the presence or absence of prescriptions for anti-cancer agents (L01) within 3 months after surgery was included as supplemental information regarding the cancer stage. These items were selected based on patient backgrounds in previous studies [12, 17].
Statistical Analyses
Data of patients who withdrew their insurance were considered censored. Continuous variables are expressed as medians and interquartile ranges (IQRs). Categorical variables are expressed as absolute numbers and percentages (%). Continuous and categorical variables were compared using the Mann–Whitney U and chi-squared tests, respectively. To balance patient backgrounds, propensity score matching was performed when the standardized mean difference between groups exceeded 0.1. Kaplan–Meier analysis was performed to evaluate the differences in the cumulative incidence of PBO, the primary endpoint, and a log-rank test was performed. P-values less than 0.05 were considered statistically significant. JMP Clinical 8.0 (SAS Institute Inc., Cary, NC, USA) was used for the statistical analysis.
Results
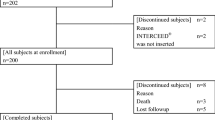
From claims data of 1.9 million people from 2005 to 2017, we identified 973 patients who underwent colectomy and excluded 386 patients based on at least one exclusion criterion. A total of 587 patients were eligible, of which 308 had anti-adhesion barriers and 279 did not (Fig. 1). Because the standardized mean difference between groups at baseline exceeded 0.1, we performed propensity score matching. Covariates were age, sex, previous abdominopelvic surgery, simultaneous surgeries, radiotherapy, postoperative radiotherapy, DM, and postoperative chemotherapy, and the propensity score was calculated by logistic regression analysis. Tables 1 and 2 summarize the patient characteristics in the unmatched cohort and matched cohort, respectively.
Overall, the median follow-up period was 26 months (IQR: 11.0–52.0) for both the unmatched and matched cohorts.
Figure 2 shows the primary endpoint findings of the unmatched cohort. The cumulative incidence of PBO throughout the follow-up period, the primary endpoint, was significantly lower in the group with barriers (log-rank test, P = 0.0483). The cumulative incidence of PBO, 37 months after the initial colectomy (when the last PBO occurred), was 6.1% and 10.9% in the barrier and no barrier groups, respectively. Figure 3 shows the primary endpoint findings of the matched cohort. The cumulative incidence of PBO throughout the follow-up period, the primary endpoint, was significantly lower in the group with barriers (log-rank test, P = 0.0153). The cumulative incidence of PBO, 32 months after the initial colectomy (when the last PBO occurred), was 5.1% and 11.8% in the barrier and no barrier groups, respectively.
Discussion
This study used an anonymized insurance claims database to evaluate the efficacy of anti-adhesion barriers in preventing PBO in patients with colon cancer undergoing colectomies. To the best of our knowledge, this is the first study to assess the preventive efficacy of anti-adhesion barriers against PBO after colectomies. The study found a significant reduction in the incidence of PBO after the first colectomy with the use of an anti-adhesion barrier compared with the use of no anti-adhesion barrier. Conducting a randomized controlled trial on a medical device that has already been approved is difficult from a patient-ethics perspective. This study is unique in that the long-term effectiveness of anti-adhesion barriers for PBO was evaluated using real-world data.
The strength of this study lies in its objective and rigorous definition of the target patients and outcomes. In this study, eligible patients and outcomes were defined using procedure codes. Although the Japanese claims database has a high specificity regarding codes for procedures, prescriptions, and devices, it has been noted that diagnostic names may contain “provisional diagnoses” for billing purposes and that codes must be combined [18, 19]. Outcomes were evaluated based on this objective definition, so the risk of detection bias was low.
In a previous study conducted on patients with colorectal cancer treated with colorectal resection, the incidence of PBO occurring beyond 30 days and up to 20 months (when the last PBO occurred) was 2.7% and 4.6% in the Seprafilm® and control groups, respectively [17]. In the present study, the cumulative incidence of PBO in the unmatched cohort was 6.1% in the group with barriers and 10.9% in the group without barriers, compared to 5.1% in the group with barriers and 11.8% in the group without barriers in the matched cohort. These cumulative incidences of PBO were both higher than in a previous study. One reason for this difference is presumably that this study could not exclude PBO caused by cancer progression due to the lack of information in the datasets. We believe that this does not affect our conclusions because PBO caused by cancer progression occurs in both the barrier and no barrier groups.
Another study conducted on patients with colon cancer treated with colectomies reported that 7.8% and 10.6% of patients in the Seprafilm® and control groups, respectively, had PBO [20], which was generally consistent with our results. In this study, both groups showed a continuous increase in the incidence of PBO over approximately 3 years. This trend is similar to those reported in previous studies [12, 20]. Postoperative adhesion formation may contribute to the development of PBO after several years after surgery, and long-term observation may be necessary to evaluate PBO.
Note that there have been changes in the treatment of colorectal cancer between 2005 and 2017 in terms of the increase in laparoscopic surgery and the first approval of molecularly targeted drugs in 2007 [21, 22]. However, these occurred in both the barrier and no barrier groups, and therefore, we do not consider them to affect our conclusions.
Laparoscopy with a scheduled second look is the primary method of evaluating and diagnosing adhesions by visualization of their presence and extent [23]. In terms of this technique, it has been noted that although a single band of adhesive tissue can cause PBO, extensive and dense intra-abdominal adhesions may also be asymptomatic [24]. However, the more important clinically relevant outcomes are PBO and operative time for reoperation, which are difficult to compare in prospective trials owing to the diversity of causes and the need for long-term follow-up data [25]. From a health-economic perspective, hospitalization for PBO reportedly costs approximately €2,000 for non-operative cases and €16,000 for cases requiring surgery [26], emphasizing the importance of evaluating clinically relevant outcomes, such as PBO. PBO is a significant mental and physical burden for patients, and it is significant to evaluate the occurrence of PBO as an outcome. The insurance database is unique in that it can identify the same individual even if he or she has been treated at different medical institutions within the same health insurance association enrollment period. In addition, data pertaining to procedure fees, drug prescriptions, and the use of specific insured medical devices are reflected with extreme accuracy, as reimbursement is not available unless a claim is filed. Evaluation using insurance claims databases may be useful for issues where the procedure is the endpoint and long-term follow-up is required and may help to compensate for aspects of efficacy that are difficult to assess using clinical trials alone.
The limitations of this study are mentioned below.
-
The data we analyzed did not include medical information, such as laboratory, imaging, and physical findings of the patients, or the diagnosis of symptoms and stage of the disease by the healthcare provider; these requirements have not been considered. Therefore, it was not possible to determine whether PBO was caused by adhesions resulting from surgery or by other factors (e.g., aggravation of the underlying disease). Determination of the cause of PBO could help in the reduction of its incidence. We do not believe that our conclusions are affected by this limitation since aggravation of the underlying disease occurs in both the barrier and no barrier groups.
-
No surgical information was provided. Therefore, information on the location of the placement of anti-adhesion barriers was not collected. A drawback of anti-adhesion barriers is that their actions are limited to the site of placement [24]. The effects of different placements are not reflected in the results. In addition, its direct application to intestinal anastomoses has been reported to increase the complications of fistula formation [27]; however, this disadvantage has not been evaluated.
-
Because people over 75 years of age are not eligible for health insurance coverage by health insurance associations, data for these individuals are not included in the JMDC database [15]. Patients over 75 years of age should be evaluated using a different data source.
-
Data from 25 health insurance associations on 1.9 million insured persons under the age of 75 years and their families, representing 1.5% of the Japanese population, were used. Data from a limited number of facilities were used, and thus, it may not be sufficient for generalization.
-
Not all potential confounders and risk factors for PBO were included in the database, and unmeasured factors have not been adjusted for. Combining this with the data from another database would provide more accurate results.
Conclusion
During colectomies for patients with colon cancer, the use of anti-adhesion barriers could significantly reduce the incidence of PBO compared to the non-use of anti-adhesion barriers. This study evaluated the effectiveness of these medical devices using outcomes of interest to patients. This study provides important information for future research on medical device evaluation.
References
Ouaïssi M, Gaujoux S, Veyrie N, et al. Post-operative adhesions after digestive surgery: their incidence and prevention: review of the literature. J Visc Surg. 2012;149(2):104–14. https://doi.org/10.1016/j.jviscsurg.2011.11.006.
Sikirica V, Bapat B, Candrilli SD, et al. The inpatient burden of abdominal and gynecological adhesiolysis in the US. BMC Surg. 2011;11(1):13. https://doi.org/10.1186/1471-2482-11-13.
Ten Broek RP, Issa Y, Van Santbrink EJ, et al. Burden of adhesions in abdominal and pelvic surgery: systematic review and meta-analysis. BMJ. 2013;347:f5588. https://doi.org/10.1136/bmj.f5588.
Krielen P, Stommel MWJ, Pargmae P, et al. Adhesion-related readmissions after open and laparoscopic surgery: a retrospective cohort study (SCAR update). Lancet. 2020;395:33–41. https://doi.org/10.1016/S0140-6736(19)32636-4.
Hernandez MC, Finnesgard EJ, Shariq OA, et al. Disease severity and cost in adhesive small bowel obstruction. World J Surg. 2019;43(12):3027–34.
Menzies D, Parker M, Hoare R, et al. Small bowel obstruction due to postoperative adhesions: treatment patterns and associated costs in 110 hospital admissions. Ann R Coll Surg Engl. 2001;83:40–6.
Seprafilm package insert (https://www.info.pmda.go.jp/downfiles/md/PDF/480348/480348_20900BZY00790000_A_14_01.pdf), accessed Mar 3, 2023.
Interceed package insert (https://www.info.pmda.go.jp/downfiles/md/PDF/340216/340216_20300BZY01058000_A_07_05.pdf), accessed Mar 3, 2023.
Ad Spray package insert (https://www.info.pmda.go.jp/downfiles/md/PDF/470034/470034_22800BZX00234000_A_01_04.pdf), accessed Mar 3, 2023.
Tenaleaf package insert (https://www.info.pmda.go.jp/downfiles/md/PDF/250090/250090_30300BZX00289000_A_01_02.pdf), accessed Mar 3, 2023.
Becker JM, Dayton MT, Fazio VW, et al. Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based bioresorbable membrane: a prospective, randomized, double‐blind multicenter study. J Am Coll Surg. 1996;183(4):297–306.
Fazio VW, Cohen Z, Fleshman JW, et al. Reduction in adhesive small bowel obstruction by Seprafilm adhesion barrier after intestinal resection. Dis Colon Rect. 2006;49(1):1–11.
Barmparas G, Branco BC, Schnüriger B, et al. The incidence and risk factors of post-laparotomy adhesive small bowel obstruction. J Gastrointest Surg. 2010;14:1619–28.
Williams SB, Greenspon J, Young HA, et al. Small bowel obstruction: conservative vs. surgical management. Dis Colon Rectum. 2005;48(6):1140–6.
Nagai K, Tanaka T, Kodaira N, et al. Data resource profile: JMDC claims database sourced from health insurance societies. J Gen Fam Med. 2021;22(3):118–27.
Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25(1):1–42.
Park CM, Lee WY, Cho YB, et al. Sodium hyaluronate-based bioresorbable membrane (Seprafilm®) reduced early postoperative intestinal obstruction after lower abdominal surgery for colorectal cancer: the preliminary report. Int J Colorectal Dis. 2009;24(3):305–10.
Okui T, Nojiri C, Kimura S, et al. Performance evaluation of case definitions of type 1 diabetes for health insurance claims data in Japan. BMC Med Inf Decis Mak. 2021;21(1):52.
Fujihara K, Yamada-Harada M, Matsubayashi Y, et al. Accuracy of Japanese claims data in identifying diabetes-related complications. Pharmacoepidemiol Drug Saf. 2021;30:594–601.
Saito G, Sadahiro S, Ogimi T, et al. Preventive effects of a synthetic absorbable antiadhesive film (seprafilm) on small bowel obstruction in patients who underwent elective surgery for colon cancer: a randomized controlled trial. J Surg Oncol. 2019;120(6):1038–43.
Kang W. Past and current status of colorectal cancer surgery. J Nihon Univ Med Ass. 2022;81(5):255–65.
Takahashi T, Matsuhashi N, Yoshida K. The development of a new molecular-targeted medicine for metastatic colorectal cancer. J Jpn Soc Coloproctol. 2018;71:417–24.
De Wilde RL, Devassy R, Broek RPGT, et al. The future of adhesion prophylaxis trials in abdominal surgery: an expert global consensus. J Clin Med. 2022;11(6):1476. https://doi.org/10.3390/jcm11061476.
Diamond MP, Wexner SD, diZereg GS, et al. Adhesion prevention and reduction: current status and future recommendations of a multinational interdisciplinary consensus conference. Surg Innov. 2010;17(3):183–8. https://doi.org/10.1177/1553350610379869.
Ten Broek RPG, Stommel MWJ, Strik C, et al. Benefits and harms of adhesion barriers for abdominal surgery: a systematic review and meta-analysis. Lancet. 2014;383(9911):48–59. https://doi.org/10.1016/S0140-6736(13)61687-6.
Krielen P, van den Beukel BA, Stommel MWJ, et al. In-hospital costs of an admission for adhesive small bowel obstruction. World J Emerg Surg. 2016;11:49. https://doi.org/10.1186/s13017-016-0109-y.
Beck DE, Cohen Z, Fleshman JW, Adhesion Study Group Steering Committee, et al. A prospective, randomized, multicenter, controlled study of the safety of Seprafilm adhesion barrier in abdominopelvic surgery of the intestine. Dis Colon Rectum. 2003;46(10):1310–9. https://doi.org/10.1007/s10350-004-6739-2.
Acknowledgements
We would like to thank Editage (www.editage.com) for the English language editing.
Funding
All authors have received no external funding.
Author information
Authors and Affiliations
Contributions
Concept and design: All. Acquisition of data: RI, TH. Data analysis: RI. Interpretation of data: All. Drafting of the manuscript: RI, NM, KT. Critical revision of manuscript: All. Final approval: All.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflicts of interest to declare. The views expressed in this article are those of the authors and do not necessarily reflect the official views of the Pharmaceuticals and Medical Devices Agency.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iwata, R., Mochizuki, S., Hasegawa, T. et al. Preventive Effects of Bioabsorbable Anti-Adhesion Barriers on Bowel Obstruction After Colectomy in Colon Cancer Patients: A Retrospective Cohort Study Using an Insurance Claims Database. Ther Innov Regul Sci 58, 831–837 (2024). https://doi.org/10.1007/s43441-024-00660-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43441-024-00660-3