Abstract
The glutamate decarboxylase (Gad) system is an important amino acid-dependent acid resistance system commonly found in microorganisms. Actinobacillus succinogenes is one of the best natural producers of succinic acid (SA) but lacks glutamate decarboxylase. This study assessed the effects of Gad system introduction into A. succinogenes. The recombinant strains gadB-SW and gadBC-SW were constructed by heterologous expression of gadB alone, or gadB together with gadC, respectively. After 1.0 and 1.5 h of acid stress at pH 4.6, cell survival of gadBC-SW was greater than gadB-SW. The growth of gadB-SW and gadBC-SW was both affected by the expression of heterologous proteins and by γ-aminobutyric acid, with gadBC-SW growth reduced at a neutral pH. SA production in acidic conditions was evaluated by a shake flask and by 3-L bioreactor fermentation. The results showed gadBC-SW to increase SA production by 8.4% in shake flask compared to the parent strain, SW. For a 3-L bioreactor batch fermentation under acidic environment, the highest conversion rate of sugar to SA was observed for gadBC-SW, reaching 96%. However, SA concentration by gadBC-SW was only 47 g/L and 31 g/L at pH 6.5 and pH 6.0, respectively. In summary, the introduction of heterologous gadB and gadC into A. succinogenes not only improved acid tolerance but also influenced the synthesis of SA and added a metabolic burden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbial production of organic acids is a promising approach by which to obtain building-block chemicals for use as renewable carbon [1, 2]. Succinic acid (SA) is an important platform intermediate in that it has two functional carboxyl groups that can be used for final product diversity. SA is widely used in chemical, pharmaceutical, food, and agricultural industries. Efficient SA fermentation, as a cheap and renewable source of carbon, may out compete petrochemical synthesis [3]. SA is a common metabolic product of many micro-organisms. Actinobacillus succinogenes is a Gram-negative facultative anaerobic bacterium that ferments a wide range of carbohydrates to succinate, acetate, and formate. The bacterium is one of the best natural sources of high concentrations of SA [4, 5]. A. succinogenes 130Z (ATCC 55618) can produce 74 g/L of succinate. The bacterium was first isolated from bovine rumen. A derived mutant, FZ53, can produce 106 g/L succinate [6, 7], which demonstrates the feasibility of microbial fermentation as a means by which to produce SA.
With A. succinogenes, high yields of SA can be achieved, although it is likely that products of fermentation inhibit both cell growth and SA production. Cell growth is affected adversely at low pH. The Aiba-Shoda model quantified inhibited production of SA by A. succinogenes ZT-130 [8]. The initial SA concentration affected final SA production, with SA yield decreased from 1.11 to 0.49 g/g with an initial 20 g/L of SA. With anaerobic culture conditions, A. succinogenes generates SA through TCA reductive pathways (C4), acidifying the medium. The optimal pH for A. succinogenes growth is 7.0, with fermentation at neutral pH [9]. pH neutralizing agents such as MgCO3, CaCO3, NaOH, or KOH are used to neutralize acidic culture medium, sustaining stable production of SA at pH ranging from 6.0 to 7.2 during fermentation [10, 11]. Acid neutralization increases fermentation cost and downstream separation [12, 13].
In our previous transmission electron microscopy study, A. succinogenes cell membranes were found to be severely damaged by acid stress at pH 4.7. As initial pH decreased, cell growth was inhibited and the activity of H+-ATPase decreased [14]. These results suggested that A. succinogenes was intolerant to acid even though it was an acidogenic bacterium. Hence, the use of A. succinogenes at an industrial scale is limited by poor cell growth, poor tolerance to acid conditions, and susceptibility to osmotic stress.
Currently, using metabolic and genome engineering, recombinant strains cultured at low pH can improve the acid resistance of A. succinogenes [15, 16]. Genome shuffling has been reported to improve A. succinogenes acid tolerance. A genome-shuffled strain, F3-21, survived at pH 5.2, accumulating 38.1 g/L of SA in a 5 L stirred bioreactor with controlled pH 5.6–6.0. SA accumulation increased by 45%, when compared to the parent strain (26.2 g/L) [17]. Another shuffled strain A. succinogenes, AS-F32, survived at pH 3.5, with a SA yield of 31.2 g/L at pH 4.8, which was 1.1-fold greater than the original strain, As-R2 [16]. A. succinogenes BC-4, a mutant derived by adaptive evolution, has improved cell growth and SA production in weak acid culture conditions. The yield of SA was 20.7 g/L with anaerobic culture conditions at pH 5.8 [18]. With these improvements, SA yield was still unsatisfactory in acidic environments.
Acid inhibition is a complex phenomenon that affects many microbial cellular structures and physiological states. Weak acids (e.g. lactic acid, acetic acid, and succinic acid) are microbial fermentation products that enter cells by free diffusion, which rapidly disperse within cells, releasing large numbers of protons and anions. These molecules adversely affect acid-sensitive DNA, increase protein degeneration, decrease enzymatic activity, and damage cell membranes. To adapt to the environment, microorganisms induce common mechanisms to resist acid stress including the glutamate decarboxylase system (Gad system), biofilm formation, the F0F1-ATPase proton pump, protection or repair of macromolecules (e.g. Dps and RecA systems), and alkali production [19]. Based on sequencing analysis of the A. succinogenes genome (NC_009655.1) [5], the acid resistance mechanisms of A. succinogenes include the F0F1-ATPase, as well as repair or protection of macromolecules by repair proteins RceF (Asuc_003), RadC (Asuc_0013), RceO (Asuc_0193), and DNA mismatch repair proteins MutS (Asuc_0345), DnaK (Asuc_1092), and AP endonuclease (Asuc_0359). The role of amino acid decarboxylation and deamination in acid resistance is unclear. A. succinogenes lacks Gad, and is reported to be a glutamate auxotroph [5]. However, glutamate (Glu) is cell protective during acid stress. Glu is involved in protein synthesis and other fundamental cellular processes including; glycolysis, gluconeogenesis, and the citric acid cycle [20]. In particular, Glu plays an important role in acid resistance for a number of microorganisms via the Gad system [21, 22]. The Gad system is Glu dependent and is the most effective acid resistance mechanism [23, 24]. This system is comprised of two components, glutamate decarboxylase (GadA and GadB), and antiporter GadC. Glu is catalyzed to γ-aminobutyric acid (GABA) by glutamate decarboxylase with consumption of one proton in Escherichia coli, Lactobacillus brevis, Bacillus cereus, and Listeria monocytogenes [21,22,23,24,25]. In this process, GABA is exchanged with extracellular Glu by the Glu/GABA antiporter GadC [26, 27]. The Gad system plays a primary role in bacteria that require stomach transit en route to host invasion. These bacteria include E. coli, Shigella flexneri, Clostridium perfringens, Bacteroides, Fusobacterium, and Eubacterium [28, 29]. As described above, when a cell is exposed to low pH, the Gad system converts a molecule of extracellular Glu to extracellular GABA, consuming an intracellular proton to reduce pH. Using the Gad system, Brucella microti resists extreme acid stress (pH 2.5) as does E. coli, surviving for several hours in extremely acidic surroundings (pH 2–3) [23].
In this study, an exogenous Gad system comprised of glutamate decarboxylase (GadB) and antiporter (GadC) genes was introduced into A. succinogenes CGMCC1593 to determine whether the introduced Gad system could improve acid resistance (Fig. 1). The effects of the Gad system on growth and SA production were investigated via heterologous expression of gadB alone, or gadB together with gadC. To our knowledge, this study is the first to determine whether the Gad system functions in acid-stressed A. succinogenes.
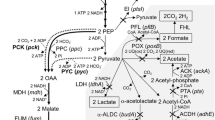
The SA synthesis pathway and introduction of the Gad system into A. succinogenes. The abbreviation of metabolites were as follows: G6P Glucose-6-phosphate, F6P fructose-6-phosphate, G3P glyceraldehyde-3-phosphate, PEP phosphoenolpyruvate, Pyr pyruvic acid, PGI phosphohexose isomerase, PFK phosphofructokinase, ENO enolase, OAA oxaloacetate, MAL malate, FUM fumarate, SUC succinate, SUCC succinyl CoA, AKG α-ketoglutarate, ICI isocitrate, CIT citrate, LA lactic acid, AA acetic acid, EA ethyl alcohol, FA formic acid, PCK phosphoenolpyruvate carboxykinase, PFL pyruvate formate-lyase, LDH lactate dehydrogenase, ACKA acetokinase, ADH alcoholdehydrogenase
Materials and methods
Strains, plasmid and media
A. succinogenes CGMCC1593 (SW) was isolated from bovine rumen by our laboratory and stored at the CGMCC (China General Microbiological Culture Collection Center). E. coli JM109 and Lactobacillus buchneri NRRL B-30929 were purchased from BNCC (BeNa culture collection, China). E. coli was cultivated in LB medium (containing the following: 10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl) at 37 °C. A. succinogenes was cultured anaerobically in TSB (Sinopharm Chemical Reagent Co., Ltd, China). L. buchneri was cultured anaerobically in MRS medium (containing the following: casein peptone 1%, beef extract 0.8%, yeast extract 0.4%, glucose 2%, MgSO4 0.02%, C2H3O2Na·3H2O 0.5%, ammonium citrate 0.2%, K2HPO4 0.2%, MnSO4 0.005%, and Triton 80 0.1%) at 37 °C. Plasmid pLGZ922 was purchased from ATCC (American Type Culture Collection, Manassas, VA, USA).
Fermentation medium for SA production by A. succinogenes contained the following: corn steep liquor 1.0–3.0%, NaH2PO4·2H2O 0.2–0.5%, K2HPO4·3H2O 0.2–0.5%, MgCl2·6H2O 0.02–0.05%, glucose 5%, CaCl2 0.01–0.03%, and Na2S 0.01–0.1%).
DNA manipulation techniques
For gadA, gadB, gadB, and gadC expression in A. succinogenes, overexpression plasmids were constructed (Fig. S1). The primers are listed in Table 1. The gadA fragments were amplified by PCR from the genome of Lactobacillus brevis, and gadB or gadC fragments were amplified by PCR from the genome of L. buchneri. Plasmids were constructed with a one-step cloning kit (Vazyme, China) following the manufacturer’s instructions and transformed into E. coli. The sequences of gadA, gadB, and gadBC were confirmed using Sanger sequencing by GENEWIZ. Inc. Plasmids were transformed into A. succinogenes by electroporation [15]. One μg of plasmid DNA was added to 100 μL of competent cells in a 0.2-cm gap cuvette and the suspension electroporated at 2.5 kV for 5–6 ms.
Protein preparation and SDS-PAGE analysis
Recombinant strains were cultured anaerobically in TSB medium at 37 °C for 16 h. Cells were harvested by centrifugation at 8000 rpm for 10 min and resuspended in sterile water. The cells received ultrasonic treatment (3 s of treatment, followed by 5 s of no treatment, for 20 min at 350 W), then the supernatant was centrifuged at 8000 rpm for 10 min. The supernatant was used as a crude protein preparation and subjected to SDS-PAGE. The SDS-PAGE was performed using a 12% separation gel and a 5% spacer gel.
Quantification of Gad system activity
The recombinant strains were inoculated into TSB medium and cultured to stationary phase. The cells were harvested by centrifugation at 8000 rpm for 10 min and washed twice with PBS buffer solution. Then, the cells were resuspended in 0.2 M sodium acetate-acetic acid buffer with 50 g/L sodium glutamate and 0.6 mM phosphopyridoxal. After incubation for 30 min at 37 °C and centrifugation at 220 rpm the reaction was terminated with 10% trichloroacetic acid (TCA). The reaction solution was filtered through a 0.22 μL filter and stored at − 20 °C before analysis. One unit of Gad activity was defined as the amount of enzyme required to generate 1 μmol of GABA per min with the above assay conditions.
Determination of acid-resisting spot
A strain cryopreserved in glycerol was inoculated into TSB medium and cultured to stationary phase at 37 °C. The cells were harvested by centrifugation at 8000 rpm for 10 min and washed twice with sterile water. Then, 100 μL of the cell suspension was added to 900 μL of TSB medium which contained 0 mM or 40 mM sodium glutamate at pH 4.6. After culturing for 1.0 h or 1.5 h at 37 °C, 5 μL of culture solution was plated on TSB solid medium and observed after 24 h at 37 °C.
Measurement of intracellular pH
The cells of stationary phase were harvested by centrifugation at 8000 rpm for 10 min and resuspended in standard curve buffer of different pH value (pH4.0, pH5.0, pH6.0, pH7.0, pH8.0) with 1 μM valinomycin and nigericin. The cells were harvested again and resuspended in standard curve buffer of different pH value. 0.25 μM fluorescent probe BCECF AM were added to the above solution for 20 min at 30 °C. The cells were harvested by centrifugation and washed three times by the same buffer. Fluorescence intensities were measured at excitation wavelengths of 490 and 440 nm by the multifunctional enzyme marker. The emission wavelength was 525 nm, and the slit widths were 20 nm. I490/I440 was fluorescence intensity I. The standard curve was drawn with pH as the abscise and lgI as the ordinate.
The sample cells of stationary phase were harvested by centrifugation at 8000 rpm for 10 min and resuspended 50 mM HEPES-K (pH 8.0). 0.25 μM fluorescent probe BCECF AM were added to the above solution for 20 min at 30 °C. The cells were harvested by centrifugation and washed three times by phosphate buffer(pH 7.0). Fluorescence intensities were measured following the standard curve method.
The standard curve buffer contained the following: 50 mM glycine, 50 mM citric acid, 50 mM Na2HPO4, 50 mM KCl.
Fermentation
Cells were cultured in a 500 mL sterile flask containing 200 mL TSB medium at 38 °C for 12–14 h in an anaerobic incubator. A 10% suspension was inoculated into fermentation medium with suitable MgCO3 for early fermentation. The pH was maintained at 6.0–6.5 by supplementation with 300 g/L Na2CO3. The temperature and agitation were 38 °C and 400 rpm, respectively. When the sugar content was less than 10 g/L, glucose was added but the concentration was kept below 20 g/L. Fermentation was stopped when sugar was exhausted.
Analysis methods
The optical density of A. succinogenes was monitored using a spectrophotometer at 660 nm (OD660). Samples were filtered through a 0.22 μm filter before application to an HPLC system. GABA was analyzed by HPLC with an Agilent system using UV detection. SA was analyzed by HPLC with a Waters system and Sepax Carbomix H-NP column using a refractive index (RI) detector. The column temperature was 55 °C and the mobile phase was 3.3 mM H2SO4 at a flow rate 0.5 mL/min.
Results and discussion
Introduction into A. succinogenes of a Gad system comprised of heterologous gadB and gadBC
Plasmid pLGZ922 and recombinant plasmids; pLGZ-gadA, pLGZ-gadB, and pLGZ-gadBC were introduced into A. succinogenes CGMCC1593 by electroporation to construct recombinant strains pLGZM-SW, gadA-SW, gadB-SW, and gadBC-SW. The expression of GadA, GadB, and GadC proteins in these recombinant strains was detected by SDS-PAGE. GadB in the gadB-SW and gadBC-SW strains was successfully expressed. Since the molecular weights of GadB and GadC of L. buchneri are 54.5 kDa and 55.4 kDa, respectively, the GadC in the gadBC-SW was not obvious in SDS-PAGE (Fig. 2A). GadA was not detected (data are not shown).
The activity of Gad in recombinant strains is assessed in Fig. 2B. Both consumption of Glu and generation of GABA were observed in gadB-SW and gadBC-SW, with their GABA yields 0.62 ± 0.053 U/mg free cells and 0.653 ± 0.073 U/mg free cells, respectively. Neither consumption of Glu nor production of GABA was detected in the control strain, which also confirmed the absence of Gad in the A. succinogenes wild strain. Thus, both the gadB-SW and gadBC-SW strains exhibited Gad activity, with the gadBC-SW strain slightly greater than the gadB-SW strain. GadC is a Glu/GABA antiporter, which transfers extracellular Glu to the cytoplasm and intracellular GABA to the extracellular milieu [30]. These results suggested that GadC in A. succinogenes increased the exchange rate between Glu and GABA, reducing cell damage during acid stress, which may be more conducive to the formation of GABA.
The effect of the Gad system on the growth of recombinant strains
To investigate the acid tolerance of Gad recombinant strains, survival in a low pH environment was assessed by an acid-resisting spot (Fig. 3). Cell survival was decreased with the prolongation of acid stress. Whether or not Glu was added, maximum survival of the double gene expression strain gadBC-SW was 1.0 h and 1.5 h at pH 4.6. These results demonstrated that heterologous expression of gadB and gadC in A. succinogenes improved acid tolerance and survival in the presence of Glu with acidic conditions. However, survival of all strains was increased with Glu supplementation. These results suggested the importance of Glu to A. succinogenes in acidic environments. The observation that Glu improved acid tolerance was similar to the known importance of Glu for microbial acid resistance. Glu enhances survival of E. coli by Gad at pH 2.5 [23]. There was no Gad system in A. succinogenes, which suggested other potential mechanisms by which Glu protected cells from acid stress. GadB catalyzed Glu to GABA for survival in an acidic environment. However, GABA could not be quickly and effectively transported outside of the recombinant gadB-SW strain, in that the strain lacked the antiporter, GadC, which resulted in increased cell death. Therefore, the tolerance of gadB-SW to acid was lower than gadBC-SW.
The intracellular pH of recombinant strains was measured (Table S1). The intracellular pH of gadB-SW and gadBC-SW was increased slightly change after suffering pH 4.6 for 1 h, but the intracellular pH of pLGZ-SW was less decreased, which indicated gadB-SW and gadBC-SW may maintain their survival by increasing the intracellular pH value under acid stress.
The growth of recombinant strains at neutral pH was assessed (Fig. 4). The OD660 of pLGZ-SW, gadB-SW and gadBC-SW was 1.809, 1.703, and 1.602, respectively, which differed from survival with acid stress. It may be that introduction of exogenous protein increased metabolic burden affecting cell growth. Furthermore, GABA may have an effect on cell growth and this possible effect was assessed by the addition of 20 mM GABA to the culture medium (Fig. 4). The resultant OD660 of pLGZ-SW, gadB-SW, and gadBC-SW were 1.788, 1.607, and 1.362, respectively. These OD660 values were less than those without GABA addition. GABA appeared to be unfavorable to cell growth at neutral pH. The gadBC-SW strain was also affected by GABA. The burden of foreign protein expression on cell growth, as well as the impact of GABA produced by the Gad system, may decrease the growth of recombinant strains, even though the survival of A. succinogenes was increased by the Gad system.
The effect of the Gad system on SA production by recombinant strains
To further study the effects of the Gad system on SA production, the effect of acidic conditions on the recombinant strains was assessed during shake flask fermentation. MgCO3 is an excellent pH regulator during fermentation. Generally, the optimum mass ratio of glucose and MgCO3 was 1:0.8 in fermentation medium. When the mass ratio of glucose and MgCO3 was 1:0.4 (Table 2), the greatest SA accumulation was by gadBC-SW at 19.2 g/L, which was slightly greater than that of SW (17.7 g/L) and an increase of 8.4%. This mass ratio also resulted in the greatest cell growth, OD (8.28). The minimum SA accumulation (16.2 g/L) was by strain gadB-SW, with both pH and OD values at the end of fermentation the lowest. It may be that GABA was produced but not efficiently transported to the extracellular milieu in a low pH environment, not conducive to cell growth or SA accumulation. The introduction of a complete Gad system into A. succinogenes maximized SA production in an acidic environment.
SA production by the recombinant strains was assessed by fermentation in a 3-L bioreactor at pH 6.5 and pH 6.0. SW, gadB-SW, and gadBC-SW produced 54.8 g/L, 50.3 g/L, and 47 g/L SA at pH 6.5, respectively (Fig. 5A). The corresponding conversion rates of sugar to SA were 74%, 74%, and 78%, respectively. Comparison of the growth of the three strains found the maximum growth OD660 value for strain gadBC-SW to be the lowest. Compared with that of SW, the OD of strain gadBC-SW decreased slowly during the middle and late fermentation period, with OD value the highest at the end of fermentation. Furthermore, the SA concentration produced by gadB-SW was not improved. This result may be related to modification of SA cellular metabolism due to the expression of heterologous proteins. When fermentation was maintained at pH 6.0, SW, gadB-SW, and gadBC-SW produced 37.35 g/L, 36.94 g/L, and 31.95 g/L of SA with corresponding conversion rates of sugar to SA of 85%, 90%, and 96%, respectively (Fig. 5B). During the fermentation process, sugar consumption by the recombinant strains was significantly slower, and interestingly, when the pH value of gadBC-SW reached 6.4, it no longer decreased. And the lowest SA concentration was by the gadBC-SW strain, which was approximately 15% lower than that of SW. The highest conversion rate of sugar to SA was by the gadBC-SW strain, which was increased 13.9% over the parent strain, even though the SA concentration was lower. This phenomenon was also observed in a fermentation environment of pH 6.5. The pH of the gadBC-SW stain was always above 6.0 during fermentation, demonstrating acid resistance with heterologous expression of gadB and gadC. Acetic acid (AA) accumulation by the gadBC-SW strain reached 4.01 g/L, which was greater than that of the other two strains. These results suggested that the introduction of gadB and gadC may have enhanced the AA biosynthetic pathway and affected SA accumulation. The gadB-SW strain produced slightly less SA than the SW strain, but more than the gadBC-SW strain. It was possible that the expression of heterologous proteins may have caused a metabolic burden that affected cell growth and SA production.
Conclusions
Recombinant strains of A. succinogenes that expressed gadB and gadBC were successfully constructed. After acid stress, the survival of A. succinogenes was improved by the introduction of a complete Gad system comprised of gadB and gadC, although cellular growth was slightly reduced at neutral pH. Despite the effect on cell growth, the introduction of gadB and gadC significantly increased the conversion rate of sugar to SA in 3-L bioreactor batch fermentation with acid conditions. The greatest rate of sugar conversion to SA was 96% by the gadBC-SW strain. The increased metabolic burden of gadB-SW and gadBC-SW influenced bacterial growth, sugar consumption, and SA production of A. succinogenes. This study provided a foundation upon which to improve strains of A. succinogenes for greater production of SA.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Sauer M, Porro D, Mattanovich D, Branduardi P. Microbial production of organic acids: expanding the markets. Trends Biotechnol. 2008;26:100–8. https://doi.org/10.1016/j.tibtech.2007.11.006.
Kover A, Kraljić D, Marinaro R, Rene ER. Processes for the valorization of food and agricultural wastes to value-added products: recent practices and perspectives. Syst Microbiol Biomanuf. 2021. https://doi.org/10.1007/s43393-021-00042-y.
Jansen ML, Gulik WMV. Towards large scale fermentative production of succinic acid. Curr Opin Biotechnol. 2014;30:190–7. https://doi.org/10.1016/j.copbio.2014.07.003.
Zhang W, Qiao Y, Wu M, et al. Metabolic regulation of organic acid biosynthesis in Actinobacillus succinogenes. Front Bioeng Biotech. 2019. https://doi.org/10.3389/fbioe.2019.00216.
McKinlay JB, Laivenieks M, Schindler BD, Mckinlay AA, Siddaramappa S, Challacombe JF, et al. A genomic perspective on the potential of Actinobacillus succinogenes for industrial succinate production. BMC Genomics. 2010;11:680. https://doi.org/10.1186/1471-2164-11-680.
Guettler MV, Jain MK, Rumler D (1996) Method for making succinic acid, bacterial variants for use in the process, and methods for obtaining variants. U. S. patent 5573931 A
Guettler MV, Rumler D, Jain MK. Actinobacillus succinogenes sp. nov., a novel succinic-acid-producing strain from the bovine rumen. Int J Syst Bacteriol. 1999;49:207–16. https://doi.org/10.1099/00207713-49-1-207.
Corona-Gonzalez RI, Bories A, González-Álvarez V, Snell-Castro R, Toriz-González G, Pelayo-Ortiz C. Succinic acid production with Actinobacillus succinogenes, zt-130 in the presence of succinic acid. Curr Microbiol. 2010;60:71–7. https://doi.org/10.1007/s00284-009-9504-x.
Tag A, Madcs A, Erd B, Los C, Rma A, Rg A. Optimization of anaerobic fermentation of Actinobacillus succinogenes for increase the succinic acid production. Biocatal Agric Biotechnol. 2020. https://doi.org/10.1016/j.bcab.2020.101718.
Liu YP, et al. Strategies of pH control and glucose-fed batch fermentation for production of succinic acid by Actinobacillus succinogenes CGMCC1593. J Chem Technol Biotechnol. 2008;83:722–9. https://doi.org/10.1002/jctb.1862.
Wang CC, Zhu LW, Li HM, et al. Performance analyses of a neutralizing agent combination strategy for the production of succinic acid by Actinobacillus succinogenes ATCC 55618. Bioproc Biosyst Eng. 2012;35(4):659–64. https://doi.org/10.1007/s00449-011-0644-6.
Dai ZX, Guo F, Zhang SJ, Zhang WM, Yang Q, Dong WL, Jiang M, et al. Bio-based succinic acid: an overview of strain development, substrate utilization, and downstream purification. Biofuel Bioprod Bior. 2019. https://doi.org/10.1002/bbb.2063.
Chong LA, Klo B, Zc C, Zsa D, Xl B, Rdp B. Promising advancement in fermentative succinic acid production by yeast hosts. J Hazard Mater. 2020. https://doi.org/10.1016/j.jhazmat.2020.123414.
Zhang Q, Chen PC, Zheng P. Physiological and transcriptional responses of Actinobacillus succigenes to acid stress. J Microbiol. 2017. https://doi.org/10.13343/j.cnki.wsxb.20170400.
Guarnieri MT, Chou YC, Salvachúa D, Mohagheghi A, Beckham GT. Metabolic engineering of Actinobacillus succinogenes provides insights into succinic acid biosynthesis. Appl Environ Microbiol. 2017. https://doi.org/10.1128/AEM.00996-17.
Hu S, You Y, Xia F, Liu J, Dai W, Liu J. Genome shuffling improved acid-tolerance and succinic acid production of Actinobacillus succinogenes. Food Sci Biotechnol. 2019;28(3):817–22. https://doi.org/10.1007/s10068-018-0505-z.
Liu X, Zheng P, Ni Y, Dong JQ, Sun ZH. Breeding Actinobacillus succinogenes with acid-tolerance by genome shuffling. Microbiol. 2009. https://doi.org/10.13344/j.microbiol.china.2009.11.008.
Zhang WM, Tao YX, Wu M, Xi FX, Dong WL, Zhou J, Gu JC, Ma JF, Jiang M, et al. Adaptive evolution improves acid tolerance and succinic acid production in Actinobacillus succinogenes. Process Biochem. 2020;98:76–82. https://doi.org/10.1016/j.procbio.2020.08.003.
Tang HZ, Zhang L, Liu YP, et al. Mechanisms of acid tolerance in bacteria and prospects in biotechnology and bioremediation. Biotechnol Adv. 2015. https://doi.org/10.1016/j.biotechadv.2015.06.001.
Feehily C, Karatzas KAG. Role of glutamate metabolism in bacterial responses towards acid and other stresses. J Appl Microbiol. 2013;114:11–24. https://doi.org/10.1111/j.1365-2672.2012.05434.x.
Li Q, Tao QY, Teixeira JS, Su SWM, Ganzle MG. Contribution of glutaminases to glutamine metabolism and acid resistance in Lactobacillus reuteri and other vertebrate host adapted lactobacilli. Food Microbiol. 2020;86:103343. https://doi.org/10.1016/j.fm.2019.103343.
Cotter PD, O’Reilly K, Hill C. Role of the glutamate decarboxylase acid resistance system in the survival of Listeria monocytogenes LO28 in low pH foods. J Food Prot. 2001;64:1362–8. https://doi.org/10.4315/0362-028x-64.9.1362.
Lu P, Ma D, Chen YL, Guo YY, Chen GQ, Deng HT, Shi YG, et al. L-glutamine provides acid resistance for Escherichia coli through enzymatic release of ammonia. Cell Res. 2013;23:635–44. https://doi.org/10.1038/cr.2013.13.
Zhao H, Feng Z, Quan X, Cao Z, Jie L. The soluble transhydrogenase UdhA affecting the glutamate-dependent acid resistance system of Escherichia coli under acetate stress. Biol Open. 2018. https://doi.org/10.1242/bio.031856.
Mancini A, Carafa I, Franciosi E, Nardin T, Tuohy KM. In vitro probiotic characterization of high GABA producing strain Lactobacilluas brevis DSM 32386 isolated from traditional “wild” Alpine cheese. Ann Microbiol. 2019;69:1435–43. https://doi.org/10.1007/s13213-019-01527-x.
Krammer EM, Prevost M. Function and regulation of acid resistance antiporters. J Membr Biol. 2019;252:465–81. https://doi.org/10.1007/s00232-019-00073-6.
Ma D, Lu P, Shi Y. Substrate selectivity of the acid-activated glutamate/γ-aminobutyric acid (GABA) antiporter GadC from Escherichia coli. J Biol Chem. 2013;288:15148–53.
Richard H, Foster JW. Escherichia coli glutamate- and arginine-dependent acid resistance systems increase internal pH and reverse transmembrane potential. J Bacteriol. 2004;186:6032–41. https://doi.org/10.1128/JB.186.18.6032-6041.2004.
Cozzani I, Barsacchi R, Dibenedetto G, et al. Regulation of breakdown and synthesis of L-glutamate decarboxylase in Clostridium perfringens. J Bacteriol. 1975;123(3):1115–23. https://doi.org/10.1128/JB.123.3.1115-1123.1975.
Krulwich T, Sachs G, Padan E. Molecular aspects of bacterial pH sensing and homeostasis. Nat Rev Microbiol. 2011;9:330–43. https://doi.org/10.1038/nrmicro2549.
Acknowledgements
The authors are grateful for the financial support from the National First-class Discipline Program of Light Industry Technology and Engineering (Grant No. LITE2018-04) and the Topnotch Academic Programs Project of Jiangsu Higher Education Institutions (TAPP).
Author information
Authors and Affiliations
Contributions
CCM had a major contribution to this work and wrote the manuscript. ZQ and QJZ did some supplementary experiments and analyzed the data. WD and CPC revised the manuscript. ZP conceived and supervised the study and contributed to the writing of the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, C., Zhang, Q., Qian, J. et al. Effect of the Gad system on Actinobacillus succinogenes during acid stress. Syst Microbiol and Biomanuf 2, 177–185 (2022). https://doi.org/10.1007/s43393-021-00054-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43393-021-00054-8