Abstract
Study design
Prospective, quality-improvement.
Objectives
To evaluate pain management following posterior spinal fusion (PSF) for adolescent idiopathic scoliosis (AIS) and Scheuermann’s Kyphosis (SK) determine the optimal opioid and benzodiazepine prescription amounts, and implement a multimodal post-operative pain regimen.
Summary of background data
The incidence of prescription opioid abuse is increasing in the United States. Orthopedic spine surgeons often prescribe large quantities of opioids post-operatively for pain control. Previous efforts on pain control have focused on in-patient post-operative regimens after PSF.
Methods
Between 2/1/17 and 5/30/18 patients with AIS or SK were sent home with pain diaries after discharge to document daily narcotic, benzodiazepine, non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen and gabapentin use following PSF. Diaries were collected at the 4 week post-operative visit. Data from two cohorts were reviewed: pre-intervention and post-intervention. Our prescription intervention went into effect 9/1/17.
Results
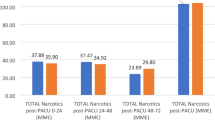
Twenty-four (30%) patients returned pain diaries. The pre-intervention cohort consisted of 12 patients (7 female; 5 males; 14.9 years (range 12–19)). Patients were prescribed on average 80 × 5 mg tabs (26–140) of oxycodone but used on average 45 tabs (12–129) over an average of 17.5 days (9–33). They were prescribed an average of 30 × 2 mg tabs (0–150) of diazepam, used on average 4.8 (0–105) tabs over 12.5 (5–25) days. The post-intervention cohort consists of 12 patients (9 female; 3 male; 14.8 years (12–19)). They were prescribed on average 50 × 5 mg tabs (35–80) of oxycodone, used 20.5 (0–39.5) tabs over 8.5 days (3–16). They were prescribed on average 18 × 2 mg tabs of diazepam (0–43), used 5.4 tabs (0–19) over 10 days (5–14).
Conclusions
This analysis has directly impacted clinical practice. Prescribed opioid and benzodiazepine doses have been decreased by over 50%, and more resources are being directed towards determining the disparity between the amount of medications prescribed and consumed in our post-operative patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of prescription opioid use and misuse has been increasing in the United States over the past few decades. From 1976 to 2015, a Monitoring the Future (MTF) survey identified peak prescription opioid use by high school seniors in 1989 (20.4%) and 2002 (20%). These percentages remained stable until 2013 and declined through 2015 (~ 15%). During the same time period, the percentage of teens reporting nonmedical use correlated with medical prescription opioid use. Additionally, the decline in opioid use from 2013 to 2015 coincided with similar declines in opioid prescriptions [1], likely due to increased awareness [2].
Excess medication not properly disposed of can increase the risk for illicit use, prescription opioid misuse disorder, overdose, and death. In 2012, the incidence of hospitalizations for opioid poisoning was 3.71 per 100,000 children: specifically 2.62 for ages 1–4 and 10.17 for ages 15–19, which was nearly double from 15 years prior [3].
Adolescents are highly susceptible to prescription opioid misuse and their first exposure is often following an orthopedic injury or surgery. For example, legitimate opioid use prior to high school graduation is associated with a 33% increased risk of future misuse [4], and 36.9% of high school seniors from the MTF cohort admitted to using their own prior prescriptions [5]. Patients receive prescriptions from providers in the ambulatory setting or the emergency department. Between 2005 and 2007, adolescents were prescribed a controlled medication (opioids, sedative-hypnotics, stimulants) at 14.5% of visits. Adolescents received a prescription at 23.4% of visits for back pain, 12.9% of visits for musculoskeletal issues, and 16.6% of visits for other injuries [6]. Similarly, recent studies have demonstrated that high contact athletes are more likely to misuse opioids, with injury speculated as the driving factor. In a study that compared 16 competitive high school sports, weightlifting, wrestling and football were identified as sports that increase the odds of opioid misuse [5, 7, 8]. A 2018 study found that 44% of ambulatory narcotic prescriptions were written by orthopedic departments. When comparing opioid use in surgical vs. nonsurgical patients, 4.8% of surgical patients used opioids for 90 + days compared to 0.1% of nonsurgical patients. Risk factors for prolonged opioid use included older age, female gender, chronic pain, preoperative opioid prescription and previous substance use disorders [2].
Orthopedic spine surgeons often prescribe large quantities of opioids post-operatively for pain control. Previous efforts on pain control have focused on decreasing variability between patients in the in-patient post-operative pain regimen, and decreasing the length of hospital stay after posterior spinal fusion (PSF). Current consensus guidelines for post-operative pain management include a post-operative PCA pump, a goal of transitioning to PO pain medication on a targeted date (e.g., POD1 or POD2), use of a muscle spasm medication (e.g., diazepam), and use of perioperative gabapentin and ketorolac to decrease opioid needs [9, 10]. Additionally, the use of ketamine and magnesium intraoperatively has also shown a significant decrease in morphine consumption post-operatively [11].
There is very little literature on the optimal post-operative pain regimen following discharge after PSF. This study focuses on pain management following PSF for adolescent idiopathic scoliosis (AIS) and Scheuermann’s Kyphosis (SK). The objectives of this study were to analyze the outpatient pain management regimen to avoid over prescribing opioid medications, determine the optimal opioid and benzodiazepine prescription amounts, and implement a multimodal post-operative pain regimen. Our goal was to find the balance between effectively managing pain and minimizing excess medication, ultimately decreasing the risk of abuse and harm without affecting patient care and comfort.
Materials and methods
Data were collected between 2/1/17 and 5/30/18 from patients with a spine deformity (AIS or SK) who were sent home with detailed pain diaries after discharge following PSF. PSF was performed by one of three surgeons at a single institution. The pain diaries documented daily narcotic, benzodiazepine, NSAID, acetaminophen and gabapentin use. Patients also recorded average daily pain score, activity level, and pain control satisfaction. Diaries were collected at the 4 week post-operative visit.
Data from two cohorts were reviewed: pre-intervention (surgeries from 2/1/17 to 8/31/17) and post-intervention (surgeries from 9/1/17 to 5/30/18). For the pre-intervention cohort, there was variability in the amount of narcotic and benzodiazepine medication prescribed and no standardized pain regimen education. Prescription intervention went into effect on 9/1/17. The intervention was multifactorial. First, all patients discussed post-operative pain expectations with an orthopedic nurse at a pre-operative clinic visit. Second, all patients had a standardized medication education and instruction session with a member of the pain team prior to discharge where the details of the pain regimen were reiterated. At this time the medications prescribed were standardized to alternating scheduled naproxen and acetaminophen for primary pain control, daily gabapentin, and only using oxycodone for breakthrough pain and diazepam for muscle spasms. All patients were to be prescribed no more than 50 × 5 mg of oxycodone and 20 × 2 mg tabs of diazepam. The post-intervention prescription amounts were determined from the pre-intervention pain diary responses.
IBM Statistical Package for Social Sciences (SPSS) software v24 was used for statistical analysis. Normalization was confirmed with both the Shapiro–Wilk and Levene’s tests. Normal continuous data were analyzed with a one way ANOVA, while nonparametric data were analyzed with the Mann–Whitney U test. Categorical data were assessed by Chi-square analysis.
Results
Eighty pain diaries were distributed over the course of the study. Forty-eight diaries were distributed prior to intervention and 32 diaries following intervention. The mean age in the pre-intervention cohort was 15 ± 2.1 years (11–20 years), while the mean age for the post-intervention cohort was 14 ± 2.8 (12–26 years). There were 34 females (71%) and 14 males (29%) in the pre-intervention cohort and 23 females (72%) and 9 males (28%) in the post-intervention cohort. Prior to intervention, the median oxycodone prescription was 80 ± 44 × 5 mg tablets (12–210 tabs) and the median diazepam prescription was 30 ± 37.6 × 2 mg tablets (10–150 tabs). Following the intervention, the median oxycodone prescription was 50 ± 16.4 × 5 mg tablets (0–80 tabs) and the median diazepam prescription was 25 ± 14 × 2 mg tablets (6–75 tabs). Prior to intervention, 89.5% of patients received a gabapentin prescription, compared to 90.1% following intervention. Before intervention 14.6% of patients requested a refill of their prescription for oxycodone or diazepam, and 12.5% requested a refill post-intervention. There were statistically significant differences in both the amount of oxycodone and diazepam prescribed between groups (both p < 0.001). There were no significant differences in age, gender, gabapentin prescription, or refill requests.
Twelve pain diaries were returned in the pre-intervention group (25% return rate). Of those who returned pain diaries, the average patient age was 14.9 ± 2.4 years (12–19 years) and there were 7 females (58.3%) and 5 males (41.7%). The median amount of 5 mg oxycodone tabs used was 45.4 ± 34 (12–129 tabs), while the median amount of 2 mg diazepam tabs used was 4.8 ± 30.0 (0–105 tabs). Oxycodone was used for a median of 17.5 ± 7.2 days (9–33 days), while diazepam was used for a median of 12.5 ± 7.8 (5–25 days). The median oxycodone prescription was 80 ± 35.7 × 5 mg tabs (26.6–140 tabs) and patients utilized 58.3 ± 27% (22–97%) of their oxycodone prescription on average. The median diazepam prescription was 30 ± 38.5 × 2 mg tabs (0–150 tabs) and patients consumed an average of 46.3 ± 41.3% (0–100%) of the prescription. On the last day of opioid use, the average pain level recorded was 3.4 ± 2.1 (0–7.5) on a 10 point scale and 83.3% of patients were satisfied with pain control. The pre-intervention cohort was satisfied with pain control 97.1 ± 16.1% (55–100) of the time during diary documentation.
Twelve pain diaries were returned in the post-intervention group (38% return rate). The average patient age was 14.8 ± 2.1 years (12–19 years) and there were 9 females (75%) and 3 males (25%). The median amount of 5 mg oxycodone tabs used was 20.5 ± 12.6 (0–39.5 tabs), while the median amount of 2 mg diazepam tabs used was 5.4 ± 5.5 (0–19 tabs). Oxycodone was used for a median of 8.5 ± 4 days (3–16 days), while diazepam was used for a median of 10 ± 3.2 days (5–14 days). The median oxycodone prescription was 50 ± 10.3 × 5 mg tabs (35–80 tabs) and patients utilized 33.4 ± 24% (0–79%) of their oxycodone prescription on average. The median diazepam prescription was 17.5 ± 6.5 × 2 mg tabs (0–42.5 tabs) and patients consumed an average of 39 ± 30.6% (0–85%) of the prescription. On the last day of opioid use, the average pain level recorded was 4.8 ± 1.5 (3–8) on a 10 point scale and 100% of patients were satisfied with pain control. The post-intervention cohort was satisfied with pain control 100 ± 16% (57–100%) of the time during diary documentation.
After intervention implementation, there was a statistically significant decrease in the amount of oxycodone prescribed (p = 0.010), amount of oxycodone consumed (p = 0.008), percentage of prescribed oxycodone used (p = 0.026), days of medication used for pain control (p = 0.003) and amount of diazepam prescribed (p = 0.045). There was no significant difference between groups in age (p = 0.856), gender (p = 0.667), diazepam used (p = 0.755), percentage of diazepam prescription consumed (p = 0.643), days of diazepam use (p = 0.573), pain on last day of oxycodone use (p = 0.148), satisfaction on last day of oxycodone use (p = 0.545) or percentage of pain control satisfaction documented by the diaries (p = 0.536).
Discussion
Following pain medication prescription intervention, the final discharge pain regimen consisted of a baseline of NSAIDs (ibuprofen or naproxen) alternating with acetaminophen, daily gabapentin, an opioid for breakthrough pain (ex: 50 × 5 mg oxycodone), and a benzodiazepine (ex: 20 × 2 mg of diazepam) for muscle spasms. Prior to discharge from the hospital, all patients received a standardized set of verbal and written instructions reinforcing that NSAIDs, acetaminophen, and gabapentin were to be used as the primary method of scheduled pain control, benzodiazepine should be taken as needed for muscle spasms, and oxycodone should only be used for breakthrough pain.
Although the diary return rate was low, there were no significant differences between the pre and post-intervention cohorts in terms of patient demographics, oxycodone prescription, diazepam prescription, gabapentin prescription, or refill requests. The intervention undertaken in this study successfully decreased the amount of narcotics prescribed, total amount used, and post-operative days of use. The amount of oxycodone used decreased from 45.4 × 5 mg tabs pre-intervention to 20.5 × 5 mg tabs post-intervention. Opioids (oxycodone) were used for 17.5 days pre-intervention and decreased to 8.5 days post-intervention. Additionally, the amount of oxycodone prescribed decreased from 80 × 5 mg tabs to 50 × 5 mg tabs. Despite the decrease in oxycodone prescription, patients still only used 33.4% of their prescription in the post-intervention cohort compared to 58.3% used in the pre-intervention cohort. The median oxycodone use decreased by 54.8%, while the days of use decreased by 51.4% following intervention. Despite this decrease, there was no difference in pain control on the last day of opioid use (3.4/10 vs. 4.8/10) or satisfaction with pain control on the last day of opioid use (83.3% vs. 100%). On average, the pre-intervention cohort was satisfied with pain control 97% of the time while the post-intervention cohort was satisfied with pain control 100% of the time.
Unlike opioid use, there was no significant decrease in benzodiazepine use. The only significant decrease was in the amount of diazepam prescribed: 30 × 2 mg tabs pre-intervention and 17.5 × 2 mg tabs post-intervention. Patients used 4.75 × 2 mg tabs of diazepam pre-intervention and 5.4 × 2 mg tabs post-intervention. Benzodiazepines were used for 12.5 days pre-intervention and 10 days post-intervention. There was also no difference in the percentage of benzodiazepine prescription consumed (46.3% vs. 39%).
Multiple studies have highlighted the importance of a multi-modal pain regimen post-operatively in the hospital following PSF. This study continues to emphasize the importance of a multi-modal pain regimen following discharge. Patients consumed significantly less opioids when the predominant pain medications were scheduled NSAIDs and acetaminophen. It is not surprising that there was no significant difference in benzodiazepine use because it is used for muscle spasms, which are not controlled by any of the other medications provided. Patient pain was well controlled on a regimen of scheduled naproxen or ibuprofen and acetaminophen, daily gabapentin, a benzodiazepine for muscle spasms, and an opioid for breakthrough pain.
In this study, we began educating patients pre-operatively in clinic and prior to discharge post-operatively on pain expectations and proper management, which played an important role in decreasing the amount of opioids prescribed. There are no studies specifically showing that preoperative counseling on pain expectation and management decreases post-surgical opioid use after PSF, but there are studies demonstrating that preoperative mental health, pain and expectations are predictive of post-surgical satisfaction in patients with AIS [12]. In a study focusing on anxiety in AIS patients undergoing PSF, it was found that patients who underwent preoperative intervention (discussion of care, explanation of the surgical procedure, and tour of the hospital’s preoperative area, ICU, and patient floors) had slightly increased anxiety post-operatively but higher patient satisfaction scores [13]. Additionally, an orthopedic trauma study found that adult patients counselled preoperatively on the amount of pain medication to be prescribed and an expected cessation at 6 weeks were 1.14 times more likely to stop using opioids at 6 weeks compared to those who received no counseling [14]. Patients would highly benefit from a preoperative class on pain management and expectations with subsequent reinforcement prior to discharge after surgery.
Although this study produced statistically significant results, there were study limitations. The sample size was relatively small; however, it is comparable to previously published studies utilizing pain diaries. The significant and substantial decrease in opioid use allows generalization to a greater population. A larger sample may lead to significance of other variables. There was also a low return rate in both the pre- and post-intervention cohorts. Only one previously published study out of Sweden was found, which utilized pain diaries post PSF in AIS patients to better understand patient experiences and the trajectory of self-reported pain. In that study only 18 of 37 patients (48.6%) completed diaries [15]. Other studies looking at post-operative pain diaries after appendectomy, tonsillectomy, and wrist arthroscopy had response rates ranging from 69 to 83% [16,17,18,19]. Studies have shown the difficulty of pain diary compliance in adolescents. A multinational study using pain diaries for patients with sickle cell crises showed that patients over the age of 12 or those living in America had lower diary compliance and completion rates [20]. A headache pain diary study demonstrated that paper diaries had significantly more missing data than electronic diaries [21]. Future studies would benefit from the use electronic diaries to increase patient participation. Similarly, data was dependent on patient compliance with documentation, with the distinction mainly between those who chose to participate versus not participate in pain diary completion. Of the patients who used the pain diaries, there were only a handful of days among all the pre and post-intervention diaries where the patient skipped documentation of a single day. Finally, multiple interventions were implemented at the same time, which could lead to inconsistent application of the interventions. Although this is a risk, the over-arching message was cohesive and consistent from patient to patient and the only variables were the prescription amounts.
This analysis has directly impacted clinical practice. There has been a significant decrease in opioid consumption with maintained patient satisfaction. This indicates that the amount prescribed can be decreased further. There is still significant work to be done on decreasing opioid over-prescription. This is an ongoing initiative and continued feedback will be used to direct resources to further delineate the disparity between the amount of medications prescribed and consumed in our post-operative patients and to determine an optimal prescription dose with the ultimate aim of decreasing the risk of future opioid addiction. The issue of opioid prescriptions is not unique to PSF and future work can look into the ideal pain regimen for other orthopedic and non-orthopedic surgical procedures.
Key points
-
Opioids are often overprescribed by orthopaedic surgeons and continued research will help determine optimal post-operative opioid prescription doses.
-
Multimodal pain regimens based on scheduled NSAIDs, acetaminophen and gabapentin with benzodiazepines for breakthrough muscle spasms and opiates for breakthrough pain are highly effective.
-
Pre and post-operative counseling on pain medication regimens and patient pain expectations greatly decreases post-operative opioid consumption.
References
McCabe SE, West BT, Veliz P et al (2017) Trends in medical and nonmedical use of prescription opioids among US adolescents: 1976–2015. Pediatrics 139:e20162387
Harbaugh CM, Lee JS, Hu HM et al (2018) Persistent opioid use among pediatric patients after surgery. Pediatrics 141:e20172439
Gaither JR, Leventhal JM, Ryan SA et al (2016) National trends in hospitalizations for opioid poisonings among children and adolescents, 1997 to 2012. JAMA Pediatr 170:1195–1201
Miech R, Johnston L, O’Malley PM et al (2015) Prescription opioids in adolescence and future opioid misuse. Pediatrics 136:e1169–e1177
McCabe SE, West BT, Boyd CJ (2013) Leftover prescription opioids and nonmedical use among high school seniors: a multi-cohort national study. J Adolesc Health 52:480–485
Fortuna RJ, Robbins BW, Caiola E et al (2010) Prescribing of controlled medications to adolescents and young adults in the United States. Pediatrics 126:1108–1116
Veliz P, Boyd CJ, McCabe SE (2017) Nonmedical use of prescription opioids and heroin use among adolescents involved in competitive sports. J Adolesc Health 60:346–349
Veliz PT, Boyd C, McCabe SE (2013) Playing through pain: sports participation and nonmedical use of opioid medications among adolescents. Am J Public Health 103:e28–e30
Fletcher ND, Glotzbecker MP, Marks M et al (2017) Development of consensus-based best practice guidelines for postoperative care following posterior spinal fusion for adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 42:E547–E554
Choudhry DK, Brenn BR, Sacks K, Shah S (2019) Evaluation of gabapentin and clonidine use in children following spinal fusion surgery for idiopathic scoliosis: a retrospective review. J Pediatr Orthop 39(9):e687–e693. https://doi.org/10.1097/BPO.0000000000000989
Jabbour HJ, Naccache NM, Jawish RJ et al (2014) Ketamine and magnesium association reduces morphine consumption after scoliosis surgery: prospective randomised double-blind study. Acta Anaesthesiol Scand 58:572–579
Sieberg CB, Manganella J, Manalo G et al (2017) Predicting postsurgical satisfaction in adolescents with idiopathic scoliosis: the role of presurgical functioning and expectations. J Pediatr Orthop 37:e548–e551
Rhodes L, Nash C, Moisan A et al (2015) Does preoperative orientation and education alleviate anxiety in posterior spinal fusion patients? A prospective, randomized study. J Pediatr Orthop 35:276–279
Holman JE, Stoddard GJ, Horwitz DS et al (2014) The effect of preoperative counseling on duration of postoperative opiate use in orthopaedic trauma surgery: a surgeon-based comparative cohort study. J Orthop Trauma 28:502–506
Rullander AC, Lundstrom M, Ostlund U et al (2017) Adolescents’ experiences of scoliosis surgery and the trajectory of self-reported pain: a mixed-methods study. Orthop Nurs 36:414–423
Borgstrom A, Nerfeldt P, Friberg D (2019) Postoperative pain and bleeding after adenotonsillectomy versus adenotonsillectomy in pediatric obstructive sleep apnea: an RCT. Eur Arch Otorhinolaryngol 276:3231–3238
Group SS, Ahmed I, Cook JA et al (2015) Single port/incision laparoscopic surgery compared with standard three-port laparoscopic surgery for appendicectomy: a randomized controlled trial. Surg Endosc 29:77–85
Hansen TB, Jakobsen IA (2008) Intra-articular bupivacaine as treatment for postoperative pain after arthroscopy of the wrist. Scand J Plast Reconstr Surg Hand Surg 42:313–315
Sidman JD, Lander TA, Finkelstein M (2008) Platelet-rich plasma for pediatric tonsillectomy patients. Laryngoscope 118:1765–1767
Heath LE, Heeney MM, Hoppe CC et al (2017) Successful utilization of an electronic pain diary in a multinational phase 3 interventional study of pediatric sickle cell anemia. Clin Trials 14:563–571
Bandarian-Balooch S, Martin PR, McNally B et al (2017) Electronic-diary for recording headaches, triggers, and medication use: development and evaluation. Headache 57:1551–1569
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
Amelia M. Lindgren: Analysis and Interpretation of Data, Manuscript Preparation/Review and Final Manuscript Approval. Vidyadhar V. Upasani, Rebecca Bennett: Design, Data Acquisition, Analysis and Interpretation of Data, Manuscript Preparation/Review and Final Manuscript Approval. Burt Yaszay, Peter O. Newton: Design, Analysis and Interpretation of Data, Manuscript Preparation/Review and Final Manuscript Approval.
Corresponding author
Ethics declarations
Ethical approval
This study was reviewed by the participating hospital’s institutional review board and was determined to be exempt from review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Study conducted at Rady Children’s Hospital.
Rights and permissions
About this article
Cite this article
Lindgren, A.M., Bennett, R., Yaszay, B. et al. Quality improvement in post-operative opioid and benzodiazepine regimen in adolescent patients after posterior spinal fusion. Spine Deform 8, 441–445 (2020). https://doi.org/10.1007/s43390-019-00002-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43390-019-00002-6