Abstract
Three different measurement methods to determine the structural phase transitions and Curie temperature of Ba0.97Ca0.03Ti1−xSnxO3 (BCTS, x = 0.025, and 0.035 mol). electroceramics are discussed. At room temperature, both compositions reveal the tetragonal perovskite lattice symmetry as evidenced by X-ray diffraction, temperature-dependent dielectric constant and Raman active modes. The temperature-dependent dielectric study reveals TR-O at − 60 °C, TO-T at 14 °C, TT-C at 126 °C for composition x = 0.025 and TR-O at − 50 °C, TO-T at 20 °C, TT-C at 118 °C for composition x = 0.035. To evident the structural changes happening at phase transitions as well as Curie temperature the variation of polarization concerning temperature is investigated which supports the temperature-dependent dielectric and Raman spectroscopy studies. The room temperature recoverable energy storage density and efficiency of BCTS are calculated by the integral area of the polarization–electric field (P-E) hysteresis loop. The observed recoverable energy storage density is 21.80 mJ/cm3 and 32.40 mJ/cm3 with the efficiency of 43.58% and 52.25% for composition x = 0.025 and 0.035 mol., respectively. These results are having practical importance, due to the higher recoverable energy storage density and efficiency with moderate Curie temperature compared to the pure BaTiO3. Thus, it can be used as a promising novel and environmentally friendly, lead-free material, for different applications in low carbon vehicles, renewable energy technologies, integrated circuits, and for the high-temperature aerospace sector.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The Curie temperature (Tc) at which spontaneous polarization of ferroelectrics starts to cease and the ferroelectric phase becomes paraelectric plays an important role to decide their operating temperature range for practical applications in multifunctional devices. At lower temperature, rhombohedral (< − 90 °C) and orthorhombic (− 90 °C to 5 °C) phase of BaTiO3 material have low symmetry; while at a higher temperature, tetragonal (5 °C to 120 °C) and in cubic phase (>120 °C) it tends toward higher symmetry. The change of symmetry and phase transition in BaTiO3 is driven by both Ti and O atoms shifting along the c axis in one direction and Ba atom in the opposite direction results in a change in dipole moment and thus polarization [1]. The change of symmetry can be driven by temperature, pressure, chemical composition, electric field, etc. With the change in temperature an inconsistency is observed in dipole moment, symmetry of unit cell, specific heat capacity, dielectric permittivity and thermal expansion at the structural transition which can be utilized to detect phase transition temperature in the material. BaTiO3 is the widely studied material that undergoes three polymorphic structural transitions as rhombohedra (R) to orthorhombic (O) at − 90 °C, orthorhombic (O) to tetragonal (T) at 5 °C and tetragonal (T) to Cubic (C) at 120 °C. Among these rhombohedral, orthorhombic and tetragonal phases are the non-centrosymmetric having polarization along [111], [011], [001] axis respectively and the cubic phase is centrosymmetric with no dipole moment as shown in Fig. 1a [2,3,4,5].
Multilayer ceramics capacitors (MLCC), sonar, transducer, and actuators need stable capacitance and polarization with large spam of temperature range to make it convenient for practical applications. Because the change in temperature changes the polarization in ferroelectric material, thus the temperature decides the upper and lower temperature limits for application. Most of the electronic components made up of BaTiO3 are used for applications having temperatures ranging from room temperature to half of its Curie temperature, so it is prime important to know the Tc of such materials [2, 6]. Furthermore, the ABO3 structured alkaline-bismuth-titanate-based materials (Bi0.5Na0.5TiO3, Bi0.5K0.5TiO3) show good dielectric and ferroelectric properties than BaTiO3. But the volatility of alkaline-bismuth-titanate-based materials during sintering hampers the stability and thus the application of these materials over the wide temperature range. As compared to other lead-free materials the BaTiO3 gives the stable dielectric, ferroelectric and piezoelectric with low Curie temperature (120 °C). Thus, with appropriate substitution of Ca2+, Zr4+, Sn4+, Hf4+ in BaTiO3 helps to improve the dielectric, ferroelectric and piezoelectric properties of Pure BaTiO3 [7]. For Ca2+ and Sn4+ substituted BaTiO3 ceramics we reported the O-T phase co-existence with d33 = 287 pC/N and d*33 = 505 pm/V and Tc ~ 126 °C [8]. In this study, we analyzed that, the Curie temperature and structural phase transitions from a non-centrosymmetric state to a centrosymmetric state is an important aspect of the ferroelectrics before employing for further application. The Curie temperature and structural transition can be determined by six different temperature-dependent characterization techniques such as (1) X-ray diffraction, (2) dielectric constant measurement, (3) Raman spectroscopic study, (4) polarization (5) differential scanning calorimetric (DSC) curve and (6) up-conversion photoluminescence [7,8,9,10]. Determination of Curie temperature by a temperature-dependent dielectric constant is mostly reported. But, to evident the value of Tc apart from temperature-dependent dielectric constant measurement other approaches should be used, which we have done in this work. We explore the determination of Curie temperature by temperature-dependent dielectric constant measurement and confirm it with temperature-dependent Raman spectroscopy and P-E hysteresis loop.
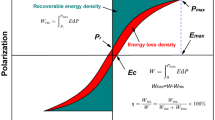
Energy storage density is an important aspect in fast-growing compact and commercially available capacitors in pulsed power applications, high-power microwave systems and power electronics devices. The remnant polarization (Pr) maximum polarization (Pmax) and break-down strength (BDS) of dielectric material are the important parameters to consider the material for energy storage density. The demonstration of recoverable energy density and loss in energy in a dielectric material is shown in Fig. 1b. There are two possible probabilities to enhance the recoverable energy storage density. One is, Low Pr value and high Pmax which increase the difference between Pr and Pmax and the other is to improve the BDS. Break down strength is the strength of material up to which it acts as dielectric/ferroelectric and above the BDS it becomes a conductor for an applied electric field. The creation of appropriate oxygen vacancies in dielectric material by suitable substituents acts as electron trap sites and once the electron trap levels become deeper it results in a significant enhancement in BDS. Most of the literature available for energy storage density is for the thin film capacitors and very little literature is available for the bulk ceramic capacitors [11, 12]. The substitution of Sn4+ for Ti4+ in BaTiO3 and (Ba,Ca)TiO3 decreases remnant polarization (Pr), coercive field (Ec) and shift the Curie temperature peak towards lower temperature with increasing room temperature permittivity [6, 7, 13]. Barium stannate titanate (BaTi1-xSnxO3) is a binary solid solution composed of BaTiO3 and BaSnO3. The substitution of higher ionic radii Sn4+ (0.69 Å) ion for the lower ionic radii Ti4+ (0.60 Å) ion in BaTiO3 leads to a decrease in the ferroelectric property [7, 13]. The reduction of Pr, Ec, and Jloss due to substituting Sn4+ for the Ti4+-site of (Ba,Ca)(Ti1-xSnx)O3 can lead to improved recoverable energy storage density (Jrecov) and energy storage efficiency (η) [13]. It has been reported that the substitution of Sn4+ enhances the diffuse phase transition and dielectric properties of the BaTiO3 and (Ba,Ca)TiO3 ceramics [6, 7, 13]. Hence, we have tried to investigate the effect of substituting Sn4+ for the Ti4+-site of (Ba,Ca)TiO3 for the energy storage properties along with determination and confirmation of the structural phase transition and Curie temperature of the BaTiO3-based lead-free perovskite materials.
2 Experimental details
The (Ba0.97Ca0.03Ti1-xSnx)O3 (BCTS, x = 0.025 and x = 0.035 mol.) ceramics were prepared by conventional solid-state reaction method. BaCO3 (99%), CaCO3 (99%), TiO2 (99%), and SnO2 (99.9%) (all are AR grade from Sigma Aldrich) were used as raw material and weighted in their stoichiometric proportion. The weighted raw materials was ball milled for 24 h. in ethanol medium then dried at 80 °C for 10 h. in the oven. This dried raw material was grounded well for 1 h. and calcined at 1100 °C for 5 h. with heating and cooling rate of 2 °C/min. To attain proper homogeneity calcined powder was further grounded for 1 h. followed by ball milling for 24 h. using ethanol medium and again drying at 80 °C for 10 h. in the oven. The calcined dried powder was pelletized in the dimension of 0.8 to 1 mm thickness and 10 mm diameter and sintered at 1300 °C for 5 h. with a heating and cooling rate of 2 °C/min in an air atmosphere. The room temperature structural phase confirmation was done using an X-ray diffractometer (D8 Advanced, Bruker Inc. Germany) with CuKα radiation (λ = 1.5406 Å). Temperature-dependent Raman measurement was performed using a He–Ne laser of 532 nm line of Raman spectroscopy (Renishaw In Via microscope Raman) and a Linkam heating–cooling stage (THMS 600). The microstructure was examined using scanning electron microscopy (JEOL-JSM 6306A, Tokyo, Japan). The dielectric permittivity (ε) as a function of temperature from −180 to 200 °C was measured at 10 kHz using an LCR meter (HIOKI- 3532–50, Nagano, Japan). The P-E hysteresis loop at various temperatures was recorded at the fixed field of 7 kV/cm at 50 Hz, using a P-E loop tracer (Marine India). The recoverable energy density and the loss in energy during the charging and discharging cycle of BCTS were calculated from the P-E loop measurement using MATLAB (R2018a) software.
3 Results and discussion
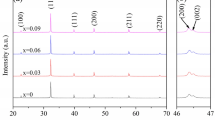
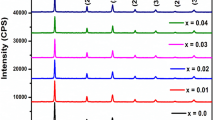
The Fig. 2a, b shows Reitveld refined X-ray diffraction patterns of (Ba0.97Ca0.03Ti1-xSnx)O3 for the composition x = 0.025 and x = 0.035, respectively. Both the composition shows the perovskite structure without any trace of secondary phase formation. The Rietveld refinement pattern for the composition x = 0.025 and x = 0.035 are well matched with the tetragonal structure P4mm (ICSD#168,783) lattice symmetry and goodness of fit parameters are shown in Table 1. The peculiar characteristic peaks, (002) and (200) splitting around 2θ = 45° with higher intensity of (200) than (002) along with splitting of (301) and (310) around 2θ = 75° with higher intensity of (310) than (301) gives supportive evidence of tetragonal structure formation as shown in Fig. 2c, d [14]. The position of the XRD peak shift to the lower 2θ value for composition x = 0.035 with increasing the interplanar distance between atoms, because the ionic radii of the Sn4+ (0.69 Å) is greater than the Ti4+ (0.605 Å), which generates the lattice strain due to replacement of Ti4+ by Sn4+ and confirmed from tetragonality (c/a) ratio given in Table 1 [15]. The theoretical and actual density given in Table 1, is calculated using lattice constant obtained from the Reitveld fitted XRD pattern and liquid immersion method (Archimedes principle) in xylen medium, respectively. The bulk density calculated using theoretical and actual density is 95.64% and 92.69% for the composition x = 0.025 and 0.035, respectively. The scanning electron micrographs reveal inhomogeneous grain distribution with an average grain size of 24 µm and 30 µm for the composition x = 0.025 and x = 0.035 as shown in Fig. 2e, f, respectively. The substitution of Sn4+ promotes the grain growth in BCT which increases the average grain size and thus the number of void spaces between the adjacent atoms which causes a decrease in bulk density [16].
a–f Rietveld refined X-ray diffraction pattern between 2θ range of 20°—80° for compositions (a) x = 0.025, (b) x = 0.035. Enlarged Rietveld refined X-ray diffraction pattern in the range of 2θ = 42°—48° and 2θ = 72°—78° for compositions (c) x = 0.025, (d) x = 0.035. SEM micrograph for compositions (e) x = 0.025 and (f) x = 0.035 of (Ba0.97Ca0.03Ti1-xSnx)O3 ceramics
Figure 3a, b shows the dielectric permittivity and first-order derivatives for the frequency 10 kHz in the vicinity of temperature − 180 °C to 200 °C. With an increase in the temperature both BCTS composition shows maxima at each structural transition and gives rise to three polymorphic structural phase transitions, R-O, O-T and T-C at − 60 °C, 14 °C, 126 °C for x = 0.025 and − 50 °C, 20 °C, 118 °C for x = 0.035, respectively. This structural transition indicates that the Sn4+ substitution raises the R-O and O-T transition temperature and decreases in T-C transition temperature [17]. The substitution of Sn4+cation leads the BCT ceramics to decrease their Curie temperature. Because the ionic radius of Sn4+cation (0.69 Å) is larger than that of the Ti4+cation (0.605 Å), this substitution of Sn4+ for Ti4+ increases the bond length of Ti–O results in decreasing the bonding force of tetrahedral of B-site cation and the oxygen anion of the ABO3 perovskite structure. As the bonding force between B–O bonds are weakening, the structural phase transition tends towards lower temperatures [18, 19]. To understand the diffuseness of the structural phase transition of both BCTS compositions, a qualitative characterization of inverse dielectric constant concerning temperature and linear plot of ln(T − Tm) versus ln( 1/ ε − 1/εm) at 10 kHz was investigated. The well-known phenomenon in normal ferroelectric materials, in which dielectric permittivity follows the Curie–Weiss law above the Curie temperature is given by following equation [20]
where C is the Curie–Weiss constant, T0 is the Curie–Weiss temperature and ε represents the dielectric constant. Figure 3c, d shows the inverse dielectric constant (1/ɛ) versus temperature at 10 kHz in the range of temperature -100 °C to 180 °C for the composition x = 0.025 and x = 0.035, respectively. The obtained value of Curie -Weiss constant (C) from the slop of the inverse dielectric constant and temperature is in the order of 105 °C which reflects the ferroelectric structural transition. Uchino and Nomura modify Curie–Weiss law to investigate the degree of ferroelectric-paraelectric diffuse phase transition and proposed the following equation [21]
where γ is the diffusion coefficient and C′ is the Curie constant. Generally, the value of γ for a normal ferroelectric material is 1, for an ideal ferroelectric relaxor material it is 2 and the variation of γ between 1 and 2 represents the incomplete diffused phase transition [18, 21]. The logarithmic form of Eq. (2) is given by the equation,
The inset diagram in Fig. 3c) and Fig. 3 (d) shows the best fit to the experimental points of the linear plot of ln(T − Tm) versus ln( 1/ ε − 1/εm) at frequency 10 kHz. The value of γ obtained from the slope of plots is 1.08 and 1.19 for the composition x = 0.025 and x = 0.035, respectively. These values of γ show the non-relaxor ferroelectric and incomplete diffused phase transition behavior for the composition x = 0.025 and x = 0.035.
Temperature-dependent Raman spectra were recorded in the temperature range − 100 °C to 130 °C to ensure about the structural phase evolution is shown in Fig. 4a, b and the phase diagram based on Raman shift for peaks υ3(LO), υ3(TO) and υ2(LO, TO) shown in Fig. 4c, d for composition x = 0.025 and x = 0.035, respectively. At − 100 °C with increasing frequency the υ1(LO), υ1(TO), υ2(LO, TO), υ4(LO), υ3(TO) and υ3(LO) Raman active modes observed at position 715 cm−1, 525 cm−1, 308 cm−1, 225 cm−1, 185 cm−1 and 168 cm−1respectively [23]. Mainly the peak υ3(LO) at 168 cm−1 along with two dips among υ3(LO) and υ3(TO) at 178 cm−1 as well as among υ3(TO) and υ4(LO) peaks at 194 cm−1 indicates the rhombohedral phase for x = 0.025 and 0.035. At − 50 °C for x = 0.025 and 0.035, the characteristic peak υ3(LO) at 168 cm−1 of rhombohedral phase get disappears, while the dip among the peaks υ3(TO) and υ4(LO) at 194 cm−1 disappear as well as υ3(TO) and other peaks show broadening. This nature of Raman spectra is an indication of the rhombohedral structure completely transforms into the orthorhombic structure. Near the temperature of 25 °C, the υ3(TO) peak at 180 cm−1 disappears for both compositions which is a sign of structural change from orthorhombic to tetragonal phase. Further increase in temperature up to 130 °C for composition x = 0.025 and 120 °C for composition x = 0.035, υ1(LO) peak at 715 cm−1 and υ2(LO, TO) at 308 cm−1 are disappearing, and the entire spectrum gives only two broad peaks, υ1(LO) at 225 cm−1 and υ1(TO) at 525 cm−1, which indicates the structural transition from tetragonal to cubic phase.
Figures 5a–d and 6a–d show the electric field-induced polarization (P-E) hysteresis loop at temperatures 30 °C, 60 °C, 90 °C, 130 °C and 30 °C, 60 °C, 90 °C, 120 °C for composition x = 0.025 and x = 0.035, respectively, at an applied electric field of 7 kV/cm. Both compositions reveal the asymmetric nature for ± Pr and ± Ec values and hence exhibit the ferroelectric imprint phenomenon [24]. When a ferroelectric material experiences high voltage or repeated pulses for a long time particularly with the increase in temperature, its polarization is not fully reversed by the application of a single voltage pulse with the opposite polarity. Thus, inadequate polarization orientation in opposite direction leads to the shift of the PE hysteresis loop along the electric field axis [24]. The P-E hysteresis loop shows non-linear nature up to the temperature of 90 °C for both compositions x = 0.025, x = 0.035, and becomes linear at temperature 130 °C for composition x = 0.025 and 120 °C for composition x = 0.035. This result indicates that the material shows non-linear nature until the presence of polarization in the perovskite tetragonal phase and above Curie temperature polarization due to dipole moment becomes zero, resulting in the linear nature of PE hysteresis loop. Figures 5e and 6e show spontaneous polarization and inverse permittivity concerning temperature for composition x = 0.025 and x = 0.035, respectively. The spontaneous polarization and inverse permittivity vanish at Curie temperature. While after Curie temperature the spontaneous polarization remains stable near to zero and the inverse permittivity again starts to increase with temperature. According to Landau theory; the presence of interface, hetero-phase state, and hysteresis are the required parameters for the first-order transition and change in any one of these parameters is sound to evidence the first-order transition as they are intimately linked with each other [25]. The temperature-dependent P-E measurement for composition x = 0.025 and x = 0.035 shows a decrease in polarization and hysteresis loss (energy loss) with an increase in temperature. Thus, a decrease in energy loss (hysteresis) of material from a nonlinear ferroelectric state to a linear paraelectric state is an indicator of the first-order phase transition of the synthesized material. Thus, the P-E hysteresis loop with non-linear ferroelectric tetragonal structure and linear cubic structure accomplished the Curie temperature 130 °C and 120 °C for composition x = 0.025 and x = 0.035, respectively, which is consistent with the temperature-dependent dielectric and Raman spectroscopic study [8, 26].
a– f Polarization verses electric field loops at temperatures (a) 30 °C, (b) 60 °C, (c) 90 °C and (d) 130 °C, (e) variation of spontaneous polarization and inverse permittivity with respect to temperature, (f) temperature dependent recoverable energy density (Jrecov), energy loss (Jloss) and efficiency (η) for composition (Ba0.97Ca0.03Ti0.975Sn0.025)O3
a– f Polarization verses electric field loops at temperatures (a) 30 °C, (b) 60 °C, (c) 90 °C and (d) 120 °C, (e) variation of spontaneous polarization and inverse permittivity with respect to temperature, (f) temperature dependent recoverable energy density (Jrecov), energy loss (Jloss) and efficiency (η) for composition (Ba0.97Ca0.03Ti0.965Sn0.035)O3
To know the probability in practical applications, the energy storage performances for the compositions x = 0.025 and x = 0.035 were studied as a function of an applied electric field at room temperature. The energy-storage density of the BCTS materials for composition x = 0.025 and x = 0.035 was investigated according to the schematic diagram given in Fig. 1b. The recoverable energy density (Jrecov) stored in the dielectric material is calculated from P-E hysteresis loops using the following equations [27,28,29]:
where, Pr and Pm are remnant and maximum polarization values respectively, and E is the applied electric field. The obtained values for Jrecov with above equation are 21.84 mJ/cm3 and 32.40 mJ/cm3 for composition x = 0.025 and x = 0.035, respectively. The loss in electrical energy (Jloss) due to inherent hysteresis calculated by the numerical integration of the closed area of the PE hysteresis loop is given by the equation,
Thus, the observed loss in electrical energy is 28.75 mJ/cm3 and 29.6 mJ/cm3 for the composition x = 0.025 and x = 0.035, respectively. The sum of electrical loss (Jloss) and the recoverable energy density gives the total amount of energy to charge a ferroelectric capacitor and given by the equation,
With the analogy of the total energy density, 50.59 mJ/cm3 and 62 mJ/cm3 are the energy required to charge the ferroelectric capacitors of the composition x = 0.025 and x = 0.035, respectively. Based on Eqs. (4) and (5), the energy storage efficiency (η) value of material can be calculated with the following equation:
Thus, the calculated value of efficiency from Eq. (7) are 43.58% and 52.25% for the compositions x = 0.025 and 0.035, respectively. The increase in the efficiency from the composition x = 0.025 to x = 0.035 is due to the decrease in the coercive electric field with composition caused by the oxygen vacancies. Figures 5f and 6f show the change in recoverable energy density (Jrecov), energy density loss (Jloss) and efficiency (η) concerning temperature. The observed values for Jrecov, η and Jloss at Curie temperature for composition x = 0.025 are 47.14 mJ/cm3, 87.78% and 6.56 mJ/cm3 respectively. Similarly for composition x = 0.035 the observed values are 66.21 mJ/cm3, 82.59% and 13.95 mJ/cm3 for Jrecov, η and Jloss, respectively. As temperature increases the ferroelectric tetragonal structure of Ba0.97Ca0.03Ti1-xSnxO3 becomes cubic at 126 ºC for composition x = 0.025 and 120 ºC for composition x = 0.035 giving rise to slimmer PE loop results in a decrease in the remnant polarization and hysteresis loss [30]. According to Eq. (4) as remnant polarization decreases the ΔP (ΔP = Pmax—Pr) value increases, which is favorable to increase recoverable energy density (Jrecov) along with the decrease in energy density loss (Jloss). The increase in Jrecov and decrease in Jloss effectively increase the efficiency (η). The observed recoverable energy density, energy density loss and efficiency of BCTS material at room temperature are compared with other lead-free polymer and bulk ceramics in Table 2. With such Jloos, Jreco and efficiency (η) values, the BCTS ceramics are comparable with other lead-free ceramics for the energy-storage capacity and have potential application in electrostatic energy-storage devices [27, 31,32,33].
4 Conclusions
The Ba0.97Ca0.03Ti1-xSnxO3 ceramics prepared by solid-state reaction reveal the formation of tetragonal perovskite structure without a trace of any secondary phase formation. The temperature-dependent dielectric and Raman spectroscopic study gives three polymorphic structural transitions corresponding to TR-O, TO-T, and TT-C. Temperature-dependent P-E hysteresis loops provide information about the first-order ferroelectric to the paraelectric structural transition of BCTS ceramics for compositions x = 0.025 and 0.035. The energy storage density 21.80 mJ/cm3 and 32.40 mJ/cm3 with efficiency (η) of 43.58% and 52.25% of composition x = 0.025 and x = 0.035 are comparable with other lead-free ceramics for the energy-storage capacity and have potential application in electrostatic energy-storage devices. Thus, this work determines and confirms the structural phase transition and Curie temperature as well as energy storage density of the BaTiO3-based lead-free perovskite materials.
References
Ch. Eisenschmidt, H.T. Langhammer, R. Steinhausen, G. Schmidt, Ferroelectrics 432, 103 (2012)
K. C. Kao, Dielectric Phenomenon in Solids with emphasis on physical concepts of electronic processes, Elsevier New York, (2004).
E. Chandrakala, J. P. Praveen, A. Kumar, A. R. James, D. Das, J. Am. Ceram. Soc. 99 11 3659 (2016).
B. Rawal, P. Dixit, N.N. Wathore, B. Praveenkumar, H.S. Panda, Bull. Mater. Sci. 43, 82 (2020)
D. Shihua, S. Tianxiu, Y. Xiaojing, L. Guanghua, Ferroelectrics 402, 55 (2010)
A. K. Nath, N. Medhi, Bull. Mater. Sci. 35 5 847 (2012).
M. Acosta, N. Novak, V. Rojas, S. Patel, R. Vaish, J. Koruza, G. A. Rossetti, J. Rödel, Appl. Phys. Rev. 4, 041305 (2017).
P. S. Kadhane, B. G. Baraskar, T. C. Darvade, A. R. James, R. C. Kambale, Solid State Commun. 306 113797 (2020).
M. A. Ansari, K. Sreenivas, Mater. Lett. 264 127294 (2020).
J. Wu, W. Mao, Z. Wu, Y. Jia, Mater. Lett. 166, 75 (2016)
V. S. Puli, D. K. Pradhan, S. Adireddy, R. Martínez, P. Silwal, J. F. Scott, C. V. Ramana, D. B. Chrisey, R. S. Katiyar, J. Phys. D: Appl. Phys. 48 355502 (2015).
Y. Zhang, W. Li, W. Cao, Y. Feng, Y. Qiao, T. Zhang, W. Fei, Appl. Phys. Lett. 110, 243901 (2017).
S. Markovi, M. Mitri, N. Cvjeticanin, D. Uskokovic, J. Eur. Ceram. Soc. 27, 505–509 (2007)
N. Chaiyo, D.P. Cann, N. Vittayakorn, Mater. Design 133, 109 (2017)
M. Khan, A. Mishra, J. Shukla, P. Sharma, AIP Conference Proceedings 2100, 020138 (2019).
T. Hoshina, S. Hatta, H. Takeda, T. Tsurumi, Jpn. J. Appl. Phys. 57 0902BB (2018).
A. Kalyani, K. Brajesh, A. Senyshyn, R. Ranjan, Appl. Phys. Lett. 104 252906 (2014).
S. J. Kuang, X. G. Tang, l. Y. Li, Y. P. Jiang, Q. X. Liu, Scripta Materialia 61 68 (2009).
I. Ramovatar, S. Coondoo, N. Satapathy, Panwar. Physica B 553, 68 (2019)
J.P. Praveen, K. Kumar, A.R. James, T. Karthik, S. Asthana, D. Das, Curr. Appl. Phys. 14, 396–402 (2014)
K. Uchino, S. Nomura, Ferroelectr. Lett. 44, 55–61 (1982)
L. Qiang, N. Li, P. Haijun, Z. Nianshun, F. Huiqing, Ceram. Int. 45, 1676 (2019)
C.H. Perry, D.B. Hall, Phys. Rev. Lett. 15, 700 (1965)
Hiroshi Ishiwara, journal of Nanoscience and Nanotechnology, Vol. 12, 7619–7627, 2012.
Y. Mnyukh, American Journal of Condensed Matter Physics 3(5) 25 142–150 (2013).
P. Jaita, A. Watcharapasorn, N. Kumar, D.P. Cann, S. Jiansirisomboon, Electron. Mater. Lett. 11, 828 (2015)
G. Jian, Y. Jiao, Q. Meng, Z. Wei, J. Zhang, C. Yan, K. S. Moon, C.P. Wong, Communications materials, doi.org/https://doi.org/10.1038/s43246-020-00092-0.
F. Akram, M. Sheeraz, A. Hussain, W. Kim, T.H. Kim, C.W. Ahn, Ceram. Int. 47, 23488–23496 (2021)
Y. Huang, F. Li, H. Hao, F. Xia, H. Liu, S. Zhang, J. Materiomics 5, 385–393 (2019)
M. Zhoua, R. Liang, Z. Zhou, X. Dong, j. ceramint, 2019, 09, 265.
T. Garg, V. Annapureddy, K.C. Sekhar, D.Y. Jeong, N. Dabra, J.S. Hundal, Polym. Compos. 41(12), 5305–5316 (2020)
Z. Zhang, S. Luo, S. Yu, Z. Guan, R. Sun, C.P. Wong, Mater. Des. 142, 106–113 (2018)
A. Kumar, K.C.J. Raju, J. Ryu, A.R. James, Appl. Phys. A 126, 175 (2020)
Acknowledgements
RCK thankfully acknowledges the Science and Engineering Research Board (SERB), Government of India (File No. EMR/2016/001750) for providing the research funds under the Extra Mural Research Funding (Individual Centric) scheme.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kadhane, P.S., Baraskar, B.G., Darvade, T.C. et al. Investigation of structural phase transition, Curie temperature and energy storage density of Ba0.97Ca0.03Ti1−xSnxO3 electroceramics. J. Korean Ceram. Soc. 59, 578–588 (2022). https://doi.org/10.1007/s43207-022-00189-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43207-022-00189-x