Abstract
Triple-negative breast cancer (TNBC) is a highly heterogeneous disease defined by the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2), resulting in poor clinical outcomes and high mortality. The present study was aimed to evaluate the efficacy of Punicalagin (PCG), a polyphenol obtained from the Punica granatum, against TNBC. We evaluated the therapeutic potential of PCG in TNBC (MDA-MB-231, BT-20) and ER + (MCF-7) breast cancer cells. A dose-dependent inhibition of MDA-MB-231 cell proliferation was observed with PCG (12.5–100 μM). However, only 50 and 100 μM doses of PCG inhibited the growth of BT-20 and MCF-7 cells. PCG significantly increased mitochondrial ROS in TNBC cells and induced autophagy across all cell lines, as evidenced by an increase in autophagic vacuoles and a decrease in the ratio of LC3-II/LC3-I. PCG suppressed PI3K/Akt and activated phosphorylated c-Jun N-terminal kinase (p-JNK) signaling. Based on these findings, it can be concluded that PCG is capable of significantly inhibiting the proliferation of TNBC cells through the suppression of the PI3K/Akt pathway as well as the initiation of the JNK pathway. PCG could thus be potentially useful as a therapeutic agent for the treatment of TNBC.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) has been reported to affect a total of 2.3 million women in 2020, making it the most common cancer diagnosed worldwide. BC has been reported to cause the highest number of deaths among females, accounting for 685,000 fatalities worldwide in 2020 [1]. BC is treated locally through surgery and radiation. Systemic therapies such as chemotherapy, hormone therapy, targeted drug therapy, and immunotherapy are used alone or in combination depending on the type of BC. However, despite all the recent advances in BC therapy, some subtypes of BC continue to be a significant challenge to monitor and treat. One such BC subtype is triple-negative breast cancer (TNBC), which accounts for about 15–20% of the BC cases [2]. TNBC does not express estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER-2), has clinical features that include high invasiveness, high metastatic potential, proneness to relapse, and poor prognosis, with a mortality rate of approximately 40% within 5 years of diagnosis [3].
The medicinal properties of plants have been recognized for millennia. According to estimates, compounds from natural sources including vinblastine, vincristine, camptothecin and its derivatives, taxanes, and their analogs, among others, account for more than half of the anti-cancer medications now used in chemotherapy [4]. In other words, natural bioactive compounds serve as a source for innovative chemotherapeutic medicines that are employed in the treatment of different cancers. Pomegranate (Punica granatum) is a member of the Punicaceae family. In many nations, pomegranates have long been used in traditional medicine to cure acidosis, diarrhea, and respiratory problems [5]. The pomegranate leaf has characteristics that make it effective against helminths, parasites, diarrhea, inflammation, and cancer. Pomegranate contains hydrolyzable tannins, primarily ellagitannins, and anthocyanins (cyaniding, delphinidin, and pelargonidin), which give the fruit its distinctive red hue. About ninety percent of the antioxidant potential of pomegranates is attributed to these polyphenols, with Punicalagin (PCG), a unique type of ellagitannin, accounting for over half of this antioxidant capacity on its own [6]. PCG, as shown in Fig. 1, is a polyphenolic tannin from the leaf and husk of pomegranates. It has historically been reported to have a variety of biological characteristics, including antioxidant [7] anti-inflammatory [8], anti-cancer [9], and antiviral activities [10]. PCG has been reported to stop the proliferation of cancer cells and cause autophagy and apoptosis in various cancer cell lines in vitro, such as thyroid cancer [11], lung cancer [9], colorectal cancer [12], and cervical cancer [13].
Extensive research has been conducted on the role of reactive oxygen species (ROS) in cancer and other human disease conditions. ROS occurs naturally as a waste product of several biological activities, including the metabolism of oxygen [14]. ROS is a collection of reactive, unstable, and partially reduced derivates of oxygen. These derivates include the hydroxyl radical (·OH), singlet oxygen (1O2), superoxide anion (O2−), hypochlorous acid (HOCl), and hydrogen peroxide (H2O2) [15]. They play an important role in cell signaling as second messengers and are required for many different cellular activities in both healthy and malignant tissues. In cancer cells, ROS have been shown to be active in a way that may be both beneficial and harmful. When present in low to moderate concentrations, ROS serve as signal transducers that promote cancer cell growth, motility, invasion, angiogenesis, and resistance to anticancer drugs [14, 16]. In other words, maintaining a healthy amount of ROS is critical for the homeostasis of cancer cells during the progression of cellular processes, such as proliferation, differentiation, migration, and apoptosis. On the other hand, cancer cells are damaged by excessive amounts of ROS, which ultimately results in cell death [17].
In response to intracellular and extracellular stressors like starvation, cells undergo a process of self-digestion known as macroautophagy (henceforth, autophagy) in which cellular proteins and organelles are digested by lysosomal enzymes. Autophagy is a process that happens at a low basal level to degrade damaged proteins and organelles within a cell to preserve cellular homeostasis even when no external stimulus is present [18]. The autophagic process has been linked to several human diseases, including cancer [19]. The autophagic death of cancer cells due to numerous chemotherapeutic drugs has already been confirmed. Drug-resistant cancer cells have also been studied for their potential as a therapeutic target through autophagy [20]. PCG has been shown to trigger autophagy in BCPAP cells, a type of human papillary thyroid carcinoma cell line [11]. Based on these reports of the anticancer activity of PCG, we aimed to analyze the role of PCG in cell growth arrest in TNBC cells in this study. The phosphorylated c-Jun N-terminal kinase (p-JNK) signaling pathway was thought to be a potential target for the action of PCG regulation in TNBC development. It was also hypothesized that the PCG might cause autophagy after triggering the JNK pathway.
Materials and methods
Reagents and chemicals
PCG was purchased from (ChemCruz, Huissen, Netherlands) and dissolved in dimethyl sulfoxide (DMSO) to make a final concentration of 10 mM sterile PCG in DMSO.
Cell culture and media
TNBC cells, MDA-MB-231 and BT-20, were procured from the Korean Cell Line Bank (KCLB; Seoul, Korea), while ER + , PR + / − , HER2 − cell line i.e., MCF-7 was acquired from the ATCC (Manassas, VA, USA). DMEM (Welgene, Gyeongsan, Korea) was used to culture cancer cell lines. DMEM for these cell lines was supplemented with 5% fetal bovine serum (FBS; BIOWEST, Nuaillea, France), 1 M HEPES buffer solution (Welgene, Gyeongsan, Korea), and 1% antibiotics (Thermo Fisher Scientific, USA) for MDA-MB-231 and MCF-7 cells. However, the BT-20 cells were provided with 10% FBS, 1 M HEPES buffer solution, and 1% antibiotics for growth. During the trial period, cells were perpetuated at 37 °C by providing a humid environment with 5% CO2. The cells were sub-cultured every three days after 70–80% confluence was achieved by treating them for 3 min at 37 °C with 1% Trypsin–EDTA (Invitrogen Life Technologies Inc., Carlsbad, CA, USA).
Cell viability assay
The 96-well culture plates were seeded with 3 × 103 cells per well and after a 24-h incubation period were treated subsequently with different concentrations of PCG (0–100 M) for 72 h. The viability of the cells was determined by using the Quanti-MAX™ WST-8 Cell Viability Assay Kit (Biomax, Korea). The plates were incubated for one hour after removal of the medium and addition of Quanti-MAX™, followed by the measurement of absorbance at 450 nm using a Synergy NEO 2 multimode plate reader (BioTek, Winnoski, VT, USA). The cytotoxicity of the PCG was compared with the cell survival ability of the cells treated with 1% DMSO.
Colony formation assay
A seeding density of 500 cells per well was used for MDA-MB-231 and MCF-7 cells, which were incubated for 24 h, while a seeding density of 1000 cells per well was used for BT-20 cells. Seeding of cells was followed by exposure to different concentrations of PCG and incubated for 10 days. Every three days, the medium was changed to provide fresh supply of the pertinent chemical until day 10. After that, the cells were washed twice with DPBS (Welgene, Gyeongsan, Korea) and then treated with 100% methanol (Sigma-Aldrich Inc., Saint Louis, USA) for 20 min. A 0.5 percent crystal violet solution was then used to stain the colonies generated from single cells. After this, the samples were rinsed with DPBS.
MitoSOX assay
The cancer cell lines were seeded in 96-well plates at 37 °C for 24 h in a humidified atmosphere containing 5% CO2 and at a density of 4 × 103 cells per well. Following that, PCG was then administered at varying concentrations (0, 6.25, 12.5, 25, 50, and 100 mM) to the cells. The cells were stained with MitoSOX™ (5 μM) and Hoechst33342 (10 μg/ml) after 48 h. Finally, the Lionheart™ FX Automated Microscope (BioTek, USA) was wielded to capture the stained cells images and further analyzed with Gen5 v 3.14.03.
Acridine orange staining
Cancer cells (4 × 103 cells) were initially incubated at 37 °C for 24 h in a humidified atmosphere containing 5% CO2 and then treated with PCG for 48 h. After that, cells were stained with 1 µg/mL acridine orange for 20 min at 37 °C to detect the presence of autophagy-specific acidic vesicular organelles (AVOs). Later, DPBS was employed to wash the cells twice before taking the images of AVO. Images of the stained AVOs were captured using Lionheart™ FX Automated Microscope (BioTek, USA) equipped with Gen5 v 3.14.03.
Flow cytometry analysis
Cancer cells (8 × 104 cells) were seeded in 6-well culture plates and incubated for 24 h before being subjected with various doses of PCG. After 48 h of PCG treatment, the rate of apoptosis was detected by using the Annexin V-FITC Apoptosis Detection Kit (Sigma-Aldrich, USA). Succinctly, Trypsin was used to collect cells, which were centrifuged for 3 min at 2000 rpm for 3 min. Cells obtained after centrifugation were suspended in FITC, PI and 1X annexin-binding buffer in dark at room temperature for 15 min. BD FACSymphony™ A3 Cell Analyzer (BD Bioscience, USA) was then employed to detect the stained cells and the data obtained were examined by the FlowJo Software v. 10.5.3 (BD Bioscience, USA).
Western blot analysis
For the western blot analysis, MDA-MB-231 cells were treated with 20 μM and 50 μM, BT-20 cells with 75 μM and 140 μM, while the MCF-7 cells with 75 μM and 100 μM for 48 h. After this, cells were treated with EzRIPA Lysis Kit (ATTO, Tokyo, Japan) to collect the proteins. Moreover, BCA protein assay was used to measure the extracted protein. After that, SDS-Page was used to load the proteins for electrophoresis. Primary antibodies (Table 1) were used to detect proteins after they had been transferred to a PVDF membrane and blocked with 5 percent skim milk. In this study, β-actin was used as a loading control. Detection of primary antibody was performed by employing horse radish peroxidase-conjugated anti-rabbit IgG (1:3,000; Bio-Rad, USA) for 2 h at 4 °C on shaker. Finally, SuperSignal™ West Pico PLUS Chemiluminescent Substrate (Thermo Scientific, USA) was used to identify the target protein bands bands in Lumino Graph 2 (ATTO, Tokyo, Japan). Fiji (Fiji is just ImageJ 1.54f) was used to quantify the relative intensities of bands on Western blots.
Statistical analysis
For consistent findings, every in vitro experiment was performed at least three times, and the data were all examined using the GraphPad Prism program (GraphPad Software Inc., San Diego, CA, USA). The results of the analysis of variance (ANOVA) and the post hoc Dunnett's multiple comparison test are shown with the means and standard deviations (SDs) or standard errors of the means (SEMs). A p-value less than 0.05 is deemed statistically significant when compared to the negative control.
Results
Marginal toxic effect of punicalagin on human lung fibroblast cells
The effect of PCG on the primary cell line was evaluated to assess its toxicity. It was found that PCG marginally decreased the growth of cells of the human lung fibroblast cell line (LL 24) at 50 and 100 µM (Fig. 2a). A high IC50 value of 1560 µM (Fig. 2b) was recorded when compared to IC50 in cancer cell lines. This indicates that the effects of PCG are specific to cancer cells, and it does not affect healthy cells.
Punicalagin dose-dependently decreases the growth of BC cells. a PCG meagerly inhibited the growth of LL 24 cells with b IC50 of 1560 µM. Furthermore, PCG suppressed the proliferation of c MDA-MB-231, e BT-20, and g MCF-7 cell lines. IC50 dose concentrations are presented in (d) for MDA-MB-231 (IC50 = 21.42 µM), f BT-20 (IC50 = 137.6 µM), and h MCF-7 (IC50 = 78.72 µM) cell lines. Following the statistical analysis, the data in the graphs have been derived from at least three repeated experiments and shown as the mean ± SD. *p ≤ 0.05 compared to control
Effect of punicalagin on the proliferation of breast cancer cells
Various concentrations of PCG were used against MDA-MB-231, BT-20, and MCF-7 cancer cell lines for 72 h. The viability and proliferation of the cells were determined using the water-soluble tetrazolium salt (WST) assay. PCG inhibited the growth of MDA-MB-231 cells in a dose-dependent manner (Fig. 2c), and in contrast, in the BT-20 and MCF-7 cancer cell lines, PCG demonstrated the same results at concentrations of 50 and 100 µM (Fig. 2e, g). Moreover, the IC50 values for the MDA-MB-231, MDA-MB-231, BT-20, and MCF-7 cancer cell lines were 21.42, 137.6, and 78.72 µM (Fig. 2d, f, h), respectively. These results indicate that PCG can cause a reduction in the proliferation of cancer cells.
Effect of punicalagin on the colony formation ability of breast cancer cells
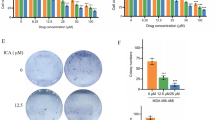
The MDA-MB-231, BT-20 (TNBC cells) and MCF-7 (ER +) breast cancer lines were challenged with different doses of PCG from 6.25 µM to 100 µM. It was noted that the colony formation ability of PCG decreased dose-dependently in the MDA-MB-231 and BT-20 cell lines (Fig. 3a, b). However, interestingly, the colony formation ability of MCF-7 increased at 6.25 and 12.5 µM concentrations of PCG, and then it started decreasing from 25 µM to 100 µM (Fig. 3c). These results are indicative of the ability of PCG to inhibit the growth of cancer cells.
Punicalagin suppressed the colony formation ability of BC cells. Comparative analysis of the colony formation ability of control with the Punicalagin in a MDA-MB-231, b BT-20, and c MCF-7 cell lines. PCG dose dependently decrease the colonies of the MDA-MB-231 and BT-20, while a dose of 25–100 µM caused a decline in the number of colonies of MCF-7 cells
Effect of punicalagin on the mitochondrial membrane potential in TNBC cells
ROS plays an essential role in the anti-cancer activity of several drugs by initiating many apoptotic pathways. Therefore, we investigated whether PCG increases the generation of mitochondrial ROS in breast cancer cells. At 50 and 100 µM, PCG induced an increase in the ROS levels in MDA-MB-231 (Fig. 4a, b) and BT-20 (Fig. 4c, d) cells and disrupted the mitochondrial membrane potential. However, only a higher dose of 100 µM induced an increase in ROS production in MCF-7 cells (Fig. 4e, f). These results demonstrate that PCG can increase the production of ROS in the TNBC cells.
Punicalagin disrupted the mitochondrial membrane potential in TNBC cells. Representative images for the analysis of mitochondrial ROS through MitoSOX assay in a, b MDA-MB-231, c, d BT-20, and e, f MCF-7 cell lines. All cells were seeded at a density of 4 × 103 per well in cell culture dishes and treated with Punicalagin (6.25 µM, 12.5 µM, 25 µM, 50 µM, and 100 µM). Following the statistical analysis, the data in the graphs have been derived from at least three repeated experiments and shown as the mean ± SD. *p ≤ 0.05 compared to control
Effect of punicalagin on the necrosis in MDA-MB-231 cells
Apart from the ROS-induced disturbance of the mitochondrial membrane potential of breast cancer cell lines, we explored the other mechanisms underlying the preferential death of MDA-MB-231, BT-20, and MCF-7 cancer cell lines due to PCG using flow cytometry. It was found that PCG is capable of selectively inducing necrosis in MDA-MB-231 cells (Fig. 5a, b). However, no significant apoptotic cells were noted in the flow cytometric analysis of BT-20 (Fig. 5c, d), and MCF-7 (Fig. 5e, f) cancer cell lines. It can be concluded that PCG can cause toxicity in cancer cells. However, this does not occur through apoptosis.
Punicalagin caused necrosis in MDA-MB-231 cells. Representative images for the analysis of Annexin V assay in a, b MDA-MB-231, c, d BT-20, and e, f MCF-7 cell lines. All cells were seeded at a density of 8 × 104 per well in cell culture dishes and treated with Punicalagin (6.25 µM, 12.5 µM, 25 µM, 50 µM, and 100 µM). Punicalagin meagerly caused necrosis in the MDA-MB-231 cell line at 100 µM concentration. Following the statistical analysis, the data in the graphs have been derived from at least three repeated experiments and shown as the mean ± SD. *p ≤ 0.05 compared to control
Effect of punicalagin on the autophagy in TNBC cells
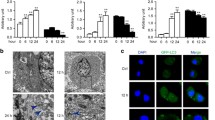
Acridine orange (AO) staining was used to further assess the method by which PCG causes cell death in breast cancer cells. Fluorescence microscopy was used to verify the formation of red acidic vesicles after staining with AO (Fig. 6). In conjunction with the results of the MitoSOX™ assay, it was observed that PCG induced autophagy in MDA-MB-231 and BT-20 cell lines at 50 and 100 µM (Fig. 6a–d). However, only the higher dose of 100 µM was able to induce autophagy in the MCF-7 cells (Fig. 6e, f). These results indicate that the death of cancer cells appeared to be due to the activation of autophagy.
Punicalagin causes autophagy in TNBC cells. Representative images for the analysis of autophagy through Acridine Orange staining in a MDA-MB-231, b BT-20, and c MCF-7 cell lines. All cells were seeded at a density of 4 × 103 per well in cell culture dishes and treated with Punicalagin (6.25 µM, 12.5 µM, 25 µM, 50 µM, and 100 µM). Punicalagin caused autophagy in the d MDA-MB-231 and e BT-20 cell lines at 50 µM and 100 µM concentrations. However, Punicalagin could only induce autophagy at 100 µM in the f MCF-7 cell line. Following the statistical analysis, the data in the graphs have been derived from at least three repeated experiments and shown as the mean ± SD. p ≤ 0.05 was considered statistically significant. *p ≤ 0.05 compared to control
Effect of punicalagin on the activation of JNK to initiate autophagy
The expression of autophagy-related proteins was investigated further to confirm the initiation of autophagy in breast cancer cell lines. Western blot results revealed that the expression of the LC3-II/LC3-I ratio was significantly increased in TNBC cells along with the autophagy-related 4 cysteine peptidase (Atg4) protein (Fig. 7a, b). The level of Atg4 protein decreased (Fig. 7c) with the increase in the dose of PCG. Moreover, the expression of phosphoinositide 3-kinase (PI3K) and AKT proteins decreased (Fig. 7e, f), while the expression of p-JNK increased (Fig. 7d) with an increase in the PCG dose. It can be concluded that PCG may cause the arrest in the growth of cells through the initiation of JNK proteins, which in turn activates the autophagic proteins to cause autophagy.
Punicalagin triggers JNK to cause autophagy in the triple negative breast cancer cell lines. a Representative images for the western blot analysis. Punicalagin caused an increase in b LC3-II/LC3-I rations c p-JNK, while the concentration of d Atg4 was increased initially and then decreased in TNBC cell lines. Furthermore, e PI3K and f AKT concentration decreased in TNBC cell lines. Following the statistical analysis (one-way ANOVA with Fisher LSD post-hoc test), the data in the graphs have been derived from at least three repeated experiments and shown as the mean ± SEM. *, **, ***, ****p ≤ 0.05, 0.01, 0.001 and 0.0001 respectively
Discussion
There is an urgent need for effective and safe therapeutic approaches for the treatment of TNBC to overcome the issue of treatment resistance and its consequent impact on the prognosis. Plant-derived natural compounds that protect against and slow the progression of certain malignancies in humans have received a lot of interest recently. These compounds derived from foods are chemo-preventive substances that are thought to be less toxic to normal cells and more effective against cancerous cells than conventional cancer treatments. Polyphenols are a viable therapeutic alternative in the treatment of cancers due to their selective toxicity against cancer cells and the reduced concerns regarding treatment resistance [21, 22]. PCG is a polyphenol found in pomegranate (Punica granatum), which has been shown to inhibit cell proliferation, cell cycle progression, and metastasis in human cancer cell lines such as lung, cervical, colorectal, and thyroid cancer [23]. However, the underlying molecular processes by which it inhibits tumor growth remain unknown. The objective of this study was to investigate the effectiveness of PCG and its underlying mechanism of action against TNBC. In this study, we found that PCG inhibits the growth of BC cells. PCG was found to be more effective in TNBC cell lines when compared with the ER+ MCF-7 cell line. Our results revealed that the PCG increases ROS generation and induces autophagy in MDA-MB-231 and BT-20 cells after triggering the JNK pathway (Fig. 8).
Overproduction of ROS, highly reactive molecules that are typical metabolic products of mitochondria, has a crucial role as a mediator of cell death in response to diverse stimuli [24, 25]. Previous studies have indicated that ROS has a role in oxidative DNA damage and cell cycle arrest, and may also decrease cancer cell migration [26,27,28]. The proliferation of MKN-45, a human gastric cancer cell line was considerably suppressed by trichosanthin (TCS) through the mediation of ROS generation and the Nuclear factor kappa B/tumor protein 53 (NF-κB/p53) pathway [29]. Curcumin treatment of cervical cancer cells resulted in an increase in the levels of ROS, which led to the induction of apoptosis, autophagy, and cellular senescence as well as the upregulation of p53 and p21 proteins [30]. Similarly, PCG has been shown to suppress lung cancer cells [31] and cervical cancer [32] via the generation of ROS. These prior findings corroborated our hypothesis that oxidative stress is linked to a decrease in cell proliferation. Our study has revealed an increase in the generation of ROS after PCG treatment. Increased intracellular ROS generation resulted in the suppression of the growth of the TNBC cell lines.
Necroptosis, or controlled necrotic cell death [33] has been suggested as a unique approach to treating tumors [34, 35]. Cancer treatment mostly involves apoptosis-based therapy with drugs such as cisplatin, carboplatin, or paclitaxel. However, the efficacy of this approach is limited due to drug resistance. This has led to the discovery of using necroptosis as a unique method of treating cancer that does not rely on apoptosis [35]. The current study revealed that PCG can selectively trigger necroptosis in breast cancer cells. Consequently, PCG, as a necrosis inducer, may offer a viable treatment option to overcome the problem of resistance in cancer cells commonly observed in apoptosis-based therapy. However, inducing necroptosis can also cause long-term inflammatory reactions that inhibit the immune system and encourage tumor spread. This suggests that inducing necroptosis may not be the best course of action for treating cancer. Necroptosis-based cancer treatment is still controversial, but more research into the necroptosis action of PCG may provide a better understanding of the role of necroptosis in cancer.
Autophagy is a mechanism of self-preservation whereby unnecessary or dysfunctional components of the cell are removed and cellular components are recycled. This mechanism protects cells from stress, oxidative stress, and low oxygen levels by digesting damaged organelles and proteins to recycle their constituent amino acids [20]. The control of autophagy is crucial for the use of anticancer agents that induce oxidative stress. A variety of extrinsic stimuli, including harmful substances like chemotherapeutic agents and ionizing radiation, can trigger autophagy in the cells. Depending on the type of cancer, the stimuli, their concentration, and the length of the treatment, autophagy can play either a pro-survival or a pro-death function in the cancer cells [36]. Despite the fact that autophagy is considered a protective mechanism, autophagy can trigger cell death under extreme conditions [18]. If the apoptotic signaling pathways are not present, cell death induced by autophagy has been suggested as an additional method for death of cells [37].
According to recent research, PCG may trigger autophagy as a pro-death or inhibitory signal to decrease the growth of cancer cells [12, 38]. PCG-induced autophagy of TNBC cells has not been extensively studied. Our findings indicate that PCG may facilitate the development of autophagosomes in BC cells. The development of a double membrane autophagosome [39] is a hallmark of autophagy, a process that has been preserved throughout evolution.
The protein marker, LC3, has been consistently linked to mature autophagosomes [40]. There are four mammalian homologs of the cysteine protease known as autophagy-related 4 (ATG4) (ATG4A-D). ATG4 proteins have catalytic and short-finger domains. ATG4 promotes autophagosome formation by reversibly lipidating and delipidating seven homologs of autophagy-related 8 (ATG8), such as the gamma-aminobutyric acid (GABA) type A receptor-associated protein (GABARAP) and LC3, to enable autophagy [41]. It was observed in this study that the PCG-caused autophagy increased the ratio of LC3-II/LC3-1 along with the Atg4B. Interestingly, the effect of autophagy due to PCG was more effective in the TNBC cells when compared with the MCF-7 breast cancer cell line. The varied reactions to PCG-induced autophagy may be attributable to the distinct genetics of the two breast cancer cell lines. Consistent with our study, MDA-MB-231 cells exhibited a greater susceptibility to autophagy triggered by rapamycin in comparison with MCF-7 cells [42]. Furthermore, with the increase in the dose of PCG, the level of Atg4B decreased (Fig. 7). This could be attributed to the blockage of the interaction of Atg4B with LC3 at high autophagic cargo presence in the cells, which ultimately leads to the stable binding of LC3 at the inner membrane of the autophagosome to facilitate proper autophagosome maturation [43, 44]. Moreover, it is also likely that the increased ROS generation resulted in the inactivation of Atg4 to promote the lipidation of LC3 at the site of autophagosome formation during autophagy induction [45].
Extensive research has established that mitogen-activated protein kinases (MAPKs) mediate cellular responses to external signals. Many anticancer treatments trigger cell death, and JNK has been proven to be a crucial mediator of this process [46]. Activation of JNK is maintained by its phosphorylation by ROS [47]. In the present study, PCG also upregulated the p-JNK (a MAPK signaling protein) in TNBC cells, which is indicative of the correlation of p-JNK with ROS [48,49,50]. Furthermore, the PI3K/Akt signaling pathway controls autophagy through the mammalian target of the rapamycin (mTOR) pathway. Inhibition of PI3K/Akt has been documented in our study, which is consistent with previous studies [51, 52]. Taken together, our study demonstrated that the PCG can stop the proliferation of TNBC cells through p-JNK and PI3K/Akt activation.
In conclusion, this study contributed significantly to our understanding of how PCG triggers autophagic cell death in TNBC cells. PCG promoted autophagy in TNBC cells by activating the ROS-mediated JNK pathway and promoted the conversion of LC3-I to LC3-II. As the growth inhibitory effect of PCG has been studied through various in-vitro assays, further studies to assess the anti-metastatic efficacy and in-vivo studies will pave the path for developing a future therapeutic strategy to treat TNBC.
Data availability
Data and material that support the findings of the study are available from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Maqbool M, Bekele F, Fekadu G (2023) Treatment strategies against triple-negative breast cancer: an updated review. Breast Cancer Targets Ther 14:15–24. https://doi.org/10.2147/BCTT.S348060
Chang-Qing Y, Jie L, Shi-Qi Z, Kun Z, Zi-Qian G, Ran X, Hui-Meng L, Ren-Bin Z, Gang Z, Da-Chuan Y, Chen-Yan Z (2020) Recent treatment progress of triple negative breast cancer. Progress Biophys Mol Biol 151:40–53. https://doi.org/10.1016/j.pbiomolbio.2019.11.007
Newman DJ, Cragg GM (2020) Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod 83:770–803. https://doi.org/10.1021/acs.jnatprod.9b01285
Shaygannia E, Bahmani M, Zamanzad B, Rafieian-Kopaei M (2016) A review study on Punica granatum L. J Evid Based Complement Altern Med 21:221–227. https://doi.org/10.1177/2156587215598039
Berköz M, Allahverdiyev O (2017) Punicalagin isolated from Punica granatum husk can decrease the inflammatory response in RAW 264.7 macrophages. East J Med 22:57–64. https://doi.org/10.5505/ejm.2017.08760
Zhang Y, Tan X, Cao Y, An X, Chen J, Yang L (2022) Punicalagin protects against diabetic liver injury by upregulating mitophagy and antioxidant enzyme activities. Nutrients 14:2782. https://doi.org/10.3390/nu14142782
Cao Y, Chen J, Ren G, Zhang Y, Tan X, Yang L (2019) Punicalagin prevents inflammation in LPS-induced RAW264.7 macrophages by inhibiting FoxO3a/autophagy signaling pathway. Nutrients 11:2794. https://doi.org/10.3390/nu11112794
Berköz M, Krośniak M (2020) Punicalagin induces apoptosis in A549 cell line through mitochondria-mediated pathway. Gen Physiol Biophys 39:557–567. https://doi.org/10.4149/gpb_2020024
Liu C-H, Kuo Y-T, Lin C-J, Lin L-T (2023) Involvement of cell surface glycosaminoglycans in chebulagic acid’s and punicalagin’s antiviral activities against Coxsackievirus A16 infection. Phytomedicine 120:155047. https://doi.org/10.1016/j.phymed.2023.155047
Cheng X, Gao Y, Yao X, Yu H, Bao J, Guan H, Sun Y, Zhang L (2016) Punicalagin induces apoptosis-independent autophagic cell death in human papillary thyroid carcinoma BCPAP cells. RSC Adv 6:68485–68493. https://doi.org/10.1039/C6RA13431A
Ganesan T, Sinniah A, Chik Z, Alshawsh MA (2020) Punicalagin regulates apoptosis-autophagy switch via modulation of annexin A1 in colorectal cancer. Nutrients 12:2430. https://doi.org/10.3390/nu12082430
Zhang L, Chinnathambi A, Alharbi SA, Veeraraghavan VP, Mohan SK, Zhang G (2020) Punicalagin promotes the apoptosis in human cervical cancer (ME-180) cells through mitochondrial pathway and by inhibiting the NF-kB signaling pathway. Saudi J Biol Sci 27:1100–1106. https://doi.org/10.1016/j.sjbs.2020.02.015
Sena LA, Chandel NS (2012) Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48:158–167. https://doi.org/10.1016/j.molcel.2012.09.025
Yang H, Villani RM, Wang H, Simpson MJ, Roberts MS, Tang M, Liang X (2018) The role of cellular reactive oxygen species in cancer chemotherapy. J Exp Clin Cancer Res 37:266. https://doi.org/10.1186/s13046-018-0909-x
Okon IS, Zou MH (2015) Mitochondrial ROS and cancer drug resistance: implications for therapy. Pharmacol Res 100:170–174. https://doi.org/10.1016/j.phrs.2015.06.013
Shah MA, Rogoff HA (2021) Implications of reactive oxygen species on cancer formation and its treatment. Semin Oncol 48:238–245. https://doi.org/10.1053/j.seminoncol.2021.05.002
Cao W, Li J, Yang K, Cao D (2021) An overview of autophagy: mechanism, regulation and research progress. Bull du Cancer 108:304–322. https://doi.org/10.1016/j.bulcan.2020.11.004
Mulcahy Levy JM, Thorburn A (2020) Autophagy in cancer: moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ 27:843–857. https://doi.org/10.1038/s41418-019-0474-7
Booth L, Roberts JL, Poklepovic A, Dent P (2024) Autophagy as a therapeutic mechanism to kill drug-resistant cancer cells. Anticancer Drugs 35:177–182. https://doi.org/10.1097/cad.0000000000001549
Sharma E, Attri DC, Sati P, Dhyani P, Szopa A, Sharifi-Rad J, Hano C, Calina D, Cho WC (2022) Recent updates on anticancer mechanisms of polyphenols. Front Cell Develop Biol 10:1005910. https://doi.org/10.3389/fcell.2022.1005910
Gupta N, Singh S, Chauhan D, Srivastava R, Singh VK (2023) Exploring the anticancer potentials of polyphenols: a comprehensive review of patents in the last five years. Recent Pat Anti-Cancer Drug Discov 18:3–10. https://doi.org/10.2174/1574892817666220512220036
Kilit AC, Aydemir E (2023) Anticancer effects of punicalagin. Haydarpasa Numune Med J 63:99–104. https://doi.org/10.14744/hnhj.2021.77044
Gao L, Wang Z, Lu D, Huang J, Liu J, Hong L (2019) Paeonol induces cytoprotective autophagy via blocking the Akt/mTOR pathway in ovarian cancer cells. Cell Death Dis 10:609. https://doi.org/10.1038/s41419-019-1849-x
Kumar K, Sabarwal A, Singh RP (2019) Mancozeb selectively induces mitochondrial-mediated apoptosis in human gastric carcinoma cells through ROS generation. Mitochondrion 48:1–10. https://doi.org/10.1016/j.mito.2018.06.003
Chen Y-F, Liu H, Luo X-J, Zhao Z, Zou Z-Y, Li J, Lin X-J, Liang Y (2017) The roles of reactive oxygen species (ROS) and autophagy in the survival and death of leukemia cells. Crit Rev Oncol/Hematol 112:21–30. https://doi.org/10.1016/j.critrevonc.2017.02.004
Liu X-J, Wang Y-Q, Shang S-Q, Xu S, Guo M (2022) TMT induces apoptosis and necroptosis in mouse kidneys through oxidative stress-induced activation of the NLRP3 inflammasome. Ecotoxicol Environ Saf 230:113167. https://doi.org/10.1016/j.ecoenv.2022.113167
Nakamura H, Takada K (2021) Reactive oxygen species in cancer: current findings and future directions. Cancer Sci 112:3945–3952. https://doi.org/10.1111/cas.15068
Wei B, Huang Q, Huang S, Mai W, Zhong X (2016) Trichosanthin-induced autophagy in gastric cancer cell MKN-45 is dependent on reactive oxygen species (ROS) and NF-κB/p53 pathway. J Pharmacol Sci 131:77–83. https://doi.org/10.1016/j.jphs.2016.03.001
Yi L-T, Dong S-Q, Wang S-S, Chen M, Li C-F, Geng D, Zhu J-X, Liu Q, Cheng J (2020) Curcumin attenuates cognitive impairment by enhancing autophagy in chemotherapy. Neurobiol Dis 136:104715. https://doi.org/10.1016/j.nbd.2019.104715
Fang L, Wang H, Zhang J, Fang X (2021) Punicalagin induces ROS-mediated apoptotic cell death through inhibiting STAT3 translocation in lung cancer A549 cells. J Biochem Mol Toxicol 35:1–10. https://doi.org/10.1002/jbt.22771
Xie X, Hu L, Liu L, Wang J, Liu Y, Ma L, Sun G, Li C, Aisa HA, Meng S (2022) Punicalagin promotes autophagic degradation of human papillomavirus E6 and E7 proteins in cervical cancer through the ROS-JNK-BCL2 pathway. Transl Oncol 19:101388. https://doi.org/10.1016/j.tranon.2022.101388
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1:112–119. https://doi.org/10.1038/nchembio711
Yan J, Wan P, Choksi S, Liu Z-G (2022) Necroptosis and tumor progression. Trends in Cancer 8:21–27. https://doi.org/10.1016/j.trecan.2021.09.003
Zang X, Song J, Li Y, Han Y (2022) Targeting necroptosis as an alternative strategy in tumor treatment: from drugs to nanoparticles. J Control Release 349:213–226. https://doi.org/10.1016/j.jconrel.2022.06.060
Yun CW, Jeon J, Go G, Lee JH, Lee SH (2020) The dual role of autophagy in cancer development and a therapeutic strategy for cancer by targeting autophagy. Int J Mol Sci 22:179. https://doi.org/10.3390/ijms22010179
Fitzwalter BE, Thorburn A (2015) Recent insights into cell death and autophagy. FEBS J 282:4279–4288. https://doi.org/10.1111/febs.13515
Subkorn P, Norkaew C, Deesrisak K, Tanyong D (2021) Punicalagin, a pomegranate compound, induces apoptosis and autophagy in acute leukemia. PeerJ 9:e12303. https://doi.org/10.7717/peerj.12303
Hollenstein DM, Kraft C (2020) Autophagosomes are formed at a distinct cellular structure. Curr Opin Cell Biol 65:50–57. https://doi.org/10.1016/j.ceb.2020.02.012
Mizushima N, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140:313–326. https://doi.org/10.1016/j.cell.2010.01.028
Park NY, Jo DS, Cho D-H (2022) Post-translational modifications of ATG4B in the regulation of autophagy. Cells 11:1330. https://doi.org/10.3390/cells11081330
Seront E, Boidot R, Bouzin C, Karroum O, Jordan BF, Gallez B, Machiels JP, Feron O (2013) Tumour hypoxia determines the potential of combining mTOR and autophagy inhibitors to treat mammary tumours. Br J Cancer 109:2597–2606. https://doi.org/10.1038/bjc.2013.644
Fujita N, Hayashi-Nishino M, Fukumoto H, Omori H, Yamamoto A, Noda T, Yoshimori T (2008) An Atg4B mutant hampers the lipidation of LC3 paralogues and causes defects in autophagosome closure. Mol Biol Cell 19:4651–4659. https://doi.org/10.1091/mbc.e08-03-0312
Zhou Y, Wang Z, Huang Y, Bai C, Zhang X, Fang M, Ju Z, Liu B (2021) Membrane dynamics of ATG4B and LC3 in autophagosome formation. J Mol Cell Biol 13:853–863. https://doi.org/10.1093/jmcb/mjab059
Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z (2007) Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J 26:1749–1760. https://doi.org/10.1038/sj.emboj.7601623
Bubici C, Papa S (2014) JNK signalling in cancer: in need of new, smarter therapeutic targets. Br J Pharmacol 171:24–37. https://doi.org/10.1111/bph.12432
Reczek CR, Chandel NS (2017) The two faces of reactive oxygen species in cancer. Ann Rev Cancer Biol 1:79–98. https://doi.org/10.1146/annurev-cancerbio-041916-065808
Zheng Q, Li Q, Zhao G, Zhang J, Yuan H, Gong D, Guo Y, Liu X, Li K, Lin P (2020) Alkannin induces cytotoxic autophagy and apoptosis by promoting ROS-mediated mitochondrial dysfunction and activation of JNK pathway. Biochem Pharmacol 180:114167. https://doi.org/10.1016/j.bcp.2020.114167
Liu G-y, Jiang X-x, Zhu X, He W-y, Kuang Y-l, Ren K, Lin Y, Gou X (2015) ROS activates JNK-mediated autophagy to counteract apoptosis in mouse mesenchymal stem cells in vitro. Acta Pharmacol Sin 36:1473–1479. https://doi.org/10.1038/aps.2015.101
Han S-H, Lee J-H, Woo J-S, Jung G-H, Jung S-H, Han E-J, Park Y-S, Kim B-S, Kim S-K, Park B-K, Choi C, Jung J-Y (2022) Myricetin induces apoptosis through the MAPK pathway and regulates JNK-mediated autophagy in SK-BR-3 cells. Int J Mol Med 49:54. https://doi.org/10.3892/ijmm.2022.5110
Kalai Selvi S, Vinoth A, Varadharajan T, Weng CF, Vijaya Padma V (2017) Neferine augments therapeutic efficacy of cisplatin through ROS-mediated non-canonical autophagy in human lung adenocarcinoma (A549 cells). Food Chem Toxicol 103:28–40. https://doi.org/10.1016/j.fct.2017.02.020
Liu F, Gao S, Yang Y, Zhao X, Fan Y, Ma W, Yang D, Yang A, Yu Y (2018) Antitumor activity of curcumin by modulation of apoptosis and autophagy in human lung cancer A549 cells through inhibiting PI3K/Akt/mTOR pathway. Oncol Rep 39:1523–1531. https://doi.org/10.3892/or.2018.6188
Funding
This work was supported by the Basic Research Lab Program (2022R1A4A1025557) through the National Research Foundation (NRF) of Korea, funded by the Ministry of Science and ICT. In addition, this study was also supported by the “Regional Innovation Strategy (RIS)” through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE; 2021RIS-001) in 2024.
Author information
Authors and Affiliations
Contributions
Conceptualization, Z.A.B. and K.-C.C.; Investigation, Z.A.B. and R.E.G; writing—original draft preparation, Z.A.B.; writing—review and editing, K.-C.C.; funding acquisition, K.-C.C. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflicts of interest to disclose.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bhutta, Z.A., Go, RE. & Choi, KC. Effect of punicalagin on the autophagic cell death in triple-negative breast cancer cells. Toxicol Res. (2024). https://doi.org/10.1007/s43188-024-00246-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43188-024-00246-z