Abstract
Polycystic ovary syndrome (PCOS) is an endocrine-metabolic syndrome that involves hyperandrogenism, menstrual irregularities, and/or small cysts in one or both ovaries which might lead to infertility in women. The genetics of PCOS is heterogenous with the involvement of several genes reported in the hypothalamic-pituitary-gonadal axis. Follicular growth and steroidogenesis regulation are both critically dependent on follicle-stimulating hormone (FSH). The variants of FSHR cause abnormal folliculogenesis, steroidogenesis, and oocyte maturation at various stages of growth and may render women more susceptible to PCOS development. The present case-control study evaluated the association of FSHR rs6165 and rs6166 variants with PCOS. A total of 743 females were recruited. PCR-RFLP method was used for the genotypic analysis of FSHR polymorphisms. Obesity was examined according to the categorization of body mass index (BMI) and waist-hip ratio (WHR). Biochemical analysis, including a lipid profile, LH, FSH, and testosterone levels, was done in both PCOS women and controls. BMI and WHR revealed a statistically significant difference between PCOS cases and controls. Overall, levels of HDL were significantly lower, whereas cholesterol, triglycerides, LDL, and VLDL levels were higher in PCOS women (p < 0.05). The genotypic and allelic frequencies of rs6165 and rs6166 did not demonstrate significant differences when PCOS women were compared with the control group. However, clinical features of PCOS including gonadotropic hormone (FSH), hyperandrogenism, and dyslipidemia were significantly correlated with variants of FSHR. The present study concludes that rs6165 and rs6166 were significantly related to clinical features of PCOS, regardless of providing direct disease risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polycystic ovary syndrome (PCOS) is an endocrine-metabolic disorder characterized by multiple hormonal imbalances that affect women at their childbearing age [1]. It is a physiological disorder that has detrimental effects on the endocrine, metabolic, psychological, and reproductive systems [2]. Classical features of PCOS include hyperandrogenism, menstrual irregularity, infertility, and polycystic ovarian (PCO) morphology [3]. However, PCOS women also experience hirsutism, acne, alopecia, and psychological issues [4]. It is estimated that 2-26% of women at their reproductive age suffer from PCOS worldwide and in India prevalence is claimed to be 11.96% [5, 6]. The most common diagnostic criteria for PCOS are the Rotterdam criteria (2003), which states that 2 out of 3 conditions should be present: (1) hyperandrogenism, (2) oligo-ovulation or anovulation (3), 12 or more cysts in one ovary, and/or ovarian volume >10 mL [7]. It was also reported that women with PCOS have a higher risk of endometrial cancer, cardiovascular disease, dyslipidemia, and type 2 diabetes mellitus [2].
PCOS was first studied by two American gynecologists in 1935 after they observed the irregularities in women with amenorrhea, hirsutism, obesity, and histological evidence of polycystic ovaries; hence, the condition was named after them as Stein-Leventhal syndrome [8]. Family studies suggested that PCOS is inherited in a pseudo-autosomal inheritance pattern with variable penetrance [9]. Studies in identical twin sisters proposed the heritability to be over 70% in women with PCOS [10, 11]. However, PCOS is a multifaceted disorder that occurs due to the interactions among multiple proteins and genes of different pathways such as steroid hormone synthesis [12], insulin-signaling pathway [13], and gonadotrophin hormone action [14] influenced by epigenetic and environmental factors [15].
Follicle-stimulating hormone (FSH) is a pituitary glycoprotein that plays a pivotal role in follicle development and regulation of steroidogenesis and triggers the oocyte maturation by binding to its receptor, FSH receptor (FSHR). FSHR belongs to the G protein coupled receptor family and is present in the granulosa cells of ovaries [16]. The biological signals of FSH are transferred to the downstream cascade through FSHR. Due to the significant role in the signal transmission of FSH, FSHR might be a candidate gene for PCOS pathogenesis. FSHR is located on the short arm of chromosome #2 (2p21) and consists of 10 exons and 9 introns [17]. The extracellular domain of the receptor is encoded by the first nine exons, while the C-terminal end of the extracellular domain, the transmembrane domain, and the intracellular domain of the FSHR are all encoded by exon 10. The C-terminal of the intracellular domain is enriched in Ser/Thr residues which can be phosphorylated by intracellular kinases and mediate the transduction of signal originating from FSH/FSHR interaction [18, 19]. FSHR is highly polymorphic and mutations in this gene can result in aberrant folliculogenesis, steroidogenesis, and oocyte maturation at several phases of follicular growth [20]. Exon 10 of the FSHR harbors two variants (rs6165 and rs6166) that alter amino acids at locations A307T and N680S respectively. The extracellular domain of FSHR is responsible for hormone binding (FSH) and it has been noted that A307T change in this domain affects hormone trafficking and signal transduction. Within the intracellular regions of FSHR, phosphorylation of the Ser and Thr residues may have an impact on protein uncoupling from adenylyl cyclase. Therefore, amino acid changes associated with the corresponding SNPs could affect the receptor’s function, including the FSH’s efficacy [21].
Thus, the present study was conducted to investigate the genetic association between the genetic variants of FSHR rs6165 and rs6166 with clinical features of PCOS in Punjab.
Materials and Methods
Selection Criteria
In the present case-control study, out of 743 subjects, 421 women with PCOS were recruited from Beri Maternity Hospital, Amritsar, Punjab and 322 age-matched women with regular menstrual cycles and no sign of PCOS were enrolled as the control group. Sample collection was done from November, 2016 to March, 2021. A Rotterdam criterion 2003 was used to diagnose PCOS women. Participants exhibiting the signs of Cushing syndrome, congenital hyperplasia, androgen secreting tumors, and thyroid dysfunction were excluded from the study. The study satisfied the guidelines of the Ethics Review Board of Guru Nanak Dev University and each participant provided written informed consent before sample collection. All the information like reproductive background, anthropometric measurements, demographic information, family history, lifestyle habits, and pedigree were taken from each individual and recorded on the predesigned questionnaire at the time of sample collection. After taking the informed consent, 5 mL of intravenous blood was withdrawn from each case and control subject. For biochemical analysis, 2 mL of blood was transferred to the vacutainer blood collection tube containing a clot activator. The remaining 3 mL of blood was poured into 0.5 M EDTA vacutainer for molecular genetic analysis. The samples were transported to the laboratory in an ice box and were kept at −20°C until further analysis.
Biochemical Measurements
Serum was isolated from 2 mL of blood by centrifuging the vial at 2000-2500 rpm for 10 min and stored at −20°C until further analysis. The hormone levels including follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testosterone (T) were measured by Calbiotech’s ELISA kits. Serum cholesterol, high-density lipoprotein (HDL), and triglycerides were also measured using specific Erba kits on Erba Mannheim biochemical analyzer. Low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) levels were calculated using Friedwald’s formula [22].
Genotype Analysis
Genomic DNA was isolated using the standard phenol-chloroform method [23]. The polymerase chain reaction was carried out in a 15 μl reaction cocktail containing 50 ng of DNA, 0.2 mM dNTP, 10 pmol of each primer, and 0.25 U Taq DNA polymerase. The primer sequences and cyclic conditions were adopted from Kambalachenu et al. [24]. Restriction fragment length polymorphism (RFLP) analysis was performed for genotyping the genetic variants of the FSHR. For the digestion of polymorphisms, rs6165 and rs6166 restriction enzymes AhdI and BseNl were used, respectively. For rs6165, products of 403 bp and 175 bp represented homozygous wild genotype (GG), a digested product of 403 bp, 175 bp, 144 bp, and 31 bp signified heterozygous genotype (AG) and bands of 403 bp, 175 bp, and 31 bp represented homozygous mutant genotype (AA). Digestion of rs6166 variant generated a product of 384 bp and 114 bp which represented homozygous GG genotype, a digested product of 498 bp and 384 bp and 114 bp signified heterozygous genotype (AG) and a band of 498 bp represented homozygous AA genotype.
Statistics
The statistical analysis was performed using SPSS (version 21, IBM SPSS, NY, USA). Chi-square (χ2) test was carried out to compare the genotypic and allelic frequencies of the PCOS cases and healthy controls. To estimate the effects of alleles, odds ratios (ORs) were calculated at a 95% confidence interval (CI) level. The student’s t-test was used to compare the clinical features of both groups. The distribution of biochemical parameters including cholesterol, triglycerides, HDL, LDL, VLDL, FSH, LH, and total testosterone levels among rs6165 and rs6166 was evaluated by one-way analysis of variance (ANOVA) test. A p-value of <0.05 was considered statistically significant. CaTS power calculator was used to determine the power of the study and sample size, which was >90% at CIs of 95% [25]. Haplotype combinations and blocks were constructed by Haploview 4.2 [26]. Linkage disequilibrium (LD) was examined, and all the haplotypes were assessed for association with PCOS.
Results
This study included 421 PCOS cases with a mean age of 24.3 ± 4.89 and 322 healthy women with a mean age of 24.6 ± 4.88 (Table 1). Early menarche was observed in PCOS cases (12.88 ± 1.33) in comparison with control women (13.09 ± 1.34). A significant difference in BMI and WHR was observed between PCOS women and controls (p = 0.00001 and p = 0.00001 respectively). Among PCOS cases, 36.5% were obese and 63.5% were non-obese; however, in controls, 20.4% were obese and 79.6% non-obese women. In our study, 58% of cases had hirsutism and 65% of them also suffered from cystic acne. Cholesterol, triglycerides, LDL, and VLDL levels were significantly higher (p = 0.00001, p = 0.00002, p = 0.009, and p = 0.00001 respectively), whereas HDL levels were lower in cases than in controls (p = 0.0001). It was seen that a sedentary lifestyle additionally increments the risk of PCOS (p = 0.00001) (Table 1).
Association Studies
The difference in allelic and genotypic frequencies of rs6165 and rs6166 was not significant between PCOS cases and controls (shown in Table 2). Further biochemical parameters were analyzed for rs6165 and rs6166 variants (Table 3). For rs6166, PCOS cases with AA genotype had significantly higher levels of cholesterol and LDL, whereas VLDL levels were significantly high in cases with the GG genotype (Table 4). HDL levels showed a significant difference in distribution with genotypes of rs6165. FSH level distribution was significantly different for rs6165 genotypes; however, total testosterone level was significantly different between genotypes of rs6166, despite non-significant association for FHSR variants (rs6165 and rs6166) and LH levels (Table 3).
Haplotype Analysis
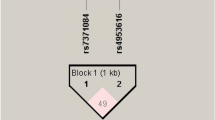
Haplotype analysis for FSHR rs6165 and rs6166 was done using the Haploview software ver 4.2. The haplotype frequency comparison is illustrated in Table 5. Two SNPs were revealed to be in linkage disequilibrium among PCOS cases and controls (D’ = 0.652, LOD = 63.25, r2 = 0.313) (Figure 1). None of the haplotypes showed a significant difference in their frequency distribution among PCOS cases and controls (Table 5).
Discussion
The present study was designed to investigate the relation of FSHR, exon 10 (rs6165 and rs6166) variants with PCOS. These two variants are crucial as protein structure analysis has shown that rs6165 affects the binding of a ligand (FSH) and rs6166 is related to the coupling of G protein for downstream signaling [20]. Besides, GWAS on Han Chinese and European populations recognized the 2p16.3 region (containing FSHR loci) to be associated with PCOS, with notable differences according to racial background [27]. In previously reported studies, these two polymorphisms have been widely studied to evaluate the potential association with PCOS and the results were inconsistent.
In the present study, age at menarche (AAM) was found to be lesser in PCOS women as compared to controls (Table 1). BMI and WHR of PCOS women were significantly higher than controls (Table 1). Levels of serum cholesterol, triglycerides, LDL-C, and VLDL-C were found to be significantly higher among PCOS women (Table 1). LH and total testosterone levels were also statistically significantly different between PCOS women and controls. According to our study, the genotypic distribution of rs6165 and rs6166 was not statistically significantly different between PCOS cases and controls (with p values 0.31 and 0.61 respectively) (Table 2). It was observed that PCOS women carrying the AA genotype of rs6166 showed elevated levels of serum cholesterol, LDL, and VLDL, and the HDL levels were also found to be significantly distributed among the rs6165 genotypes (Table 4). In the present study, LH, FSH, and total testosterone levels were also analyzed in relation to genotypes of FSHR polymorphisms and a significant increase in FSH levels in relation to the mutant genotype (AA) of rs6165 was found. In addition, it was also noted that distributions of total testosterone levels were significantly correlated with the rs6166 variant (Table 3).
Obesity leads to a hyperandrogenic state by increasing insulin resistance in the liver and boosting androgen synthesis in the ovaries [28]. To ascertain obesity, BMI is generally used as a measuring factor that helps to anticipate the risk for metabolic disorders [29]. In the current study, the BMI of PCOS women was significantly higher than controls which is known to increase the risk of diabetes mellitus, cardiovascular morbidities, and other metabolic syndromes in PCOS females (Table 1). Our results are in agreement with studies on North Indian [30], South Indian [31, 32], Chinese women [33, 34], and Turkish [21]. A study conducted on the Sardinian population disagrees with our findings [35].
The waist-to-hip ratio (WHR) is a tool that aids in assessing the probable health risks linked to obesity and being overweight. The likelihood of developing metabolic diseases like type 2 diabetes and heart disease is increased when the majority of fat accumulation occurs around the waist rather than the hips. In our study, WHR was revealed to be significantly different between both groups (Table 1). This finding is in disagreement with a study done on Iranian [27] and Polish women [36].
For all women with PCOS, lifestyle management is the first line of treatment. Physical activity is an important lifestyle management strategy that promotes better reproductive, metabolic, and mental health. The prevalence of metabolic diseases, anxiety, and mortality are greatly influenced by a sedentary lifestyle [37]. In our study, a significant correlation between a sedentary lifestyle and the development of PCOS was observed (Table 1). A study on Greek women that found that PCOS girls were physically inactive than controls confirmed our findings [38]. Lin et al. [39], in contrast to the present study, showed no statistically significant correlation between PCOS cases and physical activity in the USA.
Dyslipidemia is common in PCOS which leads to metabolic disturbances. It was reported PCOS women with higher cholesterol, triglycerides, and LDL-C levels undergoing unstimulated natural cycles were correlated with the increased number of oocytes retrieved that are of poor quality, hence resulting in less live birth outcomes [40]. Likewise, elevated cholesterol was related to the conceptive failure of PCOS women that were undergoing assisted reproduction [41]. Therefore, evaluation for dyslipidemia may not only assist with assessing cardiometabolic health but also be useful to predict reproductive outcomes after fertility treatment for women with PCOS [42]. In the present study, overall cholesterol levels, triglycerides, LDL-C, and VLDL-C levels were higher among PCOS women (Table 1). Subsequent studies in distinctive regions have stated comparable results to the current finding [43, 44]. Wild et al. did a meta-analysis and concluded that the levels of triglycerides and LDL-C were higher and HDL-C concentrations were lower in women with PCOS than in controls [45].
Exon 10 of FSHR contains rs6165 and rs6166 SNPs that lead to changes in amino acids at positions 307 and 680, respectively. These SNPs may have subtle effects on the FSHR’s sensitivity to FSH. In the present study, allelic and genotypic frequencies of rs6165 and rs6166 were compared between both groups. The genotypes and allele distribution of rs6165 were not statistically significantly different between PCOS cases and controls in our study (Table 2). This study is in line with studies on South Indian [24], Sri Lankan [46], Chinese [47], Turkish [21], Polish [48], and Korean women [16]. However, studies on Japanese [20], Italian [49], Singapore [50], and Iranian women [51] showed that rs6165 was significantly associated with PCOS development. In South Korean women, the results were contradictory; a study by Gu et al. [16] postulated that the distribution of rs6165 genotypes did not differ significantly while another study by Kim et al. [52] showed a significant difference in genotypic frequency between PCOS cases and controls.
In the current study, a non-significant association was observed for the genotypic and allelic frequency of rs6166 (Table 2). Our results were supported by studies conducted on Sri Lankan [46], Iranian [51], Chinese [47], Turkish [21], Polish [48], Dutch [53], and UK [54]. Contrary to our results, studies on South Indian [18] and Korean women [16, 52]. Kambalachenu et al. [24] conducted a case-control study and demonstrated the non-significant difference in the distribution of allele or genotypes between PCOS and controls, and found a significant association of rs6166 (Ser680Ser) genotype with PCOS in the recessive model in South Indian women. A pilot study was performed in North India, on 30 PCOS cases and 30 controls, which did not show any association between rs6166 and PCOS [55]. The conflicting results for rs6165 and rs6166 are likely due to ethnic/racial variations and the small sample sizes in some studies.
FSHR polymorphisms (rs6165 and rs6165) were revealed to be in moderate linkage disequilibrium among PCOS cases and controls (D’ = 0.652 and r2 = 0.31) as their frequency of coinherited together was 65.2% (Table 5). Also, none of the haplotypes was provided risk towards to development of PCOS in our group. Gu et al. [16] and Kim et al. [52] also revealed that both polymorphisms were in linkage disequilibrium. They showed that the haplotype having a homozygous variant combination was significantly associated with PCOS. In South India, these two polymorphisms did not show to be linked together (D’: 0.09 and r2: 0.008) which is in disagreement with our study [24].
We also assessed the possibility that FSHR polymorphisms affect clinical characteristics including lipid and hormonal profile (LH, FSH, and total testosterone). Our study showed higher levels of cholesterol, LDL, VLDL, and testosterone in association with the mutant allele of rs6166 (Table 4). A study carried out on South Indian women revealed that Ser680 was associated with high levels of FSH in PCOS [18]. Studies on the Dutch population illustrated that levels of FSH and LH were high with Ser680 polymorphism and they also concluded the mutant genotype of rs6166 was also associated with testosterone levels [53, 56]. The present study also demonstrated that higher FSH and HDL levels were also significantly associated with the mutant genotype of rs6165 (Table 3). It was observed that the level of FSH was approximately 2 times higher in the PCOS women with mutant genotypes than with wild type or heterozygote genotypes. This was in agreement with the study performed by Dolfin et al. [49] which showed that Ala to Thr polymorphism was associated with higher responsiveness to exogenous FSH in Italian women. Women with PCOS have FSH levels that are within normal ranges [53]. So, it does not appear likely that altered FSH sensitivity is a key in the ovulatory dysfunction that is typically seen in PCOS. It might be because carriers of the rs6165 mutant genotype, which had a higher FSH threshold, can increase FSH levels in a compensatory manner. Increased FSHR resistance results in lower production of E2 and inhibin B that exert an inhibitory feedback action at the level of the pituitary gland and further increases ovarian androgens and may hinder folliculogenesis [53]. We have observed a significant relationship between FSHR variants and higher levels of FSH and testosterone (Table 3).
Exon 10 polymorphisms of FSHR are known to cause FSH resistance to FSHR and that could result in hyperandrogenemia and hyperinsulinemia. In the current study, we also demonstrated that there is an interrelation between higher FSH levels and HDL levels with the AA genotype of rs6165 polymorphism of FSHR, which is unique in our study. However, a study conducted on Iraqi women demonstrated higher FSH levels with GG genotype and lower HDL levels with AG genotype of rs6165 [57]. HDL level was less strongly associated with FSH in post-menopausal women by Serviente et al. [58]. Also in our study, it is evident that women having the mutant genotypes of both polymorphisms (rs6165 and rs6166) will have higher levels of FSH, total testosterone, and dyslipidemia. Higher FSH levels in the blood may bind to the FSHR in the liver in PCOS women with the mutant genotype rs6165, which reduces LDLR expression. Low levels of LDLR have decreased LDL metabolism which may result in higher levels of LDL in circulation.
Our study has several strengths, such as the power of the study is >90%, and ethnicity of cases and controls were matched (only from the Punjab region), which reduces type I errors, common to genetic association studies and hence supports the findings. Additionally, the analysis of genetic association was done at the allele, genotype, genetic models, and haplotype levels and genotypes were also correlated with clinical features of PCOS. There are some limitations of our study. Only two polymorphisms of FSHR were evaluated and hospital-based sampling prevented the results from generalizing the entire community.
This is the first study from Punjab carried out to investigate the possible association of exon 10 variants of the FSHR gene for the development of polycystic ovary syndrome. Though many studies have been carried out on these variants, their association with the North Indian population and especially the Punjabi population was not conducted. The present study postulated that FSHR variants did not provide a significant association with PCOS, but significantly impact the clinical features of PCOS such as dyslipidemia and endocrine hormones. Thus, these polymorphisms did not directly contribute to disease progression but were linked to PCOS severity. The current study also sheds light on other PCOS risk factors (lifestyle pattern, BMI, and WHR), and identifying these factors can help with early diagnosis. A cohort study with a greater number of participants is necessary to further understand the relationship between FSHR polymorphisms and PCOS, and to produce more promising outcomes for women experiencing fertility issues.
Data Availability
The data will be available from the corresponding author on request.
References
Vishnubotla DS, Shek AP, Madireddi S. Pooled genetic analysis identifies variants that confer enhanced susceptibility to PCOS in Indian ethnicity. Gene. 2020;752:144760. https://doi.org/10.1016/j.gene.2020.144760.
Ndefo UA, Eaton A, Green MR. Polycystic ovary syndrome: a review of treatment options with a focus on pharmacological approaches. Pharmacy and therapeutics. 2013;38:336–8.
Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–31. https://doi.org/10.1038/nrendo.2010.217.
Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36:487–525. https://doi.org/10.1210/er.2015-1018.
Sarahian N, Sarvazad H, Sajadi E, Rahnejat N, Eskandari RN. Investigation of common risk factors between polycystic ovary syndrome and Alzheimer’s disease: a narrative review. Reprod Health. 2021;18:1–1. https://doi.org/10.1186/s12978-021-01203-x.
Singh A, Bora P, Krishna A. Systemic adiponectin treatment reverses polycystic ovary syndrome-like features in an animal model. Reprod Fertil Dev. 2018;30:571–84. https://doi.org/10.1071/RD17255.
Bednarska S, Siejka A. The pathogenesis and treatment of polycystic ovary syndrome: what’s new? Adv Clin Exp Med. 2017;26(2). https://doi.org/10.17219/acem/59380.
Mohamed-Hussein ZA, Harun S. Construction of a polycystic ovarian syndrome (PCOS) pathway based on the interactions of PCOS-related proteins retrieved from bibliomic data. Theor Biol Med Model. 2009;6:1–7. https://doi.org/10.1186/1742-4682-6-18.
Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37:467–520. https://doi.org/10.1210/er.2015-1104.
Leibel NI, Baumann EE, Kocherginsky M, Rosenfield RL. Relationship of adolescent polycystic ovary syndrome to parental metabolic syndrome. J Clin Endocrinol Metab. 2006;91:1275–83. https://doi.org/10.1210/jc.2005-1707.
Yilmaz B, Vellanki P, Ata B, Yildiz BO. Metabolic syndrome, hypertension, and hyperlipidemia in mothers, fathers, sisters, and brothers of women with polycystic ovary syndrome: a systematic review and meta-analysis. Fertil Steril. 2018;109:356–64. https://doi.org/10.1016/j.fertnstert.2017.10.018.
Franks S, Gharani N, Waterworth D, Batty S, White D, Williamson R, McCarthy M. The genetic basis of polycystic ovary syndrome. Hum Reprod. 1997;12:2641–8. https://doi.org/10.1093/humrep/12.12.2641.
Dunaif A, Segal KR, Shelley DR, Green G, Dobrjansky A, Licholai T. Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes. 1992;41:1257–66. https://doi.org/10.2337/diab.41.10.1257.
Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–61 https://www.nejm.org/doi/full/10.1056/NEJM199509283331307.
Rosenfield RL. Current concepts of polycystic ovary syndrome pathogenesis. Curr Opin Pediatr. 2020;32:698 https://doi.org/10.1097%2FMOP.0000000000000945.
Gu BH, Park JM, Baek KH. Genetic variations of follicle stimulating hormone receptor are associated with polycystic ovary syndrome. Int J Mol Med. 2010;26:107–12. https://doi.org/10.3892/ijmm_00000441.
Wu XQ, Xu SM, Liu JF, Bi XY, Wu YX, Liu J. Association between FSHR polymorphisms and polycystic ovary syndrome among Chinese women in north China. J Assist Reprod Genet. 2014;31:371–7. https://doi.org/10.1007/s10815-013-0166-z.
Sujatha T, Jayashankar E, Addepally U, Vijayalakshmi K, Hasan QA. Association of follicle-stimulating hormone receptor gene ser680 asn (rs6166) polymorphism with polycystic ovarian syndrome. Int J Reprod Contracept Obstet Gynecol. 2016;5:3127. https://doi.org/10.18203/2320-1770.ijrcog20162999.
Gromoll J, Simoni M. Genetic complexity of FSH receptor function. Trends Endocrinol. & Metab. 2005;16(8):368–73. https://doi.org/10.1016/j.tem.2005.05.011.
Sudo S, Kudo M, Wada SI, Sato O, Hsueh AJ, Fujimoto S. Genetic and functional analyses of polymorphisms in the human FSH receptor gene. Mol Hum Reprod. 2002;8:893–9. https://doi.org/10.1093/molehr/8.10.893.
Unsal T, Konac E, Yesilkaya E, Yilmaz A, Bideci A, Ilke Onen H, Cinaz P, Menevse A. Genetic polymorphisms of FSHR, CYP17, CYP1A1, CAPN10, INSR, SERPINE1 genes in adolescent girls with polycystic ovary syndrome. J Assist Reprod Genet. 2009;26(4):205–16. https://doi.org/10.1007/s10815-009-9308-8.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. https://doi.org/10.1093/clinchem/18.6.499.
Adeli K, Ogbonna G. Rapid purification of human DNA from whole blood for potential application in clinical chemistry laboratories. Clin Chem. 1990;36:261–4. https://doi.org/10.1093/clinchem/36.2.261.
Kambalachenu H, Durairaj Paul S, Nellepalli S, Venkatachalam P. Study on follicle stimulating hormone receptor gene polymorphism in South Indian women with polycystic ovarian syndrome. Am Med J. 2013;4:160–7. https://doi.org/10.3844/amjsp.2013.160.167.
Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet. 2006;38:209–13. https://doi.org/10.1038/ng1706.
Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype 24 maps. Bioinformatics. 2005;21:263–5. https://doi.org/10.1093/bioinformatics/bth457.
Almawi WY, Hubail B, Arekat DZ, Al-Farsi SM, Al-Kindi SK, Arekat MR, Mahmood N, Madan S. Leutinizing hormone/choriogonadotropin receptor and follicle stimulating hormone receptor gene variants in polycystic ovary syndrome. J Assit Reprod Gen. 2015;32(4):607–14. https://doi.org/10.1007/s10815-015-0427-0.
Gambineri A, Pelusi C, Vicennati V, Pagotto U, Pasquali R. Obesity and the polycystic ovary syndrome. Int J Obes. 2002;26:883–96. https://doi.org/10.1038/sj.ijo.0801994.
Kobo O, Leiba R, Avizohar O, Karban A. Normal body mass index (BMI) can rule out metabolic syndrome: an Israeli cohort study. Medicine. 2019;98(9):e14712. https://doi.org/10.1097/md.0000000000014712.
Kaur R, Kaur M, Suri V. Phenotypic presentation of PCOS with respect to BMI in a north Indian population. Clin Ter. 2021;172(5):435–7. https://doi.org/10.7417/ct.2021.2354.
Thathapudi S, Kodati V, Erukkambattu J, Katragadda A, Addepally U, Hasan Q. Anthropometric and biochemical characteristics of polycystic ovarian syndrome in South Indian women using AES-2006 criteria. Int J Endocrinol Metab. 2014;12(1). https://doi.org/10.5812/ijem.12470.
Yadav S, Tarware R. Waist hip ratio: an anatomical predictive marker of risk of PCOS. Int J Reprod Contracept Obstet Gynecol. 2019;8:1630–3 http://www.ijrcog.org/index.php.
Zhang J, Liu Y, Liu X, Xu L, Zhou L, Tang L, Zhuang J, Guo W, Hu R. High intake of energy and fat in Southwest Chinese women with PCOS: a population-based case-control study. PLoS One. 2015;10:e0127094. https://doi.org/10.1371/journal.pone.0127094.
Yuan X, Hu T, Zhao H, Huang Y, Ye R, Lin J, Zhang C, Zhang H, Wei G, Zhou H, Dong M. Brown adipose tissue transplantation ameliorates polycystic ovary syndrome. Proc Natl Acad Sci. 2016;113(10):2708–13. https://doi.org/10.1073/pnas.1523236113.
Capalbo A, Sagnella F, Apa R, Fulghesu AM, Lanzone A, Morciano A, Farcomeni A, Gangale MF, Moro F, Martinez D, Ciardulli A. The 312 N variant of the luteinizing hormone/choriogonadotropin receptor gene (LHCGR) confers up to 2· 7-fold increased risk of polycystic ovary syndrome in a Sardinian population. Clin Endocrinol. 2012;77(1):113–9. https://doi.org/10.1111/j.1365-2265.2012.04372.x.
Kałużna M, Człapka-Matyasik M, Wachowiak-Ochmańska K, Moczko J, Kaczmarek J, Janicki A, Piątek K, Ruchała M, Ziemnicka K. Effect of central obesity and hyperandrogenism on selected inflammatory markers in patients with PCOS: a WHtR-matched case-control study. J Clin Med. 2020;9(9):3024. https://doi.org/10.3390/jcm9093024.
Tay CT, Moran LJ, Harrison CL, Brown WJ, Joham AE. Physical activity and sedentary behaviour in women with and without polycystic ovary syndrome: an Australian population-based cross-sectional study. Clin Endocrinol. 2020;93(2):154-62.
Eleftheriadou M, Michala L, Stefanidis K, Iliadis I, Lykeridou A, Antsaklis A. Exercise and sedentary habits among adolescents with PCOS. J Pediatr Adolesc Gynecol. 2012;25(3):172–4. https://doi.org/10.1016/j.jpag.2011.11.009.
Lin K, Sun X, Wang X, Wang H, Chen X. Circulating adipokine levels in nonobese women with polycystic ovary syndrome and in nonobese control women: a systematic review and meta-analysis. Front Endocrinol. 2021;11:537809. https://doi.org/10.3389/fendo.2020.537809.
Liu T, Liu D, Song X, Qu J, Zheng X, Li J, Yang R, Yang S, Zhang X, Wang H, Yan L et al. Lipid metabolism was associated with oocyte in vitro maturation in women with polycystic ovarian syndrome undergoing unstimulated natural cycle. Front Cell Dev Bio. 2021;9. https://doi.org/10.3389/fcell.2021.719173.
Gao L, Li M, Wang Y, Zeng Z, Xie Y, Liu G, Li J, Zhang B, Liang X, Wei L, Yang X. Overweight and high serum total cholesterol were risk factors for the outcome of IVF/ICSI cycles in PCOS patients and a PCOS-specific predictive model of live birth rate was established. J Endocrinol Investig. 2020;43:1221–8. https://doi.org/10.1007/s40618-020-01209-5.
Luo X, Cai WY, Wu XK. Prevalence, pattern and predictors for dyslipidemia of Chinese women with polycystic ovary syndrome. Front Cardiovasc Med. 2021;8:790454. https://doi.org/10.3389/fcvm.2021.790454.
Kiranmayee D, Kavya K, Himabindu Y, Sriharibabu M, Madhuri GL, Venu S. Correlations between anthropometry and lipid profile in women with PCOS. J Hum Reprod Sci. 2017;10:167. https://doi.org/10.4103/jhrs.JHRS_108_16.
Ollila MM, Piltonen T, Puukka K, Ruokonen A, Järvelin MR, Tapanainen JS, Franks S, Morin-Papunen L. Weight gain and dyslipidemia in early adulthood associate with polycystic ovary syndrome: prospective cohort study. J Clin Endocrinol Metab. 2016;101:739–47. https://doi.org/10.1210/jc.2015-3543.
Wild RA, Rizzo M, Clifton S, Carmina E. Lipid levels in polycystic ovary syndrome: systematic review and meta-analysis. Fertil Steril. 2011;95(3):1073–9. https://doi.org/10.1016/j.fertnstert.2010.12.027.
Branavan U, Wijesundera S, Chandrasekharan V, Wijeyaratne C. Potential genetic polymorphisms predicting polycystic ovary syndrome (PCOS) in Sri Lankan women: comparison with different ethnicity. Advances in Technology. 2021;1(1):65–88. https://doi.org/10.31357/ait.v1i1.4889.
Fu L, Zhang Z, Zhang A, Xu J, Huang X, Zheng Q, Cao Y, Wang L, Du J. Association study between FSHR Ala307Thr and Ser680Asn variants and polycystic ovary syndrome (PCOS) in Northern Chinese Han women. J Assit. Reprod. Genet. 2013;30(5):717–21. https://doi.org/10.1007/s10815-013-9979-z.
Czeczuga-Semeniuk E, Jarząbek K, Galar M, Kozłowski P, Sarosiek NA, Zapolska G, Wołczyński S. Assessment of FSHR, AMH, and AMHRII variants in women with polycystic ovary syndrome. Endocrine. 2015;48:1001–4. https://doi.org/10.1007/s12020-014-0345-4.
Dolfin E, Guani B, Lussiana C, Mari C, Restagno G, Revelli A. FSH-receptor Ala307Thr polymorphism is associated to polycystic ovary syndrome and to a higher responsiveness to exogenous FSH in Italian women. J Assist Reprod Genet. 2011;28(10):925–30. https://doi.org/10.1007/s10815-011-9619-4.
Tong Y, Liao WX, Roy AC, Ng SC. Absence of mutations in the coding regions of follicle-stimulating hormone receptor gene in Singapore Chinese women with premature ovarian failure and polycystic ovary syndrome. Horm Metab Res. 2001;33:221-6. https://doi.org/10.1055/s-2001-14941
Abutorabi ES, Rashidi BH, Irani S, Haghollahi F, Bagheri M. Investigation of the FSHR, CYP11, and INSR mutations and polymorphisms in Iranian infertile women with polycystic ovary syndrome (PCOS). Rep Biochem Mol Biol. 2021;9(4):470. https://doi.org/10.52547/rbmb.9.4.470.
Kim JJ, Choi YM, Hong MA, Chae SJ, Hwang K, Yoon SH, Ku SY, Suh CS, Kim SH. FSH receptor gene p. Thr307Ala and p. Asn680Ser polymorphisms are associated with the risk of polycystic ovary syndrome. J Assist Reprod Genet. 2017;34:1087–93. https://doi.org/10.1007/s10815-017-0953-z.
Valkenburg O, Uitterlinden AG, Piersma D, Hofman A, Themmen AP, De Jong FH, Fauser BC, Laven JS. Genetic polymorphisms of GnRH and gonadotrophic hormone receptors affect the phenotype of polycystic ovary syndrome. Hum. Reprod. 2009;24(8):2014–22. https://doi.org/10.1093/humrep/dep113.
Mohiyiddeen L, Newman WG, McBurney H, Mulugeta B, Roberts SA, Nardo LG. Follicle-stimulating hormone receptor gene polymorphisms are not associated with ovarian reserve markers. Fertil Steril. 2012;97(3):677–81. https://doi.org/10.1016/j.fertnstert.2011.12.040.
Kumar S, Jain A, Goswami B, Singh A. Role of follicle-stimulating hormone receptor gene (ser680asn) polymorphism in patients with polycystic ovarian syndrome. Int J Curr Res Rev. 2020;12:61. https://doi.org/10.31782/IJCRR.2020.12158.
Overbeek A, Kuijper EA, Hendriks ML, Blankenstein MA, Ketel IJ, Twisk JW, Hompes PG, Homburg R, Lambalk CB. Clomiphene citrate resistance in relation to follicle-stimulating hormone receptor Ser680Ser-polymorphism in polycystic ovary syndrome. Hum Reprod. 2009;24(8):2007–13. https://doi.org/10.1093/humrep/dep114=.
Baban AS, Korsheed SH, Al Hayawi AY. The FSHR polymorphisms association with polycystic ovary syndrome in women of Erbil, Kurdistan in North of Iraq. Ibn AL Haitham J. Pure. Appl. Sci. 2018;24:257–72.
Serviente C, Tuomainen TP, Virtanen J, Witkowski S, Niskanen L, Bertone-Johnson E. Follicle stimulating hormone is associated with lipids in postmenopausal women. Menopause. 2019;26(5):540.
Acknowledgements
We are thankful to Beri Maternity Hospital for providing samples and to all the study subjects who were part of the study.
Availability of Data and Material
The data will be available from the corresponding author on request.
Code Availability
Not applicable.
Funding
The study was supported by UGC-JRF fellowship and RUSA 2.0 Component 4 grant.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
The study was approved by the ethical committee of Guru Nanak Dev University, consistent with provisions of the Declaration of Helsinki.
Consent to Participate
The informed consent was taken from all the study participants.
Consent for Publication
All the authors have given their consent for publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, M., Singh, S., Kaur, R. et al. Analyzing the Impact of FSHR Variants on Polycystic Ovary Syndrome—a Case-Control Study in Punjab. Reprod. Sci. 30, 2563–2572 (2023). https://doi.org/10.1007/s43032-023-01194-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-023-01194-z