Abstract
Diseases, disorders, and dysfunctions of the female reproductive tract tissues can result in either infertility and/or hormonal imbalance. Current treatment options are limited and often do not result in tissue function restoration, requiring alternative therapeutic approaches. Regenerative medicine offers potential new therapies through the bioengineering of female reproductive tissues. This review focuses on some of the current technologies that could address the restoration of functional female reproductive tissues, including the use of stem cells, biomaterial scaffolds, bio-printing, and bio-fabrication of tissues or organoids. The use of these approaches could also be used to address issues in infertility. Strategies such as cell-based hormone replacement therapy could provide a more natural means of restoring normal ovarian physiology. Engineering of reproductive tissues and organs could serve as a powerful tool for correcting developmental anomalies. Organ-on-a-chip technologies could be used to perform drug screening for personalized medicine approaches and scientific investigations of the complex physiological interactions between the female reproductive tissues and other organ systems. While some of these technologies have already been developed, others have not been translated for clinical application. The continuous evolution of biomaterials and techniques, advances in bioprinting, along with emerging ideas for new approaches, shows a promising future for treating female reproductive tract-related disorders and dysfunctions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Amidst the advancements being made in medicine, there is an increasing percentage of patients who live with chronic disorders and dysfunction of various organs and tissues. To date, most research has concentrated on disorders and dysfunctions of vital organs, while less emphasis has been placed on research into dysfunction of the organs in the reproductive system. The female reproductive system is complex, and dysfunction can occur in several organs and tissues, resulting in infertility and/or hormonal imbalance. These physiological dysfunctions impact the psychological and socio-economic status of the affected individuals. Therefore, there is a critical need for developing novel medical strategies that could restore normal physiological function and improve the quality of life of affected women.
Normal Function and Dysfunction of the Female Reproductive Tract
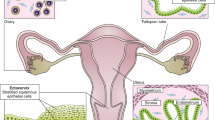
The female reproductive system is comprised of the ovaries, fallopian tubes, uterus, vagina, and external genitalia (Fig. 1), most of them are differentiated from the mesoderm-originated Müllerian duct [2]. Ovaries are known to produce (a) oocytes that fertilize during reproduction and (b) hormones that govern the various physiological processes in the body, including reproductive maturity. A long-held dogma in the ovarian biology of mammals, postulated in the early 1950s, states that in mammalian species, females are born with a defined number of eggs in their ovaries that developed during embryogenesis referred as “egg reserve” [3,4,5,6,7]. Egg reserve is the alternative name used for the primordial follicles that contains a meiotically arrested primary oocyte at the center and surrounded by one layer of cuboidal cells, which later (during the reproductive phase) differentiate into functional granulosa cells. During the reproductive phase, until the preformed egg reserves are depleted, a few of the primordial follicles from the definitive stock start to mature at the beginning of every ovarian cycle. These primordial follicles develop into ovarian follicles by differentiating the cuboidal cells into granulosa cells and recruit theca cells from the stroma of the ovary. The ovarian follicles form the functional units of the organ and produce the sex steroids estradiol (E2) and progesterone (P4) in order to prepare the uterus for implantation of a fertilized egg. The innermost layer of the uterus, the endometrium, thickens in response to E2 during the first half of the reproductive cycle prior to ovulation. Post-ovulation, the P4 produced by the corpus luteum, the transformed follicular structure after ovulation, maintains the endometrium in a secretory phase. Ovarian sex steroids E2 and P4 play a major in other physiological roles such as mammary tissue development, bone health, and sexual functions in the female [8,9,10].
The female reproductive tract includes the ovaries, fallopian tubes, uterus, and vagina. Some of the common disorders and dysfunctions of female reproductive organs are listed. Adopted from Zhao et al., 2019 [1]
Menopause refers to the progressive decline of ovarian function including the production of sex hormones, primarily E2, in women aged 45–60, resulting in cessation of menstruation [11, 12]. In addition to natural menopause due to age, younger women can also undergo induced menopause due to diseases resulting in premature ovarian insufficiency (POI) or surgically induced as a part of cancer treatments. POI is a condition that occurs with women before the age of 40, and in this condition, the ovaries do not produce normal levels of hormones (E2) or release eggs, which leads to infertility [13]. Ovarian cancer (OC) is the 5th leading cause of cancer-related death among women [14, 15]. The American College of Obstetricians and Gynecologists suggests treating the high-risk population, such as a family history of ovarian cancer, with prophylactic chemotherapy or bilateral oophorectomy. If the ovaries are retained, many of the chemotherapy treatments are gonadotoxic [16], leading to an increasing prevalence of ovarian damage, with manifestations ranging from subfertility to premature ovarian insufficiency. Women of reproductive age that have undergone oophorectomy are at high risk of developing cardiovascular disease and osteoporosis due to premature menopause [17]. In addition to cancer therapies, other causative factors for female reproductive dysfunction include ovarian insufficiency (natural or treatment-induced), polycystic ovarian syndrome (PCOS), endometriosis, fallopian tube occlusion, Asherman syndrome (AS), and other less frequent anomalies listed in Table 1 and Fig. 1 [1, 18, 19], such as the use of therapeutic alkylating agents, metabolic and autoimmune disorders, viral infections, and genetic alterations [12, 20,21,22,23,24].
Asherman’s Syndrome (AS), caused by endometriosis or repeated and aggressive curettages to remove uterine tissue, often leads to a blocked uterine cavity and/or destruction of the endometrial lining. In AS, intrauterine adhesions obliterate the uterine cavity and result in the absence of functional endometrial lining of the uterus. Infertility, resulting from dysfunctional uterine tissue, presents clinically as a recurring loss of pregnancies or hypo-menorrhea/amenorrhea [25]. Fallopian tubes that connect the ovary to the uterine cavity play a pivotal role in fertilization and transfer of the embryo to the implantation site in the uterus [26]. Fallopian tube occlusion due to neoplasms, inflammations, and tubo-ovarian abscess is the common defect that has been diagnosed in the clinics. The vagina or vaginal canal inferior to the uterus, where sperm is deposited during coitus, also undergoes cyclical changes in response to ovarian physiology changes [27, 28]. The vaginal defects and disorders include infection, the Mayer Rokitansky Kuster Hauser (MRKH) Syndrome, vaginal fistula, vaginal prolapse, and various trauma/surgical scars [1, 18, 19].
Current Therapies and Limitations
The current options to treat infertility include (a) hormone-stimulated superovulation for diminishing egg reserves; (b) intra-uterine insemination (IUI) and in vitro fertilization (IVF) to improve the chance of fertilization; (c) intra-cytoplasmic sperm injection (ICSI) for defects in sperm motility; (d) donor eggs where depleted egg reserves are diagnosed; and (e) uterine surrogacy for women with uterine defects [29]. Some of the common factors contributing to the depletion of the egg reserve include genetic syndromes, iatrogenic factors such as chemo-, radiation- or immuno-suppressive therapies [30]. Hormone replacement therapy is an option for restoring E2 and/or P4 in post-menopausal women. However, long-term HRT is controversial due to potential increased heart disease risks, stroke, and cancer [31, 32]. Despite current evidence suggesting that HRT may not be as risky as previously reported [33, 34], physicians and patients are hesitant to use HRT to treat menopausal symptoms. Moreover, recent data suggest that there is only a short period for treatment in which HRT is effective [35, 36]. Additional therapies for treating disorders of the female reproductive system include surgical corrections and tissue transplantation [37, 38]. An allogeneic uterus transplantation study published by Brannstrom et al. [39] reported a successful live birth. However, these approaches are not appropriate for every patient and can lead to various health issues. For example, the transplantation of donor tissues faces graft rejection by the host and requires life-long immunosuppressive treatment. In the case of uterine defects or deformities, the use of a surrogate mother can present moral and ethical issues for the biological parents and the surrogate.
Potential of Regenerative Medicine Approaches
Regenerative medicine offers a viable alternative for treating disorders that are currently lacking effective therapies and may have severe side effects. The bioengineering of female reproductive tissues, using various regenerative medicine and tissue engineering approaches, has the potential to revolutionize the way female reproductive dysfunction is treated. These technologies could be used for (a) therapeutic purposes, to repair/regenerate/replace dysfunctional tissue, (b) diagnostic purposes, and (c) investigative purposes to understand the underlying mechanisms of female reproductive physiology. The bioengineering strategies can be classified into two broad categories: (a) The transplantation of fresh or cryopreserved organs and (b) tissue engineering approaches that utilize a combination of cells, growth factors, and biomaterials that leverage the body’s inherent ability to regenerate/repair reproductive organs. While whole organ transplantation has demonstrated some success [39], the source of the organ and the immunogenic effects of allografts remain challenging. Tissue engineering strategies through regenerative medicine largely avoid these issues.
The field of regenerative medicine encompasses various technologies, including cell therapies, tissue engineering, and cloning, as illustrated in Fig. 2. Tissue engineering utilizes several of these technologies, including cells (Table 2), biomaterials, and bioactive factors (Table 3) and bioengineering techniques (Table 4) to generate new tissue or organs. Each of these approaches could offer novel treatments for these disorders. With proper characterization and thorough evaluation, the bioengineered tissues have promising potential to improve the quality of life of patients with reproductive tract defects. This field is rapidly growing and evolving [119].
Schematic representation of the various components of regenerative medicine. Adopted from Yalcinkaya et al., 2014 [24], with permission
Bioengineered Tissues of the Female Reproductive Tract
Several approaches have been developed to bioengineer tissues of the female reproductive system. The female reproductive tract organs have different cellular, ECM and structural compositions from one another. For example, the ovary is a solid tissue, whereas other organs such as the uterus, fallopian tube, and vagina are hollow structures. Therefore, unique approaches are needed to bioengineer the different tissues of the female reproductive tract.
Ovary
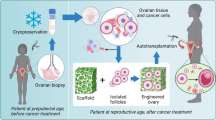
The ovary’s fundamental role is to produce oocytes and secrete hormones from ovarian follicles/corpus luteum during the ovarian cycle. Ovarian dysfunction can result in infertility, hormonal imbalance, cardiovascular disorders, neurological disorders, vasomotor symptoms, genital atrophy, and bone loss. In an attempt to restore ovarian function, various bioengineering strategies have been developed. These strategies are still at the experimental and preclinical stage and include (a) the transplantation of partial or whole ovaries that had been cryopreserved before cancer treatments as a part of oncofertility, (b) the generation of ovarian tissue from stem cells, and (c) the use of tissue engineering methods to reconstruct ovarian tissue.
Among the two major functions of the ovary, identifying novel methods for restoring egg production has a predominant need. Oogenesis, or egg production, begins during embryonic development, where cells from the yolk sac migrate to the genital ridge and contribute to the development of the ovaries [120]. This process starts as primordial germ cells (PGCs) differentiate into oogonia. Meiosis is stimulated by retinoic acid, at which point the oogonia enter meiosis and become primary oocytes, which are arrested at the prophase phase-I [121, 122]. Meiosis resumes in the follicular phase of the ovarian cycle during adult life [120]. Until recently, it had been thought that initiation of oogenesis is completed during embryological development and that all of the available primary oocytes are present at birth, leaving no oocyte stem cells in the adult ovary [61]. Therefore, research has primarily focused on developing methods to mature existing primary oocytes into fertilizable secondary oocytes utilizing in vitro techniques and/or in vivo methods. One common method used is cryopreservation of the whole ovary or cortical strips, which are later used for in vitro methods of differentiation or through in vivo transplantation of the cryopreserved ovarian tissue to resume oogenesis. Other methods include developing technologies to promote the maturation of pre-existing follicles by using 3D cell culture methods. Alternatively, recent research has suggested that oocyte stem cells exist in the adult ovary and that these cells can regenerate the oocyte pool during adult life. Therefore, a new research area has focused on developing methods to obtain stem cells from the ovary and mature them into secondary oocytes which are competent for fertilization.
Whole Ovary or Cortical Strip Cryopreservation and Transplantation
Protocols have been developed for cryopreserving ovarian tissue (whole, partial, or as cortical strips) obtained from donors as a measure to preserve fertility in individuals who are undergoing gonadotoxic treatments such as radiation or chemotherapy. This branch of research is termed “oncofertility” [123, 124]. Ovarian tissue cryopreservation (OTC) has also been adopted for patients with benign conditions such as ovarian torsion, ovarian cysts, endocrine disorders, and autoimmune diseases [38, 125]. The main objective of OTC is to maintain the structural and functional integrity of the ovary, which could be later transplanted back into the female to restore ovarian function. This is the only fertility preservation method available for prepubertal patients since there are no protocols available for ovarian stimulation and oocyte collection for young patients [125, 126]. Autologous transplantation is part of fertility preservation for those who are undergoing gonadotoxic treatments. This approach involves transplantation of cryopreserved tissue (whole or partial) back into the patient, either in an orthotopic or heterotopic fashion. Autologous transplantation of ovarian tissue following cryopreservation has a reported success rate of 63.9% in restoring ovarian activity and a natural live birth success rate of about 57.5% [118].
Cryopreservation was initially used for female reproductive tissues in the 1980s when embryos were frozen with cryoprotectant dimethyl-sulfoxide (DMSO) [127]. Later improvements in cryopreservation were made by introducing 1,2 propanediol and sucrose as cryoprotectants [128] and developing a method for gradual cooling of the tissue to –30 °C prior to long-term storage in liquid nitrogen [129]. The cryopreservation of oocytes employs various approaches to preserve the functional integrity of the eggs during long-term storage. Among these methods, slow-freezing and vitrification are the most commonly used. Slow-freezing, also known as equilibrium freezing, is carried out in a fashion that gradually replaces the fluid between the intracellular and extracellular compartment without causing any osmotic shock or deformation to cells [130]. The advantage of this technique is the use of a low concentration of cryoprotectant. However, this approach is time-consuming and requires an expensive programmable freezing machine [131,132,133,134]. Vitrification was developed by Rall and Fahy in 1985 [135] as an alternate approach. Vitrification is a non-equilibrium freezing method, where ice crystal formation is completely eliminated through the use of a high cooling rate along with higher concentrations of cryoprotectant [136]. Vitrification has been widely used to cryopreserve retrieved human oocytes [137,138,139,140,141] and in vitro matured oocytes [142, 143].
Autologous transplantation of cryopreserved whole ovary or partial ovarian tissues into rabbits [144, 145], sheep [146,147,148], and monkeys [149] were reported to show ovarian function such as follicular development, ovulation, and resumption of estrus cycle along with the restoration of hormonal balance. Kawamura et al. [150] reported a promising technique for autologous transplantation of ovary tissue that recapitulates fertility in a mouse model, where follicular growth was induced by disrupting the Hippo signaling by fragmenting ovaries followed by stimulating with Akt stimulators, which produced retrievable and fertilizable mature oocytes. This technique was termed as in vitro activation (IVA) of cryopreserved ovarian tissue. Using this technique, a 30-year-old Japanese woman was treated for infertility and successfully gave birth to a healthy baby [151]. Since, more than 130 live births have been reported worldwide using OTC techniques [152]. However, when it comes to heterologous (allogeneic and xenogeneic) transplantation, the grafted tissue requires protection from the host immune system via immune suppression.
In Vitro Maturation of Oocytes
In vitro maturation (IVM) refers to techniques developed to mature (a) pre-existing primary oocytes in the center of primordial or primary follicles or (b) neo-oogenesis, which is a technique to differentiate mature oocytes from ovarian stem/progenitor cells such as germline stem cells (GSCs) and/or oogonia stem cells (OSCs). Long-standing dogma supports the idea that women lack functional adult GSCs to replenish the pool of eggs in adult life [153, 154]. However, several groups have recently reported the presence of GSCs in adult ovaries [61, 155,156,157,158]. Oocytes derived from adult GSCs, such as OSCs or stem cells obtained through iPSC technology and somatic cell nuclear transfer (SCNT) technol ogy, may serve as an alternate for cases where there is a premature depletion of egg reserve. Despite the growing body of evidence supporting the presence of adult GSCs, the concept is still actively debated [159,160,161,162,163,164]. For adult GSCs to be universally accepted, further studies will be needed to characterize various functional aspects of oocytes derived from adult GSCs, such as oocyte commitment, meiotic progression, maturation, fertilization competency, and development competency [165].
Embryonic Stem Cells and Oogenesis
During embryonic development, the zygote develops into a blastocyst containing an inner cell mass (ICM) of pluripotent cells. At a later stage, the ICM gives rise to 2 cell types: (a) epiblasts that eventually develop into the embryo and (b) hypoblasts that develop into the yolk sac. The germline cells originate from the hypoblast-derived yolk sac and not from the epiblast-derived embryo. Since all the embroyo cells, except the germline cells, are derived from epiblasts, epiblasts are considered to be embryonic stem cells with pluripotent capability. Methods have been developed to obtain pluripotent stem cells from the mouse embryo (mouse embryonic stem cells—mESCs). Later those methods were adopted to derive human embryonic stem cells (hESCs) as well. Recently, a new type of pluripotent stem cell (PSC) has been isolated from the epiblasts of post-implanted mouse embryos (mouse epiblast-derived stem cells—mEpiSCs) [166, 167]. These cells are remarkably similar to the ESCs derived from the pre-implanted blastocyst inner cell mass (ICM) [168].
There are several striking similarities and differences among the stem cells derived from the different stage embryos. Unlike mEpiSCs, the mESCs have been shown to give rise to chimera offspring, suggesting a germline transmission potential. This feature of ICM-derived mESCs, the ability to yield germ cells, is because the mouse ICM includes cells which eventually develop into the hypoblast and serve as the precursor for germ cell lineage. Even though the mEpiSCs and mESCs exhibit similar expression patterns to some degree and share key pluripotency factors, they were reported to be derived from different embryonic stages, which are primed with other upstream signaling modules. Since hESCs share the same characteristics as mEpiSCs, which belong to a later-stage (primed and committed) embryo, these cells together are termed as “primed” PSCs, whereas the ICM-derived mESCs from the earlier stage embryo, with a potential for germline transmission, has been termed “naïve” PSCs [29]. Thus, only naïve PSCs would be appropriate for use in the tissue engineering of ovaries. Gafni et al. [169] developed culture conditions to derive naïve hESCs from blastocysts, which have germline potential. Following this path, many additional investigators have started procuring naïve hESCs [54, 170, 171] in an attempt to derive oocytes.
The development of gametes in humans can be traced to the end of the third week of gestation, where the PGCs are formed in the yolk sac wall. In response to the signals of bone morphogenetic proteins (BMPs 2, 4, and 8) from the neighboring cells, the precursors of PGC start the expression of markers such as PRDM1 (or BLMP1), PRDM14, and IFITM3, thereby acquiring the PGC phenotype. These PGCs then migrate and colonize the gonadal tissue around the fifth week of gestation. After migration, the PGCs still express some of the key markers of egg-progenitor cells such as TNAP, OCT3/4 (POU5F1), cKIT, and SSEA 1 and 3 [172,173,174,175,176]. Expression of these markers along with some additional markers such as DDX4 (VASA), NANOG, and SOX4, has been used in the field of stem cells to identify and isolate the potential precursor cells for oocytes. Around the fifth week of gestation, reactivation of the X chromosome occurs in the female germ cells [177]. The PGCs further undergo what is referred to as global epigenetic reprogramming by chromosomal remodeling, genetic imprint erasure, and extensive DNA methylation [178]. Finally, the PGCs again enter a global-remethylation phase, resulting in a partially methylated oocyte genome [179]. Understanding the molecular programming of germ cells (oocytes) has led to research to derive oocytes from various PSCs. In vitro, PGCs were derived from mESCs [42, 44, 45, 180] and iPSCs [181]. Differentiation of PSCs into oocyte-like cells were reported by several groups in monolayer culture [182, 183], in a co-culture system with fetal gonads [184], or in 3D cultures such as embryoid body culture [185] based on the expression of early germ cell markers such as VASA, DAZL, IFITM1, PRDM1A, GCNF, GDF3, and cKIT and later stage markers such as SCP3, MLH1, DMC1, GDF9, and ZP4 [186, 187]. Mouse EpiSCs were shown to lose their competency towards PGCs [188], which led to developing a novel approach to derive PGCs from EpiSCs. In this approach, the naïve ESCs obtained from the epiblasts of pre-implantation embryos were cultured in the presence of Activin A to derive intermediate epiblast-like cells. The activin A-exposed cells resembled embryonic cells that could give rise to PGCs in vivo and yielded functional oocytes [189].
Even though several groups have demonstrated that ESCs from the mouse can form follicle-like structures generating oocyte-like cells, none were able to progress forward to meiosis [42, 190]. Hayashi et al. [191] aggregated mEpiSC-derived PGCs with somatic cells from embryonic ovaries and then transplanted the cells under the ovarian bursa. This method of in vitro derivation of PGCs from ESCs, combined with in vivo oogenesis, yielded fertilizable oocytes. When the embryos from fertilized oocytes were transplanted into surrogate mothers, they developed into viable offspring, which showed that in vivo oogenesis from ESC-derived PGCs has a better outcome than the complete in vitro oogenesis process. The complete in vitro oogenesis produced fragile and abnormal-shaped oocytes, and follicles that lacked the supporting granulosa cells. In addition, the fertilization of such oocytes presented abnormal pronuclei, which could explain the observed low rate of post-implantation development. These defects were suspected to be the result of faulty epigenetic reprogramming that occurred during the in vitro oogenesis from PGCs [192]. On the other hand, oogenesis research using hESCs has shown only limited progress. The oocyte-like cells derived from hESCs using mouse fibroblast as feeder layer produced oocyte-like cells and expressed (a) markers related to meiosis such as SYCP3 and MLH1, (b) germ cell-related genes such as DDX4 (VASA), and (c) pluripotency-related genes such as POU5F1 (OCT4), IFITM3, and NANOG [193, 194]. An improved method of using ovarian fibroblasts as feeder layer for hESCs also generated large cells with oocyte markers including VASA, DAZL, GDF3, GDF9, MLH1, SCP1, and POU5F1 [195]. Other than generating oocyte-like cells, there has been no success in generating human oocytes in vitro that could be applied for any assisted reproductive technologies (ARTs) utilizing these types of stem cells. Therefore, the technology of obtaining oocytes from pluripotent stem cells needs further improvement.
Adult Stem Cells (OSC) and Oogenesis
Long-standing dogma states that all the oogonia in a developing ovary either differentiate into primary oocytes that become a part of dormant primordial follicle or undergo degeneration resulting in an adult ovary that lacks any renewable source of germ cells. However, this belief was challenged by a recent study that showed adult ovaries carry mitotically active germ cells referred to as OSCs that are capable of producing new oocytes throughout adult life [61]. White et al. [196] reported that similar to adult mice, women of reproductive age contain mitotically active germ cells such as OSCs. When these putative germ cells were aggregated with dispersed adult ovarian tissue in vitro, they formed follicle-like structures and produced oocyte-like cells. Virant-Klun et al. [197, 198] obtained the OSCs from the ovarian surface epithelium and demonstrated the production of oocyte-like cells that expressed molecular markers such as OCT4A, OCT4B, C-KIT, VASA, STELLA, and ZP2. While some researchers do not agree with the theory of the existence of OSCs in adult ovaries, others try to offer alternate views for oocyte-like cell-producing stem cells. These theories state that OSCs could be the de-differentiated cells capable of developing into germ cells under appropriate conditions [199] or that the OSCs could be small embryonic-like stem cells that reside in the ovaries [200]. Conversely, using an endogenous genetic approach, another group demonstrated that the germ cells present in adult mice were neither capable of dividing by mitosis nor could contribute to de novo oogenesis [201]. The existing controversy and disagreement of the presence of OSCs require more studies before the technology reaches clinical applications.
Addressing Hormone Imbalance
Besides oocyte production, the ovaries secrete sex steroids such as E2 and P4, which play a role in reproduction and participate in various physiological processes. Follicles are the functional unit of the ovary and are responsible for producing and releasing sex steroids. An ovarian follicle encompasses an oocyte in the center surrounded by several layers of granulosa cells (GCs), which in turn is enclosed by a layer of theca cells on the periphery of the follicle (Fig. 3a). The regulation of the endocrine function in ovarian follicles has been explained by the two-hormone two-cell theory, where LH and FSH, from the anterior pituitary gland, stimulate theca cells and granulosa cells to produce E2, respectively. LH, through the LH receptor, stimulates the expression of StAR, HSD3B1, CYP11A1, and CYP17A1 (the key steroidogenic enzymes) in theca cells (TCs), which aids in the uptake of cholesterol and converts it into pregnenolone then into progesterone, which then is converted into androgens such as androstenedione or testosterone. The androgens that are produced by TCs are then converted into estrogen by the action of aromatase (CYP19A1) that is present in the granulosa cells (GCs). CYP19A1 expression in GC is under the control of FSH; thereby, the two endocrine cells of the ovarian follicle operate in a tandem fashion [202]. The LH surge during ovulation induce the expression of StAR and 3β-HSD in granulosa and the loss of CYP17A1 (in TCs) and CYP19A1 (in GCs). Thus, the follicle losses the ability to produce androgen and estrogen after ovulation and acquire progesterone producing capacity referred as luteinization, and the whole structure of the follicle is termed corpus luteum [120]. Therefore, the steroidogenesis predominantly produces estrogen during the follicular phase of the ovarian cycle, which then switches to progesterone production during the luteal phase of the ovarian cycle as a consequence of luteinization caused by the LH surge. The switching of E2 secretion to P4 secretion is unidirectional; the cyclicity of sex steroid secretion observed in physiology is due to the emergence of the next set of follicles from the alternate ovary.
Cell-based hormone therapy to correct abnormalities caused by dysfunctional ovaries. A. The basic architecture of ovarian follicle with OC in the center surrounded by GCs and TCs; B. bio-engineering design to recapitulate the structural architecture of follicles; C. Bio-engineered endocrine tissue of ovary with GCs (pre-stained with cell-tracker green) in the middle and TCs (pre-stained with cell-tracker red) on the periphery of the constructs; Endocrine functions of these bioengineered endocrine tissue secreted E2 and P4 into the plasma of ovx rats for 90 days as evident from the sustained levels of these steroids (D and E). The μCT images of femur bone shows the correction of ovarian failure-associated trabecular bone abnormalities of with the re-established hormonal level (F-H). The safety nature of cHRT has been demonstrated by assessing the morphometry and histology of sex steroid-sensitive uterine tissue, where it showed no signs of any hypertrophy or hyperplasia of uterine tissue (I-N). GC granulosa cell, OC oocyte, Ovx ovariectomy, PLO poly-L-ornithine, TC theca cell. Adopted from Sittadjody et al., 2017 [117], with permission
The cyclicity of the ovarian cycle alternates the development of the endometrial lining and function for implantation purposes. However, the protective role of ovarian sex steroids on other organ systems is contributed by lower steroid hormone levels than what is required for reproductive function. The combination of E2 and P4 inhibits the secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus and LH from the pituitary. Similarly, inhibin, a protein hormone secreted by ovarian follicles, inhibits the secretion of the FSH-releasing pattern of GnRH through the Kissipeptin neurons and thereby FSH secretion from the pituitary. Therefore, the hypothalamus-pituitary-ovarian (HPO) axis and its negative feedback loops keep tight control on the ovarian function.
The inevitable phenomenon of ovarian failure, whether by natural processes such as age or disease or by treatment-induced dysfunction, leads to an imbalance of hormones, thereby causing various pathophysiology. The current treatment to correct the hormonal imbalance is to administer exogenous hormones, referred to as hormone replacement therapy (HRT), which has been reported to cause side effects ranging from cardiovascular issues to cancers. As an alternative, hormone delivery from ovarian tissue has been studied in an experimental setting. Transplanting the whole ovary in rodents was reported as early as the 1960s, where Krohn studied the young ovary’s ability to restore the ovarian functions in old ovariectomized mice [203]. Later, others performed similar experiments to demonstrate the potential of ovarian transplantation to restore the hormonal imbalance in aged rodents [204,205,206]. Despite the evidence supporting the correction of hormonal imbalance in rodents, the practical applicability of ovary transplantation in humans is challenging. Autologous transplantation of cryopreserved ovarian tissues in heterotrophic sites was also reported to restore the endocrine function of the ovary in humans [207, 208]. Several groups have shown the restoration of ovarian endocrine function following ovary transplantation [209,210,211,212,213,214,215]. Notably, it was reported that the endocrine function continued for several years following transplantation of ovarian tissue [216]. While autologous transplantation may not be possible in most cases where the native ovary of the affected person is dysfunctional or diseased, the option of heterologous transplantation requires life-long immunosuppressive treatment, which is known to damage ovarian function. Regenerative medicine offers an alternative solution for such conditions. The endocrine unit of the ovary is bio-engineered and immune-isolated by microencapsulation techniques or engineered from the patient’s cells, which does not require immunosuppression.
Several groups, including ours, have developed a bio-engineered ovarian tissue to provide a more natural method of hormone production in ovarian dysfunction models [115,116,117, 217]. The advantage of the cell-based HRT (cHRT) over the pharmacological HRT (pHRT) is that the bio-engineered endocrine tissue has the ability to restore the HPO axis in rat models as they were shown to reduce the ovariectomy-induced increase in circulating gonadotropins (FSH and LH) [116, 117]. In addition, the bio-engineered tissue constructs can be transplanted at different orthotopic sites, including subcutaneous tissue where there is vasculature to support the survival of the grafted constructs and allow for the circulation of secreted hormones.
Osteoporosis is known to be induced by a decline in circulating sex steroids and the elevation in circulating FSH levels caused by the disrupted negative feedback loop [218]. A cHRT approach has been developed by our group through delivering endocrine cells of ovarian follicles using alginate microcapsules into an ovariectomized rodent model. The endocrine cells of ovary, namely, GC and TC, when bio-fabricated into a structure that closely mimicked the native follicular structure, demonstrated a long-term secretion of hormones and re-established the disrupted HPO axis in the ovariectomized model. This cHRT model on one hand demonstrated the protective effect of hormones on the bone, and it also showed the safe-nature of the approach as evident from the absence of any utero-trophic effects from the transplanted bio-engineered endocrine tissue (Fig. 3)[117, 217]. Hence, the regulation of HPO axis and its feedback loop through bio-engineered endocrine tissue provide a more physiological and natural way of correcting hormonal imbalance.
The major challenge in such cHRT approach is the limited life span of transplanted endocrine cells and the lack of a source to replenish these cells once they decline in their function. Therefore, this approach requires periodical implants. In a rat model, the bio-engineered endocrine tissue was shown to secrete hormones for up to 90 days. When this is extrapolated to the human life span, it would be reasonable to have new implants of bio-engineered ovarian tissue once or twice a year to correct the hormonal imbalance. However, it might be somewhat precocious to comment on the clinical applications of cHRT as it still requires thorough investigations on various aspects of this application, including the side effects, the fate of the implanted cells, and the immune reaction before use in the clinic.
Uterus
The uterus is the largest organ in the female reproductive tract. It is composed of a three-layered wall containing the perimetrium on the periphery, the myometrium in the middle, and the endometrium towards the lumen of the uterine cavity. The endometrium, consisting of the luminal and glandular epithelium surrounded by stroma, undergoes approximately 400 cycles of regulated cell proliferation, differentiation, angiogenesis, tissue breakdown, and shedding during the menstrual cycles throughout the reproductive life of women [219]. Additionally, the uterine tissue is also known to undergo cellular differentiation, hypertrophy, and hyperplasia associated with an increase in uterine weight up to 4–5 times that of its original weight during pregnancy and undergoes remodeling once again to return to its original condition soon after parturition [220]. Based on the physiological phenomena of uterine tissue, it is evident that this organ (particularly endometrium) has a high regenerative capacity and is believed to harbor stem cells that are responsible for this extraordinary regenerative property. These actively dividing cells and the distribution of their surrounding niche are believed to be associated with endometrial diseases such as endometriosis, endometrial cancer, and inappropriate tissue remodeling resulting in a dysfunctional uterus that affects embryonic implantation and fetal development [221].
In a dysfunctional uterus, gestational surrogacy is the only available option, which comes with its downsides, such as personal, religious, ethical, and legal factors that make this decision difficult [222,223,224,225,226]. Another potential option is an allogeneic uterine transplant. Brannstrom et al. reported a live birth after the whole uterus transplantation [39]. However, the shortage of donors and/or complications associated with long-term usage of immune suppression drugs makes this approach challenging [227,228,229,230,231]. Hence, the use of bioengineered uterine tissue (whole organ or partial tissue) could be a novel option for these patients. Bioengineering uterine tissue could be achieved by (a) cell therapy, where only stem cells are used to correct the defective tissue; (b) non-cell laden scaffold, where the scaffold provides the framework for the native stem cells of the uterine tissue to populate and repair the damaged area; and (c) the use of a cell-laden scaffold to engineer a partial or whole new organ.
The innermost lining of the uterus is known for its unique regenerative capacity during the reproductive cycle. This regenerative property of the endometrium has been attributed to a small population of adult stem cells (ASCs) in the tissue [232,233,234,235]. The ASCs present in the uterine endometrium possess the properties of MSCs, and they are known for the regeneration of the tissue both in vitro and in vivo [234, 236,237,238,239,240]. It is believed that the MSCs from bone marrow and circulating blood migrate to the endometrium. In addition, there prevails a niche near the perivascular region of the endometrium that is similar to that of ESCs. Therefore, any stem cell that reaches this region or exists in that niche has the potential to give rise to multiple cell types of uterine tissue. Thus, the ESC niche at the basal layer of endometrium near the perivascular region and the migration of MSCs from bone marrow were reported to augment endometrial regeneration [241]. BMSCs have been reported as a viable source for non-hematopoietic stem cells to repair and regenerate different cellular compartments including luminal epithelium, glandular epithelium and stroma. Among the different cellular compartments, the BMSCs contribute mainly to the regeneration of the stromal compartment and, to a lesser extent, to both glandular and luminal epithelia [62,63,64,65]. Even though BMSCs have been shown to improve endometrium regeneration in mouse models [242,243,244,245], they failed to deliver positive outcomes in either a non-human primate model or in human studies [246]. Alternatively, stem cells native to the endometrial lining were reported to correct the defects in a pre-clinical model [247]. There is currently no effective stem cell therapy for endometrial pathologies; however, approaches using the stem cells native to endometrium hold great opportunity in regenerate uterine tissue.
Synthetic polymer scaffolds created by various techniques, including electrospinning or decellularized uterus, have been used to provide a 3D network for the native stem cells to populate and regenerate tissue [248, 249]. When stromal cells of endometrium origin were obtained from human samples and embedded in COL-1-enriched Matrigel followed by seeding with endometrial epithelial cells, a 3D structure was formed that resembled the endometrium of the uterus [250]. Schutte et al. [251] reported a similar 3D bioengineered cell construct. In their study, immortalized human endometrial stromal cells were embedded in COL-Matrigel and then layered with epithelial endometrium cells to study cell-cell interactions in the uterus [251, 252]. MacKintosh et al. [253] used electrospun PGA scaffolds seeded with primary bovine endometrial cells. They reported that the structural integrity of the bioengineered tissue was not ideal for in vivo use because of the choice of the material used. Park et al. [254] used primary endometrial cells in a hydrogel mixed with COL-1 to study the migration of uterine cells. A study by Wang et al. used primary human cells from the endometrium embedded in a human plasma-derived fibrin-agarose matrix to investigate the implantation of trophoblast spheroids, which demonstrated the formation of glandular structures of the uterus [255]. In addition to the endometrium of the uterus, myometrium was included in a few studies using human cells. Decellularized myometrium was used to incorporate neo-myometrium [256] or a smooth muscle layer to derive myometrium [257] to recapitulate the structural architecture of the uterus.
Sections of uterine tissue have been bioengineered for therapeutic purposes and tested in animal models. Synthetic materials such as polyetherurethane (PU/PLLA) and polytetrafluoroethylene (PTFE) were also tested to bioengineer partial uterine walls [227]. In another approach, Campbell et al. used the peritoneal cavity as a bioreactor to bioengineer hollow organs with smooth muscle-layered walls such as the uterus. They have implanted boiled blood clots in the shape of a tube and allowed myofibroblasts to infiltrate and populate the tubular structure transforming it into a functional muscular tube [258]. COL scaffolds in different forms have been used to bioengineer uterine tissue. Hu et al. showed successful restoration of uterine function in several studies [259,260,261,262]. Li et al. functionalized a collagen scaffold by incorporating bFGF to restore a functional uterus in a rat model [260].
Even though the use of natural materials such as COL has several advantages, including biocompatibility, they mostly failed to mimic the complexity of the native matrix. The use of decellularized ECM scaffolds offers the full structural complexity of the uterine tissue. It might serve as a better biomaterial framework to recapitulate uterine tissue that structurally and functionally resembles the native tissue. Santos et al. investigated three decellularization methods to bioengineer segments of rat uterine horn in vitro [263], which resulted in better structural integrity compared with studies using natural materials such as SIS as reported by Taveau et al. [264]. Full-length uterine tube reconstruction has also been attempted in a few studies using decellularized scaffolds. Miyazaki et al. [265] used decellularized uterine tissue to reconstruct the tissue by recellularization in vitro. They investigated its function in vivo by excision/replacement method, which yielded a 75% success rate for pregnancy. Similarly, Hellstrom et al. [266, 267] developed a decellularization process to obtain scaffolds that were completely devoid of MHC complexes on which GFP-incorporated BM-MSCs were injected to regenerate uterine tissue. The bioengineered uterus, when transplanted, supported full-term pregnancy in rats [266]. Previous work on regeneration of the uterus has proved successful only for small defects in rodent models, as they possess an inherent regenerative capacity. Furthermore, rodent models do not have the critical size needed for clinical translation. Attempts at regenerating bigger uterine defects in larger animal models, such as rabbits, have failed. Taveau et al. used porcine small intestine submucosa (SIS) to reconstruct the uterine wall in rabbits; however, it was noted that grafts 1 cm or longer collapsed, resulting in total architectural disruption [264].
Our group recently demonstrated that a PGA scaffold seeded with autologous cells, when transplanted into the rabbit uterus with a critical size defect in an excision/replacement method successfully regenerated, achieved normal tissue development and supported embryo implantation and fetal growth to full-term pregnancy and birth [248]. Animals implanted with the scaffold alone did not show normal uterine development. The study showed that both biodegradable scaffolds and autologous uterine cells are essential for the achievement of normal tissue. The bioengineering of functional uterine tissue for clinical applications is still in development.
Fallopian tube
The occlusion of the fallopian tube can occur due to inflammation, damage caused by disease, or trauma caused by an ectopic pregnancy. 3D models have been developed for investigating various physiological processes of the tissue. In one such study, ASCs of the fallopian tube or differentiated iPSCs were used to recreate the complex architecture of the fallopian tube in a 3D organoid model. In this model, hollow spheres of cells were bioengineered to mimic the structural architecture of the fallopian tube. The artificial model included stem cells, ciliated cells, and secretory cells of the native tissue. These artificial fallopian tube tissues have been reported to respond to hormones and nutrients [268]. These 3D fallopian tube organoids could be used for diagnostic and investigative purposes.
Cervical and vaginal tissue
In a normal pregnancy, maintenance of the anatomical architecture of the cervix is crucial during the development of the fetus and the growth of the uterus [22]. Cervical aplasia is one condition for which regenerative medicine approaches are being developed. Alborzi et al. [269] developed an approach that used a peritoneal graft to treat cervical aplasia. Initially, a plastic stent covered with a peritoneal graft was placed between the uterine cavity and upper vaginal region. Using this approach, at least four patients were successfully treated for cervical aplasia. Similarly, using an amniotic membrane and a decellularized porcine SIS have also been reported for such cervical reconstructions [270, 271]. House et al. bioengineered a cervix-like tissue construct for investigative purposes using cervical cells from two premenopausal women undergoing hysterectomy. In this bioengineered model, the cervical cells proliferated and produced ECM with a similar biochemical profile to native tissue [272]. Several other studies used bioengineered constructs in rabbit, rat, and mouse models [66,67,68, 269,270,271,272,273,274,275,276,277,278,279,280,281,282,283,284,285,286].
One in 5000 women suffers from a congenital disorder of the vagina called vaginal agenesis. The vagina does not develop properly and results in either an incomplete or partial development of the uterus [284,285,286]. The condition is also referred to as Mayer-Rokitansky-Kuster-Hauser (MRKH) syndrome. Some of the standard corrective procedures for vaginal defects include surgery and/or transplantation of synthetic or biological meshes, which eventually leads to fibrosis, poor vascularization, or graft rejection [287].
Despite the multiple uses of stem cells in other tissues of the female tract, stem cells utilized in the repair of vaginal tissue have been poorly investigated. A few studies have been reported to use stem cells in vaginal regeneration. Muscle-derived stem cells (MDSCs), when seeded on SIS scaffolds and transplanted into the vaginal stump region, have been shown to reduce fibrosis and enhance epithelial lining formation [278]. Similarly, MSCs have shown to aid in the recovery of urinary continence when administered after vaginal distension in rats [288]. They have been shown to increase the number of stem cells at the injury site [289]. However, no promising human studies have been reported to date. In one study, bone marrow-derived MSCs were first induced to express the phenotype of vaginal epithelial cells, combined with SIS, and then transplanted in place of the native vagina and demonstrated the incorporation of the neovagina [66].
Additional pre-clinical approaches have also been reported using biomaterials for vaginal reconstruction, such as dermal matrix [280,281,282,283, 285, 286], human amniotic membrane [274], and decellularized SIS [66, 270, 275,276,277,278,279]. Several cell sources have been explored in the bioengineering vaginal tissue. Biomaterials have been also used with cells for the engineering of vaginal tissue. Our groups showed that vaginal epithelial and smooth muscle cells obtained from rabbits could be expanded and seeded on PGA scaffolds. When the bioengineered scaffolds were subcutaneously implanted in nude mice, they showed assembly of multilayered tissue architecture with functional contractility in response to electrical stimulus [273]. Using a similar strategy, engineered vaginal tissue was implanted to achieve partial vaginal replacement in a rabbit model [273], and then a full replacement of the vaginal organ, also in a rabbit model [290, 291]. These studies eventually led to a human clinical experience in female patients with the MRKH Syndrome who had vaginal aplasia. A small biopsy of vaginal tissue was obtained from the rudimentary structure, the patient’s cells were isolated, expanded, and seeded onto biodegradable scaffolds, and then used to create a neovaginal organ [66]. The study was published with up to a 5-year follow-up. The engineered vaginal organs showed a tri-layered arrangement of the epithelium, matrix, and smooth muscle similar to native tissue. In addition to structural restoration, functional restoration was also confirmed in women transplanted with these bioengineered vaginal tissues [67]. This technology is currently being developed for further clinical use.
Organ-On-Chip technologies
Organ-on-chip (OOC) is a microfluidics-based technology that serves as a novel 3D cell culture device to investigate the interactions between tissues of various organ systems in an in vitro setup. This organoid-based research is currently being utilized as an investigative tool to better understand the physiological and pharmacological interactions of tissues and organs [292]. This recent-developed technology has been applied in various organ systems, including cancer models, also used in female reproductive tissue research. The OOC technology is ideal for studying tissue interactions and could serve as a great tool to design customized drug screening for a particular patient since it uses functional unit organoids of each tissue instead of the whole tissue. The OOC platform evolved along with the advancement in microfluidics. Several groups have recently reported the development of 3D micro-engineered devices to recapitulate the complexity of the female reproductive system. The studies using OOC of the female reproductive system include the modeling of the human placenta [293, 294], uterus [295], and developing a model of menstrual cycle physiology on a chip [296].
Blundell et al. [294] have developed the “placenta-on-a-chip” model where primary human placental villous endothelial cells (HPVECs) obtained from term placenta and BeWo b30 human trophoblast cell lines were used. The HPVECs were made to attach on the lower side of a porous membrane in a hanging down fashion in the bottom chamber of a two-layered microfluidic device, and in the top chamber, the BeWo b30 cells were enclosed. The two cell types in this chip device formed a placental-barrier model to study the function of the placenta and interaction of fetal-maternal cells. Other similar models were utilized to study the complex physiology of the placenta in a miniature OOC model where human trophoblasts (JEG-3) and human umbilical vein endothelial cells (HUVECs) [293]. Li et al. [295] developed the “uterus-on-a-chip” to replicate the functions of the uterus and to study the IVF-embryo transplantation (IVF-ET) process. The uterus-on-a-chip device was composed of two PDMS layers separated by a porous polycarbonate (PC) membrane to support the endometrial cell culture. The device’s top layer was designed with a zigzag-shaped channel containing a series of interlaced micro-sieves to capture the oocytes. The bottom chamber included four parallel perfusion channels resembling the microcapillary bed to support the porous membrane. The study offers a viable alternative for conventional IVF-ET methods and demonstrates the beneficial aspects of co-culturing the embryo with endometrial cells and facilitate embryonic development. Although the device was developed to study the uterine function in a mouse model, it has great potential in applying for human studies. Woodruff and colleagues developed an organ-on-a-chip system to investigate the functional complexity of the 28-day menstrual cycle of humans, which was tested with mouse cells [296]. Organoids of various female reproductive tissues were first created and interconnected in a micro-engineered device. When the interconnected organoids were exposed to hormones, mimicking the menstrual cycle, the organoids functioned similarly to in vivo. Hence this organ-on-a-chip that recapitulates the interactive functions of different tissues of the female reproductive system provides another avenue to investigating diseases of female reproductive tract such as cervical cancer and infertility.
Conclusion and Future Prospective
Infertility and hormonal deprivation are the two significant outcomes of the female reproductive system’s dysfunctional organs and tissues. While infertility affects a notable percentage of women in their reproductive age, hormone deprivation is inevitable to all women as they lose their ovarian functions in one way or other due to natural and unnatural causes. Regenerative medicine offers a viable alternative with biological and physiological outcomes compared to the current treatment options. Many regenerative technologies have been developed to bioengineer female reproductive tissues by taking the complexity of the organs and tissues into careful consideration. Bioengineering reproductive tissues provide therapeutic solutions to treat the various dysfunctions and add diagnostic modalities, including drug screening and investigative values, such as research in understanding the pathophysiology of the organ systems.
Simple approaches such as stem cell-based technologies have been shown to rectify a number of dysfunctions, repair the deformities, or regenerate and restore the tissue function. Researchers have developed regenerative medicine technologies to offer solutions for the two critical dysfunctions of ovaries, non-oogenetic infertility, and hormonal imbalance. Even though certain stem cells, including the postnatal oogonia stem cells, have been debatable, the concept of using adult OSCs opens new avenues in treating infertility for women with premature ovarian failure in their reproductive age. In vitro oogenesis from stem cells or from pre-existing immature oocytes has been improved and is believed to be ready for clinical application in the near future. Similarly, to correct hormonal imbalances due to ovarian failure, approaches such cell-based hormone replacement therapy would offer a natural remedy. This emerging technology with a thorough investigation would benefit women in the near future.
Although various bioengineering technologies have been developed to address clinical problems, the practical applicability in the clinic is of prime importance. In that aspect, among the strategies to address the disorders of female reproductive tissues including infertility, the use of autologous adult stem cells that are native to the tissue such as ovarian stem cells holds several merits over others. For example, in vitro oogenesis from adult ovarian stem cells seems to be a better and safer options compared to others. Existence of PGCs or OSCs in adult ovary that are gradually accepted with some skepticisms has provided a potential and viable option to treat infertility caused by ovarian dysfunction. Cells native to the tissue are more physiologically compatible and less deviated from the original cells. Since the in vitro oogenesis is completely an ex vivo process, it provides more control in monitoring and regulating the maturation process. The oocytes thus generated will be utilized for immediate or future use in ART such as IVF. Unlike in transplanting cryopreserved- or bioengineered-ovarian tissue into the recipient to resume reproductive function, the threat of reintroducing any potential carcinogenic cell is minimized in this approach. In addition, several clinical steps that are routinely employed in ART including ovarian stimulation, egg retrieval and preparation of eggs for IVF could be bypassed in this approach as the end product itself is a fertilizable egg. Apart from deriving oocytes from ovarian stem cells, these adult ovarian stem cells have the potentials to differentiate into other supporting cells of ovarian follicles and therefore could be utilized for cell-based hormone therapies to rectify hormonal imbalance caused by ovarian failure.
When it comes to strategies to rectify any structure deformities, regenerative medicine provides options through either autologous stem cell therapy or corrective procedures using bioengineered tissues to reconstruct the female reproductive tract tissues. Although various scaffolds have produced some desirable outcomes, bioengineering tissues by combining stem cells with natural or synthetic biomaterials have successfully repaired tissue abnormalities and restored physiological functions. Many advancing technologies, such as bio-printing of structurally complex reproductive tissues, have been actively investigated to expedite the translation into the clinic. The emerging technology of organ-on-chip with bioengineered tissues would be a powerful tool to device customized medicine, drug screening, and investigate the complex interaction between various tissues in an in vitro setup. Despite the advanced technologies developed in regenerative medicine, many scientific, technological, and regulatory challenges must be identified and addressed to deliver safe and effective therapies to patients.
Availability of Data and Material
Not applicable
Code Availability
Not applicable
References
Zhao YX, Chen SR, Su PP, Huang FH, Shi YC, Shi QY, et al. Using Mesenchymal Stem Cells to Treat Female Infertility: An Update on Female Reproductive Diseases. Stem Cells Int. 2019;2019:9071720. https://doi.org/10.1155/2019/9071720.
Ye L, Mayberry R, Lo CY, Britt KL, Stanley EG, Elefanty AG, et al. Generation of human female reproductive tract epithelium from human embryonic stem cells. PLoS One. 2011;6(6):e21136. https://doi.org/10.1371/journal.pone.0021136.
Borum K. Oogenesis in the mouse. A study of the meiotic prophase. Exp Cell Res. 1961;24:495–507. https://doi.org/10.1016/0014-4827(61)90449-9.
Faddy MJ, Jones EC, Edwards RG. An analytical model for ovarian follicle dynamics. J Exp Zool. 1976;197(2):173–85. https://doi.org/10.1002/jez.1401970203.
Green SH, Zuckerman S. The number of oocytes in the mature rhesus monkey (Macaca mulatta). J Endocrinol. 1951;7(2):194–202. https://doi.org/10.1677/joe.0.0070194.
Green SH, Zuckerman S. Further observations on oocyte numbers in mature rhesus monkeys (Macaca mulatta). J Endocrinol. 1954;10(3):284–90. https://doi.org/10.1677/joe.0.0100284.
Pan B, Li J. The art of oocyte meiotic arrest regulation. Reprod Biol Endocrinol. 2019;17(1):8. https://doi.org/10.1186/s12958-018-0445-8.
Messinis IE, Messini CI, Dafopoulos K. Novel aspects of the endocrinology of the menstrual cycle. Reprod BioMed Online. 2014;28(6):714–22. https://doi.org/10.1016/j.rbmo.2014.02.003.
Mihm M, Gangooly S, Muttukrishna S. The normal menstrual cycle in women. Anim Reprod Sci. 2011;124(3-4):229–36. https://doi.org/10.1016/j.anireprosci.2010.08.030.
Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update. 2012;18(1):73–91. https://doi.org/10.1093/humupd/dmr039.
Hewlett M, Mahalingaiah S. Update on primary ovarian insufficiency. Curr Opin Endocrinol Diabetes Obes. 2015;22(6):483–9. https://doi.org/10.1097/MED.0000000000000206.
Qin Y, Jiao X, Simpson JL, Chen ZJ. Genetics of primary ovarian insufficiency: new developments and opportunities. Hum Reprod Update. 2015;21(6):787–808. https://doi.org/10.1093/humupd/dmv036.
Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update. 2005;11(4):391–410. https://doi.org/10.1093/humupd/dmi012.
Momenimovahed Z, Tiznobaik A, Taheri S, Salehiniya H. Ovarian cancer in the world: epidemiology and risk factors. Int J Women's Health. 2019;11:287–99. https://doi.org/10.2147/ijwh.S197604.
Arora N, Talhouk A, McAlpine JN, Law MR, Hanley GE. Long-term mortality among women with epithelial ovarian cancer: a population-based study in British Columbia, Canada. BMC Cancer. 2018;18(1):1039. https://doi.org/10.1186/s12885-018-4970-9.
Orr BE, R. P. Diagnosis and Treatment of Ovarian Cancer. Hematol Oncol Clin North Am. 2018;32(6):943–64.
Takahashi TAJ, K. M. Menopause. Med Clin North Am. 2015;99(3):521–34.
Roy A, Matzuk MM. Reproductive tract function and dysfunction in women. Nat Rev Endocrinol. 2011;7(9):517–25. https://doi.org/10.1038/nrendo.2011.79.
Soderberg SF. Vaginal disorders. Vet Clin North Am Small Anim Pract. 1986;16(3):543–59. https://doi.org/10.1016/s0195-5616(86)50060-7.
Anderson RA, Wallace WH. Fertility preservation in girls and young women. Clin Endocrinol. 2011;75(4):409–19. https://doi.org/10.1111/j.1365-2265.2011.04100.x.
Donnez J, Dolmans MM, Pellicer A, Diaz-Garcia C, Sanchez Serrano M, Schmidt KT, et al. Restoration of ovarian activity and pregnancy after transplantation of cryopreserved ovarian tissue: a review of 60 cases of reimplantation. Fertil Steril. 2013;99(6):1503–13. https://doi.org/10.1016/j.fertnstert.2013.03.030.
Sadri-Ardekani H, Atala A. Regenerative medicine for the treatment of reproductive system disorders: current and potential options. Adv Drug Deliv Rev. 2015;82-83:145–52. https://doi.org/10.1016/j.addr.2014.10.019.
Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6(4):209–18. https://doi.org/10.1016/S1470-2045(05)70092-9.
Yalcinkaya TM, Sittadjody S, Opara EC. Scientific principles of regenerative medicine and their application in the female reproductive system. Maturitas. 2014;77(1):12–9. https://doi.org/10.1016/j.maturitas.2013.10.007.
Yu D, Li TC, Xia E, Huang X, Liu Y, Peng X. Factors affecting reproductive outcome of hysteroscopic adhesiolysis for Asherman's syndrome. Fertil Steril. 2008;89(3):715–22. https://doi.org/10.1016/j.fertnstert.2007.03.070.
Cunha GR, Sinclair A, Ricke WA, Robboy SJ, Cao M, Baskin LS. Reproductive tract biology: Of mice and men. Differentiation. 2019;110:49–63. https://doi.org/10.1016/j.diff.2019.07.004.
Poonia B, Walter L, Dufour J, Harrison R, Marx PA, Veazey RS. Cyclic changes in the vaginal epithelium of normal rhesus macaques. J Endocrinol. 2006;190(3):829–35. https://doi.org/10.1677/joe.1.06873.
Sjöberg I, Cajander S, Rylander E. Morphometric characteristics of the vaginal epithelium during the menstrual cycle. Gynecol Obstet Investig. 1988;26(2):136–44. https://doi.org/10.1159/000293685.
Vassena R, Eguizabal C, Heindryckx B, Sermon K, Simon C, van Pelt AM, et al. Stem cells in reproductive medicine: ready for the patient? Hum Reprod. 2015;30(9):2014–21. https://doi.org/10.1093/humrep/dev181.
Schlegel PN. Evaluation of male infertility. Minerva Ginecol. 2009;61(4):261–83.
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. https://doi.org/10.1001/jama.288.3.321.
Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–12. https://doi.org/10.1001/jama.291.14.1701.
Beshay SMR, G.; Balthasar, J.; Florea, N. Efficacy and clinical value of commonly compounded hormone replacement therapy: a literature review. Int J Pharm Compd. 2015;19(1):6–12.
Lobo RA. Hormone-replacement therapy: current thinking. Nat Rev Endocrinol. 2017;13(4):220–31.
Hodis HNM, W. J. The timing hypothesis and hormone replacement therapy: a paradigm shift in the primary prevention of coronary heart disease in women. Part 1: comparison of therapeutic efficacy. J Am Geriatr Soc. 2013;61(6):1005–10.
Hodis HNM, W. J. The timing hypothesis and hormone replacement therapy: a paradigm shift in the primary prevention of coronary heart disease in women. Part 2: comparative risks. J Am Geriatr Soc. 2013;61(6):1011–8.
Tulandi T, Marzal A. Redefining reproductive surgery. J Minim Invasive Gynecol. 2012;19(3):296–306. https://doi.org/10.1016/j.jmig.2012.01.010.
Gellert SE, Pors SE, Kristensen SG, Bay-Bjorn AM, Ernst E, Yding AC. Transplantation of frozen-thawed ovarian tissue: an update on worldwide activity published in peer-reviewed papers and on the Danish cohort. J Assist Reprod Genet. 2018;35(4):561–70. https://doi.org/10.1007/s10815-018-1144-2.
Brannstrom MBH, Dahm-Kahler P, Olausson M, Olofsson JI, Rodriguez-Wallberg K. One uterus bridging three generations: first live birth after mother-to-daughter uterus transplantation. Fertil Steril. 2016;106(2):261–6.
Thomson JAI-EJ, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(539):1145–7.
Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–62. https://doi.org/10.1146/annurev.cellbio.17.1.435.
Hubner K, Fuhrmann G, Christenson LK, Kehler J, Reinbold R, De La Fuente R, et al. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300(5623):1251–6. https://doi.org/10.1126/science.1083452.
Nayernia K, Nolte J, Michelmann HW, Lee JH, Rathsack K, Drusenheimer N, et al. In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell. 2006;11(1):125–32. https://doi.org/10.1016/j.devcel.2006.05.010.
Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, Daley GQ. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427(6970):148–54. https://doi.org/10.1038/nature02247.
Toyooka Y, Tsunekawa N, Akasu R, Noce T. Embryonic stem cells can form germ cells in vitro. Proc Natl Acad Sci U S A. 2003;100(20):11457–62. https://doi.org/10.1073/pnas.1932826100.
Chen HF, Kuo HC, Chien CL, Shun CT, Yao YL, Ip PL, et al. Derivation, characterization and differentiation of human embryonic stem cells: comparing serum-containing versus serum-free media and evidence of germ cell differentiation. Hum Reprod. 2007;22(2):567–77. https://doi.org/10.1093/humrep/del412.
Kee K, Gonsalves JM, Clark AT, Pera RA. Bone morphogenetic proteins induce germ cell differentiation from human embryonic stem cells. Stem Cells Dev. 2006;15(6):831–7. https://doi.org/10.1089/scd.2006.15.831.
Clark AT, Bodnar MS, Fox M, Rodriquez RT, Abeyta MJ, Firpo MT, et al. Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum Mol Genet. 2004;13(7):727–39. https://doi.org/10.1093/hmg/ddh088.
Wang T. Human fetal endometrium--light and electron microscopic study. Arch Gynecol Obstet. 1989;246(3):169–79. https://doi.org/10.1007/BF00934078.
Barberini F, Makabe S, Franchitto G, Correr S, Relucenti M, Heyn R, et al. Ultrastructural dynamics of the human endometrium from 14 to 22 weeks of gestation. Arch Histol Cytol. 2007;70(1):21–8. https://doi.org/10.1679/aohc.70.21.
Okada A, Sato T, Ohta Y, Iguchi T. Sex steroid hormone receptors in the developing female reproductive tract of laboratory rodents. J Toxicol Sci. 2005;30(2):75–89. https://doi.org/10.2131/jts.30.75.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–20. https://doi.org/10.1126/science.1151526.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. https://doi.org/10.1016/j.cell.2007.11.019.
Takahashi K, Okita K, Nakagawa M, Yamanaka S. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2(12):3081–9. https://doi.org/10.1038/nprot.2007.418.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. https://doi.org/10.1016/j.cell.2006.07.024.
Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322(5903):949–53. https://doi.org/10.1126/science.1164270.
Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322(5903):945–9. https://doi.org/10.1126/science.1162494.
Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153(6):1228–38. https://doi.org/10.1016/j.cell.2013.05.006.
De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25(1):100–6. https://doi.org/10.1038/nbt1274.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. https://doi.org/10.1126/science.284.5411.143.
Johnson J, Bagley J, Skaznik-Wikiel M, Lee HJ, Adams GB, Niikura Y, et al. Oocyte generation in adult mammalian ovaries by putative germ cells in bone marrow and peripheral blood. Cell. 2005;122(2):303–15. https://doi.org/10.1016/j.cell.2005.06.031.
Mints M, Jansson M, Sadeghi B, Westgren M, Uzunel M, Hassan M, et al. Endometrial endothelial cells are derived from donor stem cells in a bone marrow transplant recipient. Hum Reprod. 2008;23(1):139–43. https://doi.org/10.1093/humrep/dem342.
Ikoma T, Kyo S, Maida Y, Ozaki S, Takakura M, Nakao S, et al. Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. Am J Obstet Gynecol. 2009;201(6):608 e1–8. https://doi.org/10.1016/j.ajog.2009.07.026.
Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25(8):2082–6. https://doi.org/10.1634/stemcells.2006-0828.
Cervello I, Gil-Sanchis C, Mas A, Faus A, Sanz J, Moscardo F, et al. Bone marrow-derived cells from male donors do not contribute to the endometrial side population of the recipient. PLoS One. 2012;7(1):e30260. https://doi.org/10.1371/journal.pone.0030260.
Li Y, Liu F, Zhang Z, Zhang M, Cao S, Li Y, et al. Bone marrow mesenchymal stem cells could acquire the phenotypes of epithelial cells and accelerate vaginal reconstruction combined with small intestinal submucosa. Cell Biol Int. 2015;39(11):1225–33. https://doi.org/10.1002/cbin.10495.
Raya-Rivera AM, Esquiliano D, Fierro-Pastrana R, Lopez-Bayghen E, Valencia P, Ordorica-Flores R, et al. Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet. 2014;384(9940):329–36. https://doi.org/10.1016/S0140-6736(14)60542-0.
Li Q, Wang J, Liu H, Xie B, Wei L. Tissue-engineered mesh for pelvic floor reconstruction fabricated from silk fibroin scaffold with adipose-derived mesenchymal stem cells. Cell Tissue Res. 2013;354(2):471–80. https://doi.org/10.1007/s00441-013-1719-2.
Santos AR Jr. Bioresorbable polymers for tissue engineering. In: Rijeka dE, editor. IntechOpen: Tissue Engineering; 2010.
Leal-Egana A, Scheibel T. Silk-based materials for biomedical applications. Biotechnol Appl Biochem. 2010;55(3):155–67. https://doi.org/10.1042/BA20090229.
Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, et al. Silk-based biomaterials. Biomaterials. 2003;24(3):401–16. https://doi.org/10.1016/s0142-9612(02)00353-8.
Vepari C, Kaplan DL. Silk as a Biomaterial. Prog Polym Sci. 2007;32(8-9):991–1007. https://doi.org/10.1016/j.progpolymsci.2007.05.013.
Kundu B, Rajkhowa R, Kundu SC, Wang X. Silk fibroin biomaterials for tissue regenerations. Adv Drug Deliv Rev. 2013;65(4):457–70. https://doi.org/10.1016/j.addr.2012.09.043.
Pawar SN, Edgar KJ. Alginate derivatization: a review of chemistry, properties and applications. Biomaterials. 2012;33(11):3279–305. https://doi.org/10.1016/j.biomaterials.2012.01.007.
Valente JFAV, T. A. M, Alves P, Ferreira P, Silva A, Correia IJ. Alginate based scaffolds for bone tissue engineering. Mater Sci Eng C. 2012;32(8):2596–603.
Sun J, Tan H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials (Basel). 2013;6(4):1285–309. https://doi.org/10.3390/ma6041285.
Standeven KF, Ariens RA, Whitaker P, Ashcroft AE, Weisel JW, Grant PJ. The effect of dimethylbiguanide on thrombin activity, FXIII activation, fibrin polymerization, and fibrin clot formation. Diabetes. 2002;51(1):189–97. https://doi.org/10.2337/diabetes.51.1.189.
Bensaid W, Triffitt JT, Blanchat C, Oudina K, Sedel L, Petite H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24(14):2497–502. https://doi.org/10.1016/s0142-9612(02)00618-x.
Noth U, Rackwitz L, Steinert AF, Tuan RS. Cell delivery therapeutics for musculoskeletal regeneration. Adv Drug Deliv Rev. 2010;62(7-8):765–83. https://doi.org/10.1016/j.addr.2010.04.004.
Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23(12):H41–56. https://doi.org/10.1002/adma.201003963.
Hemshekhar M, Thushara RM, Chandranayaka S, Sherman LS, Kemparaju K, Girish KS. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int J Biol Macromol. 2016;86:917–28. https://doi.org/10.1016/j.ijbiomac.2016.02.032.
Kim IL, Khetan S, Baker BM, Chen CS, Burdick JA. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials. 2013;34(22):5571–80. https://doi.org/10.1016/j.biomaterials.2013.04.004.
Kim B-SP I-K, Hoshiba T, Jiang H-J, Akaike T, Cho C-S. Design of artificial extracellular matrices for tissue engineering. Progress Polymer Sci. 2011;36(2):238–68.
Lee EJ, Kasper FK, Mikos AG. Biomaterials for tissue engineering. Ann Biomed Eng. 2014;42(2):323–37. https://doi.org/10.1007/s10439-013-0859-6.
Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27(18):3413–31. https://doi.org/10.1016/j.biomaterials.2006.01.039.
Atala A. Tissue engineering of reproductive tissues and organs. Fertil Steril. 2012;98(1):21–9. https://doi.org/10.1016/j.fertnstert.2012.05.038.
Yu HY, Tang ZQ, Huang L, Cheng G, Li W, Zhou J, et al. Surface modification of polypropylene macroporous membrane to improve its antifouling characteristics in a submerged membrane-bioreactor: H(2)O plasma treatment. Water Res. 2008;42(16):4341–7. https://doi.org/10.1016/j.watres.2008.05.028.
Hadjizadeh A, Ajji A, Bureau MN. Preparation and characterization of NaOH treated micro-fibrous polyethylene terephthalate nonwovens for biomedical application. J Mech Behav Biomed Mater. 2010;3(8):574–83. https://doi.org/10.1016/j.jmbbm.2010.07.002.
Brown B, Lindberg K, Reing J, Stolz DB, Badylak SF. The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng. 2006;12(3):519–26. https://doi.org/10.1089/ten.2006.12.519.
Hodde J, Record R, Tullius R, Badylak S. Fibronectin peptides mediate HMEC adhesion to porcine-derived extracellular matrix. Biomaterials. 2002;23(8):1841–8. https://doi.org/10.1016/s0142-9612(01)00310-6.
Ribeiro-Filho LA, Sievert KD. Acellular matrix in urethral reconstruction. Adv Drug Deliv Rev. 2015;82-83:38–46. https://doi.org/10.1016/j.addr.2014.11.019.
Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009;5(1):1–13. https://doi.org/10.1016/j.actbio.2008.09.013.
Reing JE, Brown BN, Daly KA, Freund JM, Gilbert TW, Hsiong SX, et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials. 2010;31(33):8626–33. https://doi.org/10.1016/j.biomaterials.2010.07.083.
Catto VF, S.; Freddi, G.; Tanzi, C. M. Recent Advances in Small Diameter Blood Vessel Regeneration. ISRN Vascular Medicine: Vascular Tissue Engineering; 2014.
Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27(19):3675–83. https://doi.org/10.1016/j.biomaterials.2006.02.014.
Alaiti MA, Ishikawa M, Costa MA. Bone marrow and circulating stem/progenitor cells for regenerative cardiovascular therapy. Transl Res. 2010;156(3):112–29. https://doi.org/10.1016/j.trsl.2010.06.008.
Opie SR, Dib N. Surgical and catheter delivery of autologous myoblasts in patients with congestive heart failure. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S42–5. https://doi.org/10.1038/ncpcardio0399.
Menasche P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117(9):1189–200. https://doi.org/10.1161/CIRCULATIONAHA.107.734103.
Menasche P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, et al. Myoblast transplantation for heart failure. Lancet. 2001;357(9252):279–80. https://doi.org/10.1016/S0140-6736(00)03617-5.
Mandla SRM; Cardiac Tissue. In: Atala AL, R.; Mikos, A. G.; Nerem, R., editor. Principles of Regenerative Medicine. Third Edition ed. Cambridge, MA, USA: Academic Press, Elsevier Inc.; 2019. p. 1073-99.
Zimmermann WH, Didie M, Doker S, Melnychenko I, Naito H, Rogge C, et al. Heart muscle engineering: an update on cardiac muscle replacement therapy. Cardiovasc Res. 2006;71(3):419–29. https://doi.org/10.1016/j.cardiores.2006.03.023.
Leor J, Aboulafia-Etzion S, Dar A, Shapiro L, Barbash IM, Battler A, et al. Bioengineered cardiac grafts: A new approach to repair the infarcted myocardium? Circulation. 2000;102(19 Suppl 3):III56–61. https://doi.org/10.1161/01.cir.102.suppl_3.iii-56.
Li RK, Jia ZQ, Weisel RD, Mickle DA, Choi A, Yau TM. Survival and function of bioengineered cardiac grafts. Circulation. 1999;100(19 Suppl):II63–9. https://doi.org/10.1161/01.cir.100.suppl_2.ii-63.
Matsuda NST, Yamato M, Okano T. Tissue Engineering Based on Cell Sheet Technology2007.
Masuda S, Shimizu T, Yamato M, Okano T. Cell sheet engineering for heart tissue repair. Adv Drug Deliv Rev. 2008;60(2):277–85. https://doi.org/10.1016/j.addr.2007.08.031.
Kang HW, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat Biotechnol. 2016;34(3):312–9. https://doi.org/10.1038/nbt.3413.
Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, et al. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun. 2017;8:15261. https://doi.org/10.1038/ncomms15261.
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–5. https://doi.org/10.1038/nature07935.
Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125(Pt 13):3015–24. https://doi.org/10.1242/jcs.079509.
Lancaster MA, Huch M. Disease modelling in human organoids. Dis Model Mech. 2019;12(7). https://doi.org/10.1242/dmm.039347.
Joo S, Oh SH, Sittadjody S, Opara EC, Jackson JD, Lee SJ, et al. The effect of collagen hydrogel on 3D culture of ovarian follicles. Biomed Mater. 2016;11(6):065009. https://doi.org/10.1088/1748-6041/11/6/065009.
Alzamil L, Nikolakopoulou K, Turco MY. Organoid systems to study the human female reproductive tract and pregnancy. Cell Death Differ. 2020. https://doi.org/10.1038/s41418-020-0565-5.
Kang S-M, Lee J-H, Huh YS, Takayama S. Alginate Microencapsulation for Three-Dimensional In Vitro Cell Culture. ACS Biomater Sci Eng. 2020. https://doi.org/10.1021/acsbiomaterials.0c00457.
Ashimova A, Yegorov S, Negmetzhanov B, Hortelano G. Cell Encapsulation Within Alginate Microcapsules: Immunological Challenges and Outlook. Front Bioeng Biotechnol. 2019;7(380). https://doi.org/10.3389/fbioe.2019.00380.
Sittadjody S, Saul JM, Joo S, Yoo JJ, Atala A, Opara EC. Engineered multilayer ovarian tissue that secretes sex steroids and peptide hormones in response to gonadotropins. Biomaterials. 2013;34(10):2412–20. https://doi.org/10.1016/j.biomaterials.2012.11.059.
Sittadjody S, Enck KM, Wells A, Yoo JJ, Atala A, Saul JM, et al. Encapsulation of Mesenchymal Stem Cells in 3D Ovarian Cell Constructs Promotes Stable and Long-Term Hormone Secretion with Improved Physiological Outcomes in a Syngeneic Rat Model. Ann Biomed Eng. 2020;48(3):1058–70. https://doi.org/10.1007/s10439-019-02334-w.
Sittadjody S, Saul JM, McQuilling JP, Joo S, Register TC, Yoo JJ, et al. In vivo transplantation of 3D encapsulated ovarian constructs in rats corrects abnormalities of ovarian failure. Nat Commun. 2017;8(1):1858. https://doi.org/10.1038/s41467-017-01851-3.
Pacheco F, Oktay K. Current Success and Efficiency of Autologous Ovarian Transplantation: A Meta-Analysis. Reprod Sci. 2017;24(8):1111–20. https://doi.org/10.1177/1933719117702251.
Amorim CA. Special Issue Devoted to a New Field of Regenerative Medicine: Reproductive Tissue Engineering. Ann Biomed Eng. 2017;45(7):1589–91. https://doi.org/10.1007/s10439-017-1862-0.
Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30(6):624–712. https://doi.org/10.1210/er.2009-0012.
Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234(2):339–51. https://doi.org/10.1006/dbio.2001.0269.
Pepling ME, Spradling AC. Female mouse germ cells form synchronously dividing cysts. Development. 1998;125(17):3323–8.
Turan V, Oktay K. Sexual and fertility adverse effects associated with chemotherapy treatment in women. Expert Opin Drug Saf. 2014;13(6):775–83. https://doi.org/10.1517/14740338.2014.915940.
Vassilakopoulou M, Boostandoost E, Papaxoinis G, de La Motte RT, Khayat D, Psyrri A. Anticancer treatment and fertility: Effect of therapeutic modalities on reproductive system and functions. Crit Rev Oncol Hematol. 2016;97:328–34. https://doi.org/10.1016/j.critrevonc.2015.08.002.
Donnez J. Fertility preservation in women, focusing on cancer, benign diseases and social reasons. Minerva Ginecol. 2018;70(4):385–6. https://doi.org/10.23736/S0026-4784.18.04245-4.
Donnez J, Dolmans MM. Fertility Preservation in Women. N Engl J Med. 2018;378(4):400–1. https://doi.org/10.1056/NEJMc1715731.
Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. 1983;305(5936):707–9. https://doi.org/10.1038/305707a0.
Lassalle B, Testart J, Renard JP. Human embryo features that influence the success of cryopreservation with the use of 1,2 propanediol. Fertil Steril. 1985;44(5):645–51. https://doi.org/10.1016/s0015-0282(16)48981-8.
Anderson AR, Wilkinson SS, Price S, Crain JL. Reduction of high order multiples in frozen embryo transfers. Reprod BioMed Online. 2005;10(3):402–5. https://doi.org/10.1016/s1472-6483(10)61803-2.
Mazur P. Equilibrium, quasi-equilibrium, and nonequilibrium freezing of mammalian embryos. Cell Biophys. 1990;17(1):53–92. https://doi.org/10.1007/BF02989804.
Kuwayama M, Vajta G, Ieda S, Kato O. Comparison of open and closed methods for vitrification of human embryos and the elimination of potential contamination. Reprod BioMed Online. 2005;11(5):608–14. https://doi.org/10.1016/s1472-6483(10)61169-8.
Balaban B, Urman B, Ata B, Isiklar A, Larman MG, Hamilton R, et al. A randomized controlled study of human Day 3 embryo cryopreservation by slow freezing or vitrification: vitrification is associated with higher survival, metabolism and blastocyst formation. Hum Reprod. 2008;23(9):1976–82. https://doi.org/10.1093/humrep/den222.
Al-Hasani S, Ozmen B, Koutlaki N, Schoepper B, Diedrich K, Schultze-Mosgau A. Three years of routine vitrification of human zygotes: is it still fair to advocate slow-rate freezing? Reprod BioMed Online. 2007;14(3):288–93. https://doi.org/10.1016/s1472-6483(10)60869-3.
Rama Raju GA, Haranath GB, Krishna KM, Prakash GJ, Madan K. Vitrification of human 8-cell embryos, a modified protocol for better pregnancy rates. Reprod BioMed Online. 2005;11(4):434–7. https://doi.org/10.1016/s1472-6483(10)61135-2.
Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at -196 degrees C by vitrification. Nature. 1985;313(6003):573–5. https://doi.org/10.1038/313573a0.
Vajta G, Nagy ZP. Are programmable freezers still needed in the embryo laboratory? Review on vitrification. Reprod BioMed Online. 2006;12(6):779–96. https://doi.org/10.1016/s1472-6483(10)61091-7.
Garcia GSR, Arenas ML, Gonzalez O, Ramirez P, Patrizio P. Comparative study of human oocyte cryopreservation by vitrification or slow freezing. Fertil Steril. 2008;90:S291–S2.
AbdelHafez FDN, Ali MY, Sayed EH, Abu-Alhassan AM, Bedaiwy MA. Oocyte cryopreservation: a technical and clinical update. Expert Rev Obstet Gynecol. 2009;4(4):443–54.
Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod BioMed Online. 2005;11(3):300–8. https://doi.org/10.1016/s1472-6483(10)60837-1.
Yoon TK, Kim TJ, Park SE, Hong SW, Ko JJ, Chung HM, et al. Live births after vitrification of oocytes in a stimulated in vitro fertilization-embryo transfer program. Fertil Steril. 2003;79(6):1323–6. https://doi.org/10.1016/s0015-0282(03)00258-9.
Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology. 2007;67(1):73–80. https://doi.org/10.1016/j.theriogenology.2006.09.014.
Chian RC, Gilbert L, Huang JY, Demirtas E, Holzer H, Benjamin A, et al. Live birth after vitrification of in vitro matured human oocytes. Fertil Steril. 2009;91(2):372–6. https://doi.org/10.1016/j.fertnstert.2007.11.088.
Chian RC, Huang JY, Gilbert L, Son WY, Holzer H, Cui SJ, et al. Obstetric outcomes following vitrification of in vitro and in vivo matured oocytes. Fertil Steril. 2009;91(6):2391–8. https://doi.org/10.1016/j.fertnstert.2008.04.014.
Chen CH, Chen SG, Wu GJ, Wang J, Yu CP, Liu JY. Autologous heterotopic transplantation of intact rabbit ovary after frozen banking at -196 degrees C. Fertil Steril. 2006;86(4 Suppl):1059–66. https://doi.org/10.1016/j.fertnstert.2006.04.019.
Deng XH, Xu AR, Chao L, Yu HL, Zhen JH, Hashimoto S, et al. Effect of different sites for cryopreserved ovarian tissue implantation in rabbit. Hum Reprod. 2007;22(3):662–8. https://doi.org/10.1093/humrep/del430.
Onions VJWR, McNeilly AS, Campbell BK. Ovarian endocrine profile and long-term vascular patency following heterotopic autotransplantation of cryopreserved whole ovine ovaries. Hum Reprod 2009;24(11):2845-55. .
Grazul-Bilska AT, Banerjee J, Yazici I, Borowczyk E, Bilski JJ, Sharma RK, et al. Morphology and function of cryopreserved whole ovine ovaries after heterotopic autotransplantation. Reprod Biol Endocrinol. 2008;6(1):16. https://doi.org/10.1186/1477-7827-6-16.
Denschlag D, Knobloch CKA, Baessler A, Goebel H, Wellens E, Haberstroh J, et al. Autologous heterotopic transplantation of ovarian tissue in sheep. Fertil Steril. 2005;83(2):501–3.
Díaz-García CMM, Groth K, Dahm-Kähler P, Olausson M, Brännström M. Ovarian cortex transplantation in the baboon: comparison of four different intra-abdominal transplantation sites. Hum Reprod. 2011;26(12).
Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci. 2013;110(43):17474–9. https://doi.org/10.1073/pnas.1312830110.
Conger K. Stanford-developed technique induces egg growth in infer-tile women, and one gives birth. 2013.
Donnez J, Dolmans MM. Fertility Preservation in Women. N Engl J Med. 2017;377(17):1657–65. https://doi.org/10.1056/NEJMra1614676.
Kossack N, Meneses J, Shefi S, Nguyen HN, Chavez S, Nicholas C, et al. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27(1):138–49. https://doi.org/10.1634/stemcells.2008-0439.
Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456(7220):344–9. https://doi.org/10.1038/nature07404.
Begum S, Papaioannou VE, Gosden RG. The oocyte population is not renewed in transplanted or irradiated adult ovaries. Hum Reprod. 2008;23(10):2326–30. https://doi.org/10.1093/humrep/den249.
Eggan K, Jurga S, Gosden R, Min IM, Wagers AJ. Ovulated oocytes in adult mice derive from non-circulating germ cells. Nature. 2006;441(7097):1109–14. https://doi.org/10.1038/nature04929.
Telfer EE, Gosden RG, Byskov AG, Spears N, Albertini D, Andersen CY, et al. On regenerating the ovary and generating controversy. Cell. 2005;122(6):821–2. https://doi.org/10.1016/j.cell.2005.09.004.
Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428(6979):145–50. https://doi.org/10.1038/nature02316.
Virant-Klun I, Rozman P, Cvjeticanin B, Vrtacnik-Bokal E, Novakovic S, Rulicke T, et al. Parthenogenetic embryo-like structures in the human ovarian surface epithelium cell culture in postmenopausal women with no naturally present follicles and oocytes. Stem Cells Dev. 2009;18(1):137–49. https://doi.org/10.1089/scd.2007.0238.
Zhang D, Fouad H, Zoma WD, Salama SA, Wentz MJ, Al-Hendy A. Expression of stem and germ cell markers within nonfollicle structures in adult mouse ovary. Reprod Sci. 2008;15(2):139–46. https://doi.org/10.1177/1933719107310708.
Liu Y, Wu C, Lyu Q, Yang D, Albertini DF, Keefe DL, et al. Germline stem cells and neo-oogenesis in the adult human ovary. Dev Biol. 2007;306(1):112–20. https://doi.org/10.1016/j.ydbio.2007.03.006.
Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Mayo KE, Shea LD, et al. Fate of the initial follicle pool: empirical and mathematical evidence supporting its sufficiency for adult fertility. Dev Biol. 2006;298(1):149–54. https://doi.org/10.1016/j.ydbio.2006.06.023.
Kerr JB, Duckett R, Myers M, Britt KL, Mladenovska T, Findlay JK. Quantification of healthy follicles in the neonatal and adult mouse ovary: evidence for maintenance of primordial follicle supply. Reproduction. 2006;132(1):95–109. https://doi.org/10.1530/rep.1.01128.
Bukovsky A, Caudle MR, Svetlikova M, Wimalasena J, Ayala ME, Dominguez R. Oogenesis in adult mammals, including humans: a review. Endocrine. 2005;26(3):301–16. https://doi.org/10.1385/ENDO:26:3:301.
Tilly JL, Niikura Y, Rueda BR. The current status of evidence for and against postnatal oogenesis in mammals: a case of ovarian optimism versus pessimism? Biol Reprod. 2009;80(1):2–12. https://doi.org/10.1095/biolreprod.108.069088.
Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B. Chuva de Sousa Lopes SM et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448(7150):191–5. https://doi.org/10.1038/nature05950.
Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448(7150):196–9. https://doi.org/10.1038/nature05972.
Nichols J, Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4(6):487–92. https://doi.org/10.1016/j.stem.2009.05.015.
Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504(7479):282–6. https://doi.org/10.1038/nature12745.
Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J, et al. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell. 2014;15(4):471–87. https://doi.org/10.1016/j.stem.2014.07.002.
Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, et al. Derivation of naive human embryonic stem cells. Proc Natl Acad Sci U S A. 2014;111(12):4484–9. https://doi.org/10.1073/pnas.1319738111.
Bendel-Stenzel M, Anderson R, Heasman J, Wylie C. The origin and migration of primordial germ cells in the mouse. Semin Cell Dev Biol. 1998;9(4):393–400. https://doi.org/10.1006/scdb.1998.0204.
Chuva de Sousa Lopes SM, Roelen BA. On the formation of germ cells: The good, the bad and the ugly. Differentiation. 2010;79(3):131–40. https://doi.org/10.1016/j.diff.2009.11.003.
Matsui Y. The molecular mechanisms regulating germ cell development and potential. J Androl. 2010;31(1):61–5. https://doi.org/10.2164/jandrol.109.008094.
Saitou M, Yamaji M. Germ cell specification in mice: signaling, transcription regulation, and epigenetic consequences. Reproduction. 2010;139(6):931–42. https://doi.org/10.1530/rep-10-0043.
De Felici M, Di Carlo A, Pesce M. Role of stem cell factor in somatic–germ cell interactions during prenatal oogenesis. Zygote. 1996;4(4):349–51. https://doi.org/10.1017/S0967199400003373.
Seki Y, Hayashi K, Itoh K, Mizugaki M, Saitou M, Matsui Y. Extensive and orderly reprogramming of genome-wide chromatin modifications associated with specification and early development of germ cells in mice. Dev Biol. 2005;278(2):440–58. https://doi.org/10.1016/j.ydbio.2004.11.025.
Hajkova P, Ancelin K, Waldmann T, Lacoste N, Lange UC, Cesari F, et al. Chromatin dynamics during epigenetic reprogramming in the mouse germ line. Nature. 2008;452(7189):877–81. https://doi.org/10.1038/nature06714.
Smith ZD, Chan MM, Mikkelsen TS, Gu H, Gnirke A, Regev A, et al. A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature. 2012;484(7394):339–44. https://doi.org/10.1038/nature10960.
Eguizabal C, Shovlin TC, Durcova-Hills G, Surani A, McLaren A. Generation of primordial germ cells from pluripotent stem cells. Differentiation. 2009;78(2-3):116–23. https://doi.org/10.1016/j.diff.2009.07.001.
Imamura T, Uesaka M, Nakashima K. Epigenetic setting and reprogramming for neural cell fate determination and differentiation. Philos Trans R Soc Lond B Biol Sci. 2014;369(1652). https://doi.org/10.1098/rstb.2013.0511.
Tilgner K, Atkinson SP, Golebiewska A, Stojkovic M, Lako M, Armstrong L. Isolation of primordial germ cells from differentiating human embryonic stem cells. Stem Cells. 2008;26(12):3075–85. https://doi.org/10.1634/stemcells.2008-0289.
Tilgner K, Atkinson SP, Yung S, Golebiewska A, Stojkovic M, Moreno R, et al. Expression of GFP under the control of the RNA helicase VASA permits fluorescence-activated cell sorting isolation of human primordial germ cells. Stem Cells. 2010;28(1):84–92. https://doi.org/10.1002/stem.263.
Park TS, Galic Z, Conway AE, Lindgren A, van Handel BJ, Magnusson M, et al. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells. 2009;27(4):783–95. https://doi.org/10.1002/stem.13.
Bucay N, Yebra M, Cirulli V, Afrikanova I, Kaido T, Hayek A, et al. A novel approach for the derivation of putative primordial germ cells and sertoli cells from human embryonic stem cells. Stem Cells. 2009;27(1):68–77. https://doi.org/10.1634/stemcells.2007-1018.
Eguizabal C, Montserrat N, Vassena R, Barragan M, Garreta E, Garcia-Quevedo L, et al. Complete meiosis from human induced pluripotent stem cells. Stem Cells. 2011;29(8):1186–95. https://doi.org/10.1002/stem.672.
Medrano JV, Ramathal C, Nguyen HN, Simon C, Reijo Pera RA. Divergent RNA-binding proteins, DAZL and VASA, induce meiotic progression in human germ cells derived in vitro. Stem Cells. 2012;30(3):441–51. https://doi.org/10.1002/stem.1012.
Hayashi K, Surani MA. Self-renewing epiblast stem cells exhibit continual delineation of germ cells with epigenetic reprogramming in vitro. Development. 2009;136(21):3549–56. https://doi.org/10.1242/dev.037747.
Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146(4):519–32. https://doi.org/10.1016/j.cell.2011.06.052.
Lacham-Kaplan O, Chy H, Trounson A. Testicular cell conditioned medium supports differentiation of embryonic stem cells into ovarian structures containing oocytes. Stem Cells. 2006;24(2):266–73. https://doi.org/10.1634/stemcells.2005-0204.
Hayashi K, Ogushi S, Kurimoto K, Shimamoto S, Ohta H, Saitou M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science. 2012;338(6109):971–5. https://doi.org/10.1126/science.1226889.
Cyranoski D. Stem cells: Egg engineers. Nature. 2013;500(7463):392–4. https://doi.org/10.1038/500392a.
West FD, Machacek DW, Boyd NL, Pandiyan K, Robbins KR, Stice SL. Enrichment and differentiation of human germ-like cells mediated by feeder cells and basic fibroblast growth factor signaling. Stem Cells. 2008;26(11):2768–76. https://doi.org/10.1634/stemcells.2008-0124.
West FD, Roche-Rios MI, Abraham S, Rao RR, Natrajan MS, Bacanamwo M, et al. KIT ligand and bone morphogenetic protein signaling enhances human embryonic stem cell to germ-like cell differentiation. Hum Reprod. 2010;25(1):168–78. https://doi.org/10.1093/humrep/dep338.
Richards M, Fong CY, Bongso A. Comparative evaluation of different in vitro systems that stimulate germ cell differentiation in human embryonic stem cells. Fertil Steril. 2010;93(3):986–94. https://doi.org/10.1016/j.fertnstert.2008.10.030.
White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18(3):413–21. https://doi.org/10.1038/nm.2669.
Virant-Klun I, Zech N, Rozman P, Vogler A, Cvjeticanin B, Klemenc P, et al. Putative stem cells with an embryonic character isolated from the ovarian surface epithelium of women with no naturally present follicles and oocytes. Differentiation. 2008;76(8):843–56. https://doi.org/10.1111/j.1432-0436.2008.00268.x.
Virant-Klun I, Skutella T, Stimpfel M, Sinkovec J. Ovarian surface epithelium in patients with severe ovarian infertility: a potential source of cells expressing markers of pluripotent/multipotent stem cells. J Biomed Biotechnol. 2011;2011:381928. https://doi.org/10.1155/2011/381928.
Lei L, Spradling AC. Female mice lack adult germ-line stem cells but sustain oogenesis using stable primordial follicles. Proc Natl Acad Sci U S A. 2013;110(21):8585–90. https://doi.org/10.1073/pnas.1306189110.
Bhartiya D, Sriraman K, Parte S, Patel H. Ovarian stem cells: absence of evidence is not evidence of absence. J Ovarian Res. 2013;6(1):65. https://doi.org/10.1186/1757-2215-6-65.
Zhang H, Zheng W, Shen Y, Adhikari D, Ueno H, Liu K. Experimental evidence showing that no mitotically active female germline progenitors exist in postnatal mouse ovaries. Proc Natl Acad Sci U S A. 2012;109(31):12580–5. https://doi.org/10.1073/pnas.1206600109.
Magoffin DA. Ovarian theca cell. Int J Biochem Cell Biol. 2005;37(7):1344–9. https://doi.org/10.1016/j.biocel.2005.01.016.
Krohn PL. Review lectures on senescence. II. Heterochronic transplantation in the study of ageing. Proc R Soc Lond B Biol Sci. 1962;157:128–47. https://doi.org/10.1098/rspb.1962.0066.
Perez GI, Jurisicova A, Wise L, Lipina T, Kanisek M, Bechard A, et al. Absence of the proapoptotic Bax protein extends fertility and alleviates age-related health complications in female mice. Proc Natl Acad Sci U S A. 2007;104(12):5229–34. https://doi.org/10.1073/pnas.0608557104.
Mason JB, Cargill SL, Anderson GB, Carey JR. Transplantation of young ovaries to old mice increased life span in transplant recipients. J Gerontol A Biol Sci Med Sci. 2009;64(12):1207–11. https://doi.org/10.1093/gerona/glp134.
Cargill SL, Carey JR, Muller HG, Anderson G. Age of ovary determines remaining life expectancy in old ovariectomized mice. Aging Cell. 2003;2(3):185–90. https://doi.org/10.1046/j.1474-9728.2003.00049.x.
Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363(9412):837–40. https://doi.org/10.1016/S0140-6736(04)15728-0.
Oktay K. Ovarian tissue cryopreservation and transplantation: preliminary findings and implications for cancer patients. Hum Reprod Update. 2001;7(6):526–34. https://doi.org/10.1093/humupd/7.6.526.
Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30(3):608–15. https://doi.org/10.1093/humrep/deu353.
Rodriguez-Wallberg KA, Karlstrom PO, Rezapour M, Castellanos E, Hreinsson J, Rasmussen C, et al. Full-term newborn after repeated ovarian tissue transplants in a patient treated for Ewing sarcoma by sterilizing pelvic irradiation and chemotherapy. Acta Obstet Gynecol Scand. 2015;94(3):324–8. https://doi.org/10.1111/aogs.12568.
Oktay K, Bedoschi G, Pacheco F, Turan V, Emirdar V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am J Obstet Gynecol. 2016;214(1):94 e1–9. https://doi.org/10.1016/j.ajog.2015.10.001.
Macklon KT, Jensen AK, Loft A, Ernst E, Andersen CY. Treatment history and outcome of 24 deliveries worldwide after autotransplantation of cryopreserved ovarian tissue, including two new Danish deliveries years after autotransplantation. J Assist Reprod Genet. 2014;31(11):1557–64. https://doi.org/10.1007/s10815-014-0331-z.
Donnez J, Squifflet J, Pirard C, Demylle D, Delbaere A, Armenio L, et al. Live birth after allografting of ovarian cortex between genetically non-identical sisters. Hum Reprod. 2011;26(6):1384–8. https://doi.org/10.1093/humrep/der089.
Donnez J, Squifflet J, Pirard C, Jadoul P, Dolmans MM. Restoration of ovarian function after allografting of ovarian cortex between genetically non-identical sisters. Hum Reprod. 2010;25(10):2489–95. https://doi.org/10.1093/humrep/deq186.
Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364(9443):1405–10. https://doi.org/10.1016/S0140-6736(04)17222-X.
Andersen CY, Silber SJ, Bergholdt SH, Jorgensen JS, Ernst E. Long-term duration of function of ovarian tissue transplants: case reports. Reprod BioMed Online. 2012;25(2):128–32. https://doi.org/10.1016/j.rbmo.2012.03.014.
Guo XX, Zhou JL, Xu Q, Lu X, Liang YJ, Weng J, et al. Prevention of osteoporosis in mice after ovariectomy via allograft of microencapsulated ovarian cells. Anat Rec (Hoboken). 2010;293(2):200–7. https://doi.org/10.1002/ar.21036.
Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, et al. FSH directly regulates bone mass. Cell. 2006;125(2):247–60. https://doi.org/10.1016/j.cell.2006.01.051.
Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev. 2006;27(1):17–46. https://doi.org/10.1210/er.2004-0021.
Teixeira JR, B. R.; Pru JK. Uterine stem cells. StemBook. Cambridge, MA, USA2008.
Gargett CE, Chan RW, Schwab KE. Hormone and growth factor signaling in endometrial renewal: role of stem/progenitor cells. Mol Cell Endocrinol. 2008;288(1-2):22–9. https://doi.org/10.1016/j.mce.2008.02.026.
Semba Y, Chang C, Hong H, Kamisato A, Kokado M, Muto K. Surrogacy: donor conception regulation in Japan. Bioethics. 2010;24(7):348–57. https://doi.org/10.1111/j.1467-8519.2009.01780.x.
Dar S, Lazer T, Swanson S, Silverman J, Wasser C, Moskovtsev SI, et al. Assisted reproduction involving gestational surrogacy: an analysis of the medical, psychosocial and legal issues: experience from a large surrogacy program. Hum Reprod. 2015;30(2):345–52. https://doi.org/10.1093/humrep/deu333.
Brinsden PR. Gestational surrogacy. Hum Reprod Update. 2003;9(5):483–91. https://doi.org/10.1093/humupd/dmg033.
Shenfield F, Pennings G, Cohen J, Devroey P, de Wert G, Tarlatzis B, et al. ESHRE Task Force on Ethics and Law 10: surrogacy. Hum Reprod. 2005;20(10):2705–7. https://doi.org/10.1093/humrep/dei147.
Gardner DKWA, Howles CM, Shoham Z. The impact of legislation and socioeconomic factors in the access tp and global practice of ART. In: Gardner DKW, A.; Howles, C. M.; Shoham, Z. , editor. Textbook of Assisted Reproductive Techniques: Laboratory and Clinical Perspectives. Boca Raton: CRC Press; 2001.
Jonkman MF, Kauer FM, Nieuwenhuis P, Molenaar I. Segmental uterine horn replacement in the rat using a biodegradable microporous synthetic tube. Artif Organs. 1986;10(6):475–80. https://doi.org/10.1111/j.1525-1594.1986.tb02607.x.
Brannstrom M, Johannesson L, Bokstrom H, Kvarnstrom N, Molne J, Dahm-Kahler P, et al. Livebirth after uterus transplantation. Lancet. 2015;385(9968):607–16. https://doi.org/10.1016/S0140-6736(14)61728-1.
Lesieur O, Leloup M, Gonzalez F, Mamzer MF, Group ES. Eligibility for organ donation following end-of-life decisions: a study performed in 43 French intensive care units. Intensive Care Med. 2014;40(9):1323–31. https://doi.org/10.1007/s00134-014-3409-2.
Azimzadeh AM, Lees JR, Ding Y, Bromberg JS. Immunobiology of transplantation: impact on targets for large and small molecules. Clin Pharmacol Ther. 2011;90(2):229–42. https://doi.org/10.1038/clpt.2011.106.
Rubin R. Why The First American Uterus Transplant Failed. 2016.
Chan RW, Gargett CE. Identification of label-retaining cells in mouse endometrium. Stem Cells. 2006;24(6):1529–38. https://doi.org/10.1634/stemcells.2005-0411.
Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 2009;80(6):1136–45. https://doi.org/10.1095/biolreprod.108.075226.
Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 2004;70(6):1738–50. https://doi.org/10.1095/biolreprod.103.024109.
Cha J, Vilella F, Dey SK, Simon C., editor. Molecular interplay in successful implantation. Ten critical topics in rproductive Medicine; 2012; Washington, DC: Science/AAA.
Schwab KE, Hutchinson P, Gargett CE. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum Reprod. 2008;23(4):934–43. https://doi.org/10.1093/humrep/den051.
Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod. 2007;22(11):2903–11. https://doi.org/10.1093/humrep/dem265.
Matsuda H, Shi YB. An essential and evolutionarily conserved role of protein arginine methyltransferase 1 for adult intestinal stem cells during postembryonic development. Stem Cells. 2010;28(11):2073–83. https://doi.org/10.1002/stem.529.
Cervello I, Mas A, Gil-Sanchis C, Peris L, Faus A, Saunders PT, et al. Reconstruction of endometrium from human endometrial side population cell lines. PLoS One. 2011;6(6):e21221. https://doi.org/10.1371/journal.pone.0021221.
Cervello I, Gil-Sanchis C, Mas A, Delgado-Rosas F, Martinez-Conejero JA, Galan A, et al. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS One. 2010;5(6):e10964. https://doi.org/10.1371/journal.pone.0010964.
Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292(1):81–5. https://doi.org/10.1001/jama.292.1.81.
Zhao J, Zhang Q, Wang Y, Li Y. Uterine infusion with bone marrow mesenchymal stem cells improves endometrium thickness in a rat model of thin endometrium. Reprod Sci. 2015;22(2):181–8. https://doi.org/10.1177/1933719114537715.
Kilic S, Yuksel B, Pinarli F, Albayrak A, Boztok B, Delibasi T. Effect of stem cell application on Asherman syndrome, an experimental rat model. J Assist Reprod Genet. 2014;31(8):975–82. https://doi.org/10.1007/s10815-014-0268-2.
Jing Z, Qiong Z, Yonggang W, Yanping L. Rat bone marrow mesenchymal stem cells improve regeneration of thin endometrium in rat. Fertil Steril. 2014;101(2):587–94. https://doi.org/10.1016/j.fertnstert.2013.10.053.
Alawadhi F, Du H, Cakmak H, Taylor HS. Bone Marrow-Derived Stem Cell (BMDSC) transplantation improves fertility in a murine model of Asherman's syndrome. PLoS One. 2014;9(5):e96662. https://doi.org/10.1371/journal.pone.0096662.
Wolff EF, Uchida N, Donahue RE, Metzger ME, Hsieh MM, Libfraind LL, et al. Peripheral blood stem cell transplants do not result in endometrial stromal engraftment. Fertil Steril. 2013;99(2):526–32. https://doi.org/10.1016/j.fertnstert.2012.09.045.
Deane JA, Gualano RC, Gargett CE. Regenerating endometrium from stem/progenitor cells: is it abnormal in endometriosis, Asherman's syndrome and infertility? Curr Opin Obstet Gynecol. 2013;25(3):193–200. https://doi.org/10.1097/GCO.0b013e32836024e7.
Magalhaes RS, Williams JK, Yoo KW, Yoo JJ, Atala A. A tissue-engineered uterus supports live births in rabbits. Nat Biotechnol. 2020;38(11):1280–7. https://doi.org/10.1038/s41587-020-0547-7.
Magalhaes RSA, A. Regenerative Medicine for the Female Reproductive System. In: Atala AL, R.; Mikos, A. G.; Nerem, R., editor. Principles of Regenerative Medicine. Third Edition ed. Cambridege, MA 02139, USA: Academic Press, Elsevier; 2019. p. 1237-50.
Bentin-Ley U, Pedersen B, Lindenberg S, Larsen JF, Hamberger L, Horn T. Isolation and culture of human endometrial cells in a three-dimensional culture system. J Reprod Fertil. 1994;101(2):327–32. https://doi.org/10.1530/jrf.0.1010327.
Schutte SC, Taylor RN. A tissue-engineered human endometrial stroma that responds to cues for secretory differentiation, decidualization, and menstruation. Fertil Steril. 2012;97(4):997–1003. https://doi.org/10.1016/j.fertnstert.2012.01.098.
Schutte SC, James CO, Sidell N, Taylor RN. Tissue-engineered endometrial model for the study of cell-cell interactions. Reprod Sci. 2015;22(3):308–15. https://doi.org/10.1177/1933719114542008.
MacKintosh SB, Serino LP, Iddon PD, Brown R, Conlan RS, Wright CJ, et al. A three-dimensional model of primary bovine endometrium using an electrospun scaffold. Biofabrication. 2015;7(2):025010. https://doi.org/10.1088/1758-5090/7/2/025010.
Park DW, Choi DS, Ryu HS, Kwon HC, Joo H, Min CK. A well-defined in vitro three-dimensional culture of human endometrium and its applicability to endometrial cancer invasion. Cancer Lett. 2003;195(2):185–92. https://doi.org/10.1016/s0304-3835(03)00131-9.
Wang H, Pilla F, Anderson S, Martinez-Escribano S, Herrer I, Moreno-Moya JM, et al. A novel model of human implantation: 3D endometrium-like culture system to study attachment of human trophoblast (Jar) cell spheroids. Mol Hum Reprod. 2012;18(1):33–43. https://doi.org/10.1093/molehr/gar064.
Young RC, Goloman G. Allo- and xeno-reassembly of human and rat myometrium from cells and scaffolds. Tissue Eng Part A. 2013;19(19-20):2112–9. https://doi.org/10.1089/ten.TEA.2012.0549.
Lu SH, Wang HB, Liu H, Wang HP, Lin QX, Li DX, et al. Reconstruction of engineered uterine tissues containing smooth muscle layer in collagen/matrigel scaffold in vitro. Tissue Eng Part A. 2009;15(7):1611–8. https://doi.org/10.1089/ten.tea.2008.0187.
Campbell GR, Turnbull G, Xiang L, Haines M, Armstrong S, Rolfe BE, et al. The peritoneal cavity as a bioreactor for tissue engineering visceral organs: bladder, uterus and vas deferens. J Tissue Eng Regen Med. 2008;2(1):50–60. https://doi.org/10.1002/term.66.
Lin N, Li X, Song T, Wang J, Meng K, Yang J, et al. The effect of collagen-binding vascular endothelial growth factor on the remodeling of scarred rat uterus following full-thickness injury. Biomaterials. 2012;33(6):1801–7. https://doi.org/10.1016/j.biomaterials.2011.11.038.
Li X, Sun H, Lin N, Hou X, Wang J, Zhou B, et al. Regeneration of uterine horns in rats by collagen scaffolds loaded with collagen-binding human basic fibroblast growth factor. Biomaterials. 2011;32(32):8172–81. https://doi.org/10.1016/j.biomaterials.2011.07.050.
Ding L, Li X, Sun H, Su J, Lin N, Peault B, et al. Transplantation of bone marrow mesenchymal stem cells on collagen scaffolds for the functional regeneration of injured rat uterus. Biomaterials. 2014;35(18):4888–900. https://doi.org/10.1016/j.biomaterials.2014.02.046.
Song T, Zhao X, Sun H, Li X, Lin N, Ding L, et al. Regeneration of uterine horns in rats using collagen scaffolds loaded with human embryonic stem cell-derived endometrium-like cells. Tissue Eng Part A. 2015;21(1-2):353–61. https://doi.org/10.1089/ten.TEA.2014.0052.
Santoso EG, Yoshida K, Hirota Y, Aizawa M, Yoshino O, Kishida A, et al. Application of detergents or high hydrostatic pressure as decellularization processes in uterine tissues and their subsequent effects on in vivo uterine regeneration in murine models. PLoS One. 2014;9(7):e103201. https://doi.org/10.1371/journal.pone.0103201.
Taveau JW, Tartaglia M, Buchannan D, Smith B, Koenig G, Thomfohrde K, et al. Regeneration of uterine horn using porcine small intestinal submucosa grafts in rabbits. J Investig Surg. 2004;17(2):81–92. https://doi.org/10.1080/08941930490422456.
Miyazaki K, Maruyama T. Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials. 2014;35(31):8791–800. https://doi.org/10.1016/j.biomaterials.2014.06.052.
Hellstrom M, Moreno-Moya JM, Bandstein S, Bom E, Akouri RR, Miyazaki K, et al. Bioengineered uterine tissue supports pregnancy in a rat model. Fertil Steril. 2016;106(2):487–96 e1. https://doi.org/10.1016/j.fertnstert.2016.03.048.
Hellstrom M, El-Akouri RR, Sihlbom C, Olsson BM, Lengqvist J, Backdahl H, et al. Towards the development of a bioengineered uterus: comparison of different protocols for rat uterus decellularization. Acta Biomater. 2014;10(12):5034–42. https://doi.org/10.1016/j.actbio.2014.08.018.
Kessler M, Hoffmann K, Brinkmann V, Thieck O, Jackisch S, Toelle B, et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun. 2015;6:8989. https://doi.org/10.1038/ncomms9989.
Alborzi S, Momtahan M, Parsanezhad ME, Yazdani M. Successful treatment of cervical aplasia using a peritoneal graft. Int J Gynaecol Obstet. 2005;88(3):299–302. https://doi.org/10.1016/j.ijgo.2004.12.016.
Ding JX, Chen XJ, Zhang XY, Zhang Y, Hua KQ. Acellular porcine small intestinal submucosa graft for cervicovaginal reconstruction in eight patients with malformation of the uterine cervix. Hum Reprod. 2014;29(4):677–82. https://doi.org/10.1093/humrep/det470.
Mhaskar R. Amniotic membrane for cervical reconstruction. Int J Gynaecol Obstet. 2005;90(2):123–7. https://doi.org/10.1016/j.ijgo.2005.04.015.
House M, Sanchez CC, Rice WL, Socrate S, Kaplan DL. Cervical tissue engineering using silk scaffolds and human cervical cells. Tissue Eng Part A. 2010;16(6):2101–12. https://doi.org/10.1089/ten.TEA.2009.0457.
De Filippo RE, Yoo JJ, Atala A. Engineering of vaginal tissue in vivo. Tissue Eng. 2003;9(2):301–6. https://doi.org/10.1089/107632703764664765.
Farahat YA, Elbendary MA, Elgamal OM, Tawfik AM, Bastawisy MG, Radwan MH, Rasheed M. Application of small intestinal submucosa graft for repair of complicated vesicovaginal fistula: a pilot study. J Urol. 2012;188(3):861–4. https://doi.org/10.1016/j.juro.2012.05.019.
Farahat YA, Elbendary MA, Elgamal OM, Tawfik AM, Bastawisy MG, Radwan MH, et al. Application of small intestinal submucosa graft for repair of complicated vesicovaginal fistula: a pilot study. J Urol. 2012;188(3):861–4. https://doi.org/10.1016/j.juro.2012.05.019.
Zhou Q, Chen X, Luo X, Ding J, Zhang G, Ren Y, et al. Laparoscopic-assisted uterovaginal anastomosis for uterine cervix atresia with vaginal aplasia using a silicone stent lined with acellular porcine small intestinal submucosa graft inserted using a 16F Foley catheter. J Minim Invasive Gynecol. 2013;20(5):710–3. https://doi.org/10.1016/j.jmig.2013.03.010.
Lemos NL, Kamergorodsky G, Faria AL, Ribeiro PA, Auge AP, Aoki T. Small intestinal submucosa patch for extensive vaginal endometriosis resection. J Minim Invasive Gynecol. 2009;16(6):765–7. https://doi.org/10.1016/j.jmig.2009.07.007.
Ho MH, Heydarkhan S, Vernet D, Kovanecz I, Ferrini MG, Bhatia NN, et al. Stimulating vaginal repair in rats through skeletal muscle-derived stem cells seeded on small intestinal submucosal scaffolds. Obstet Gynecol. 2009;114(2 Pt 1):300–9. https://doi.org/10.1097/AOG.0b013e3181af6abd.
Ding JX, Zhang XY, Chen LM, Hua KQ. Vaginoplasty using acellular porcine small intestinal submucosa graft in two patients with Meyer-von-Rokitansky-Kuster-Hauser syndrome: a prospective new technique for vaginal reconstruction. Gynecol Obstet Investig. 2013;75(2):93–6. https://doi.org/10.1159/000343233.
Hilger WS, Walter A, Zobitz ME, Leslie KO, Magtibay P, Cornella J. Histological and biomechanical evaluation of implanted graft materials in a rabbit vaginal and abdominal model. Am J Obstet Gynecol. 2006;195(6):1826–31. https://doi.org/10.1016/j.ajog.2006.07.006.
Zhu L, Zhou H, Sun Z, Lou W, Lang J. Anatomic and sexual outcomes after vaginoplasty using tissue-engineered biomaterial graft in patients with Mayer-Rokitansky-Kuster-Hauser syndrome: a new minimally invasive and effective surgery. J Sex Med. 2013;10(6):1652–8. https://doi.org/10.1111/jsm.12143.
Lee CL, Wang CJ, Liu YH, Yen CF, Lai YL, Soong YK. Laparoscopically assisted full thickness skin graft for reconstruction in congenital agenesis of vagina and uterine cervix. Hum Reprod. 1999;14(4):928–30. https://doi.org/10.1093/humrep/14.4.928.
Mahdy A, Karp D, Davila GW, Ghoniem GM. The outcome of transobturator anterior vaginal wall prolapse repair using porcine dermis graft: intermediate term follow-up. Int Braz J Urol. 2013;39(4):506–12. https://doi.org/10.1590/S1677-5538.IBJU.2013.04.08.
Imparato E, Alfei A, Aspesi G, Meus AL, Spinillo A. Long-term results of sigmoid vaginoplasty in a consecutive series of 62 patients. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18(12):1465–9. https://doi.org/10.1007/s00192-007-0358-0.
Baytekin C, Menderes A, Mola F, Balik O, Tayfur V, Vayvada H. Total vaginal reconstruction with combined 'Split Labia Minora Flaps' and full-thickness skin grafts. J Obstet Gynaecol Res. 2007;33(4):524–8. https://doi.org/10.1111/j.1447-0756.2007.00575.x.
Hallberg H, Holmstrom H. Vaginal construction with skin grafts and vacuum-assisted closure. Scand J Plast Reconstr Surg Hand Surg. 2003;37(2):97–101. https://doi.org/10.1080/02844310310005621.
Umoh UE, Arya LA. Surgery in urogynecology. Minerva Med. 2012;103(1):23–36.
Dissaranan C, Cruz MA, Kiedrowski MJ, Balog BM, Gill BC, Penn MS, et al. Rat mesenchymal stem cell secretome promotes elastogenesis and facilitates recovery from simulated childbirth injury. Cell Transplant. 2014;23(11):1395–406. https://doi.org/10.3727/096368913X670921.
Cruz M, Dissaranan C, Cotleur A, Kiedrowski M, Penn M, Damaser M. Pelvic organ distribution of mesenchymal stem cells injected intravenously after simulated childbirth injury in female rats. Obstet Gynecol Int. 2012;2012:612946. https://doi.org/10.1155/2012/612946.
De Filippo RE, Bishop CE, Filho LF, Yoo JJ, Atala A. Tissue engineering a complete vaginal replacement from a small biopsy of autologous tissue. Transplantation. 2008;86(2):208–14. https://doi.org/10.1097/TP.0b013e31817f1686.
Dorin RP, Atala A, Defilippo RE. Bioengineering a vaginal replacement using a small biopsy of autologous tissue. Semin Reprod Med. 2011;29(1):38–44. https://doi.org/10.1055/s-0030-1268702.
Eddie SL, Kim JJ, Woodruff TK, Burdette JE. Microphysiological modeling of the reproductive tract: a fertile endeavor. Exp Biol Med (Maywood). 2014;239(9):1192–202. https://doi.org/10.1177/1535370214529387.
Lee JS, Romero R, Han YM, Kim HC, Kim CJ, Hong JS, et al. Placenta-on-a-chip: a novel platform to study the biology of the human placenta. J Matern Fetal Neonatal Med. 2016;29(7):1046–54. https://doi.org/10.3109/14767058.2015.1038518.
Blundell C, Tess ER, Schanzer AS, Coutifaris C, Su EJ, Parry S, et al. A microphysiological model of the human placental barrier. Lab Chip. 2016;16(16):3065–73. https://doi.org/10.1039/c6lc00259e.
Li WLG, Yan W, Zhang Q, Wang W, Zhou X, Liu D. Artificial Uterus on a Microfluidic Chip. Chin J Anal Chem. 2013;41(4):467–72.
Xiao SCJR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, McKinnon KE, et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun. 2017;8:14584.
Acknowledgments
The author(s) acknowledge the following financial support for the research, authorship, and/or publication of this article: The present study was supported, in part, by the Shulsky Foundation and a grant from The State of North Carolina.
Funding
This work was funded by The Shulsky Foundation and a grant from The State of North Carolina.
Author information
Authors and Affiliations
Contributions
James J. Yoo and Anthony Atala conceived the subject of this review. Sivanandane Sittadjody performed the literature search and wrote the manuscript. Tracy Criswell and John D. Jackson critically revised the draft.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable
Consent to Participate
Not applicable
Consent for Publication
All authors have reviewed the final manuscript and consent for publication.
Conflict of Interest
The author(s) declare no potential conflicts of interest with respect to research, authorship, and/or publication of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sittadjody, S., Criswell, T., Jackson, J.D. et al. Regenerative Medicine Approaches in Bioengineering Female Reproductive Tissues. Reprod. Sci. 28, 1573–1595 (2021). https://doi.org/10.1007/s43032-021-00548-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00548-9