Abstract
We undertook meta-analyses on MTHFR 1298A>C substitution for critically evaluating its association with recurrent pregnancy loss (RPL). MTHFR genotype data for 5888 cases and 8401 controls from 39 studies were pooled to perform this meta-analyses. Genotype data were screened, scrutinized, pooled, analysed and subjected to sensitivity analysis to carefully evaluate the association between MTHFR 1298A>C and recurrent pregnancy loss. Genetic associations were sought using dominant, recessive and co-dominant models of genetic testing with odds ratio and 95% Confidence interval (CI) as the effect measures. Further analyses were undertaken by classifying the studies into Caucasian and East Asian sub-groups. Genetic heterogeneity was tested before pooling the data across studies. For assessing publication bias, Egger’s intercept test was undertaken. We found a significant association of 1298A>C substitution with increased risk of RPL in the dominant (P=0.000; OR = 1.58; 95% CI =1.25–1.99) as well as recessive (P=0.000; OR = 1.66; 95% CI =1.25–2.20) models. In sub-group analysis, we observed a significant association of the polymorphism with RPL in the Caucasian populations using dominant (P=0.000; OR = 1.98; 95% CI =1.42–2.76) and recessive (P=0.000; OR = 2.20; 95% CI =1.49–3.24) models. However, this substitution showed no association with RPL in the East Asian populations (P=0.149; OR = 1.187; 95% CI =0.94–1.50). MTHFR 1298A>C substitution shows association with the risk of recurrent pregnancy loss. The association is in a population-specific manner with the substitution being a strong risk factor only in the Caucasian populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spontaneous loss of a pregnancy before the foetus reaches viability is known as a miscarriage. By definition, “recurrent” pregnancy loss (RPL) is defined as the loss of two or more pregnancies occurring within 20–24 weeks of conception. RPL generally occurs with an incidence as high as 3–5% of all pregnancies [1, 2]. The cause of RPL in as high as 50% of the patients cannot be ascertained [3, 4]. Miscarriage is a loss of a newborn for most people and their families as well as the loss of dreams and aspirations they believe in [4, 5]. It is a traumatic event which affects every woman differently, but can lead to grief, anxiety, depression, and even symptoms of post-traumatic stress disorder (PTSD) [6]. Pregnancy loss can also affects male partners equally significantly [7].
Folic acid is essential for pregnant women as it is critical for the synthesis of DNA, RNA and metabolism of amino acids. Single carbon derivatives of folate participate in DNA synthesis [8]. After three consequent reductions, folate forms 5,10-methylenetetrahydrofolate by the addition of a methylene bridge from formate, serine or glycine. Subsequently, 5,10-methylenetetrahydrofolate is irreversibly reduced to 5-methyltetrahydrofolate by the action of methylenetetrahydrofolate reductase (MTHFR). 5,10-Methylenetetrahydrofolate is used by methionine synthase to transform homocysteine (Hcy) into methionine, which retains and preserves homocysteine in the blood at a normal level [9]. A high level of homocysteine, termed hyperhomocysteinemia, has been reported to associate with complications in pregnancy [10] and adverse events in the cardiovascular system [11]. This suggests an important role of folic acid and one carbon cycle in foetal development and the success of pregnancy.
Several studies found that MTHFR (methylenetetrahydrofolate reductase) gene mutations increase the risk of recurrent pregnancy loss (RPL) before completion of 20 weeks of the gestation period [12]. MTHFR polymorphisms 677C>T (rs1801133) and 1298A>C (rs1801131) have been widely studied in RPL and found to increase RPL risk [13, 14]. Elevated homocysteine levels and low folic acid levels can lead to complications of pregnancy, including miscarriage, preeclampsia, and other congenital disabilities [15,16,17]. MTHFR 677C>T or 1298A>C polymorphisms have also been correlated with hyperhomocysteinemia. Eventually, MTHFR 677C>T has been shown to significantly increase the risk of miscarriage in Asians, which was also supported by meta-analyses [18,19,20,21]. Second important polymorphism in this gene, 1298A>C, has been considered to be a risk factor for pregnancy loss [22, 23].
In a previous study, we reported highly significant association of 1298A>C substitution with RPL [22]. With the availability of human genome sequence, comprehensive genetic testing of mothers would be an attractive option. However, in RPL, most of the studies have focussed on the foetal causes of pregnancy loss, which emphasizes on chromosomal aneuploidies as a major contributor [24, 25]. Nonetheless, maternal causes would also contribute significantly to pregnancy loss and genetic testing of the mother can provide significant clues for the management of RPL. While genetic testing panels for a number of human diseases have been launched, RPL in this regard is in infancy [26,27,28]. In order to rigorously evaluate the candidature of MTHFR 1298A>C polymorphism for genetic testing in RPL, we undertook this meta-analysis on 39 studies with 5888 cases and 8401 controls. We found that 1298A>C substitution increases the risk of RPL significantly and this variant could be adopted for analysis in clinical settings. Interestingly, this can help in appropriate management of the RPL cases by adjusting the folate and vitamin B12 dosage of the patients.
Material and Methods
Search Strategy
A comprehensive screening of published studies in the online repositories of Medline (PubMed), GoogleScholar, EmBase, ScienceDirect, Ovid Science Citation Index (SCI) and Cochrane Library was conducted for identifying studies published until October 2019. Keywords MTHFR, persistent pregnancy loss, 1298A>C polymorphism, methylenetetrahydrofolate reductase, miscarriage and spontaneously aborted embryos were searched in different combinations. All articles thus identified were manually reviewed for the identification of additional citations. This was followed by acquiring full text of relevant articles.
Inclusion and Exclusion Criteria
Studies fulfilling the following criteria were included: (i) case-control studies analysing MTHFR 1298A>C polymorphism in recurrent pregnancy loss; (ii) genotype analysis should involve the use of standard genotyping methods; (iii) sufficient details of genotype data were provided for calculation of the odds ratio (ORs) and confidence interval (CI). We omitted articles including review, meta-analysis and those on other pregnancies complications such as recurrent implant failure, foetal death and spontaneous abortion with foetal chromosomal aneuploidy. We also excluded those studies which were covered under broad title of adverse pregnancy outcomes.
Data Extraction
The following information were extracted from each of the full text articles: first author’s name, year of publication, country of origin, race/ethnicity, number of cases/controls and genotypes. Information was independently extracted by two authors (PM and RV) to avoid errors. In the event of a dispute, the senior author (SR) was consulted. Data extracted from each included study are detailed in Table 1. Genotype frequencies of control samples for each study were checked for their goodness of fit in the Hardy-Weinberg equilibrium. The genotype frequencies and controls was compared using 95% CI, OR and chi-square. P < 0.05 was judged to be significant.
Data Analysis
We used odds ratio (ORs) and 95% confidence interval for assessment of the association of 1298A>C substitution with RPL. The association was tested using dominant (AA vs AC+CC), recessive (AA+AC vs CC) and co-dominant (AC vs AC; AA vs CC; AC vs CC) models of analysis. Since different studies were conducted under different settings, significant heterogeneity across studies may affect the results of pooled analysis. For this, we analysed data using fixed effect and random effects models of pooled data analysis. The heterogeneity between the studies was quantitatively assessed using Q statistics, considering P value less than 0.10 as statistically significant. Heterogeneity index (I2) value < 25% means no heterogeneity, 50% means moderate heterogeneity and 75% and above corresponds to high heterogeneity [67]. Pooled OR was calculated using both the fixed effect and random effects models and high-resolution plots (forest plot) were generated. The inference was made using fixed or random effects model of analysis based upon the level of heterogeneity.
The publication bias was tested by funnel plot asymmetry and the Egger’s Regression Intercept Test. Sensitivity analysis was conducted by eliminating one study at a time and re-estimating the OR on the remaining data sets. We also conducted sensitivity analysis on studies using three or more abortions as the criteria defining recurrent pregnancy loss.
For sub-group analysis, ethnicity was classified as East Asian, Caucasian and others, but sub-group meta-analysis was conducted only in the groups having more than three studies. We identified that 16 studies were conducted on East Asian populations, 20 were conducted on Caucasian populations, and three studies did not fit in any major ethnic group.
Results
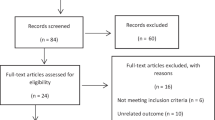
Since the objective of this analysis was to test the association of 1298A>C polymorphism with RPL, we selected only those studies which had clearly stated analysis on recurrent pregnancy loss cases only. After applying inclusion and exclusion criteria, data from 5888 cases and 8401 controls from 39 studies were included in the analysis (Fig. 1).
1298 A>C Substitution Increases the Risk of RPL
In the dominant model (AA vs AC+CC), the I2 value was 87.44, showing a high level of heterogeneity (Q =302.56; P=0.000; I2 = 87.44; t2 = 0.44; SE =0.15). Therefore, the random effects model was applied for drawing the inference (Table 2). The pooled analysis using random effects model showed that the mutant genotype was a significant risk factor for recurrent pregnancy loss (P=0.000; OR = 1.58; 95% CI =1.25–1.99) (Fig. 2). An almost symmetric distribution of studies on the funnel plot suggested the absence of publication bias (Supplementary Fig. 1). Egger’s test for intercept confirmed no publication bias (intercept = 1.85; SE = 1.06; 95% CI = −0.30–4.00; P2-tailed=0.08) (Table 2).
Forest plot for association of MTHFR 1298A>C polymorphism with RPL risk using the dominant (AA vs AC+CC) model in all populations. The Z value shows the degree and direction of relationship, whereas the P value shows the significance of the relationship. The horizontal bar shows the 95% CI with OR in the centre. The direction of projection of the horizontal bar shows the direction of association. The diamond-shaped box shows the pooled OR and its width indicates the 95% CI
In the recessive model (AA+ AC versus CC), the I2 value was 62.14, showing a high level of heterogeneity (Q =97.73; P=0.000; I2 = 62.14; t2 = 0.36; SE =0.17). Therefore, we used the random effects model for drawing the inference (Table 2). The pooled analysis using random effects model showed a highly significant increase in the risk of recurrent pregnancy loss in mutation carriers (P=0.000; OR = 1.66; 95% CI =1.25–2.20) (Fig. 3). Funnel plot showed asymmetrical distribution of the studies, suggesting publication bias (Supplementary Fig. 1), which was confirmed by Egger’s test for intercept (intercept = 1.64; SE = 0.41; 95% CI = 0.81–2.50; P2-tailed=0.000) (Table 2).
1298 A>C Substitution Increases the Risk of RPL in Caucasians
A total of 24 studies were conducted on Caucasian (Austrian, Czech Republican, Egyptian, Iranian, Indian, Italian, Mexican, Polish, Spanish, Syrian, Tunisian and Turkish) populations, consisting of 3953 cases and 6251 controls.
In the dominant model (AA vs AC+CC), the I2 value was 90.91, suggesting a high level of heterogeneity (Q =253.22; P=0.000; I2 = 90.91; t2 = 0.582; SE =0.25). Therefore, we used the random effects model for drawing the inference (Table 2). The pooled analysis using random effects model showed a highly significant association of 1298A>C substitution with increased risk of recurrent pregnancy loss (P=0.000; OR = 1.98; 95% CI =1.42–2.76) (Fig. 4). An almost symmetric distribution of studies on the funnel plot shows the absence of publication bias (Supplementary Fig. 1). Egger’s test for intercept confirmed no publication bias (intercept = 2.91; SE = 1.46; 95% CI = −0.11–5.95; P2-tailed=0.58) (Table 2).
In the recessive model (AA+AC vs CC), the I2 value was 71.30, suggesting a high level of heterogeneity (Q =76.65; P=0.000; I2 = 71.30; t2 = 0.50; SE =0.07). Therefore, we used random effects model for overall inference (Table 2). The pooled analysis using random effects model showed a highly significant association of 1298A>C substitution with increased risk of recurrent pregnancy loss (P=0.000; OR = 2.20; 95% CI =1.49–3.24) (Fig. 5). Funnel plot showed asymmetric distribution of the studies (Supplementary Fig. 1), suggesting publication bias, which was confirmed by Egger’s test for intercept (intercept = 2.35; SE = 0.50 95% CI = 1.37–3.33; P2-tailed=0.000) (Table 2).
1298 A>C Substitution Does Not Affect RPL Risk in East Asians
A total of 12 studies were conducted on East Asian (Chinese and Korean) populations, consisting of 1468 cases and 1664 controls. In the dominant model (AA vs AC+CC), the I2 value was 44.50, suggesting a moderate, but significant level of heterogeneity (Q =19.82; P=0.048; I2 = 44.50; t2 = 0.07; SE =0.07). Therefore, we used the random effects model for inference (Table 2). The pooled analysis using random effects model showed no significant association between 1298A>C substitution and the risk of recurrent pregnancy loss (P=0.149; OR = 1.187; 95% CI =0.94–1.50) (Fig. 6). An almost symmetric distribution of studies on the funnel plot suggested the absence of publication bias (Supplementary Fig. 1). Egger’s test for intercept confirmed no publication bias (intercept = 0.99; SE = 1.00; 95% CI = −1.23–3.23; P2-tailed=0.34) (Table 2).
In the recessive model (AA vs AC+CC), the I2 value was 30.79 in the East Asian group, suggesting no significant heterogeneity (Q =15.90; P=0.14; I2 = 30.79; t2 = 0.20; SE =0.30). Therefore, we used the fixed effect model for inference (Table 2). The pooled analysis using fixed effect model showed no significant association of 1298A>C with the risk of recurrent pregnancy loss (P=0.88; OR = 1.03; 95% CI =0.71–1.50) (Fig. 7). An almost symmetric distribution of studies on the funnel plot shows the absence of publication bias (Supplementary Fig. 1). Egger test for intercept confirmed no publication bias (intercept = 1.22; SE = 1.10 95% CI = −1.15–3.60; P2-tailed=0.28) (Table 2).
Sensitivity Analysis
Since the presence of sensitive studies can affect the overall inference, we conducted sensitivity analysis using two methods. The studies failing to fit in the Hardy-Weinberg equilibrium and those with very low sample size (<100 in either group) were excluded, followed by re-analysis of data each time. However, this did not change the results significantly and there was no change in the inference (Tables 3 and 4). Hence, no study was considered to be sensitive for inclusion in the pooled analysis.
In another set of sensitivity analysis, we undertook meta-analyses on studies recruiting cases with three or more pregnancy losses as the defining criteria. This also showed a significant association of 1298A>C polymorphism with recurrent pregnancy loss in both dominant and recessive models of genetic analyses (Fig. 8)
Discussion
Post-fertilization development requires massive cellular proliferation and differentiation to support the rapid pace of foetal development. DNA replication during this process demands nucleotide synthesis, which is critically dependent upon the folate cycle. The importance of folate in pregnancy has been studied since long with its first ever study published in the year 1964 [68]. Folic acid is taken from diet, which is converted to its active form, i.e. 5-MTHF. Dietary folate upon conversion to intermediate products regulates the synthesis of precursor molecules for DNA synthesis, protein synthesis and DNA methylation and gene imprinting (Fig. 9). DNA methylation is essential for regulating gene expression, which is critical for early development [69]. Methylene tetrahydrofolate enzyme is central to the folate cycle, one carbon cycle and methionine cycle (Fig. 9). Functional polymorphism, 677C>T causing Ala222Val substitution, results in a thermolabile enzyme with reduced activity [70]. Similarly, another functional polymorphism, 1298A>C causing Glu429Ala substitution, results in decreased enzyme activity [71]. As a result of disturbed folate cycle, MTHFR mutations have also been linked to chromosomal anomalies in the developing foetus [39, 72], hypo-methylation during trophoblast development [73] and pregnancy loss or neural tube defects in live birth cases [74]. Foetal chromosomal abnormalities and developmental defects are a major cause of recurrent pregnancy loss [24, 25]. Therefore, these polymorphisms have a mechanistic link with pregnancy loss.
Due to its undisputed importance, folic acid is prescribed before and during pregnancy. According to the Center for Disease Control and Prevention (CDC), 400 mcg/day of folic acid is required, which is critical during pregnancy as it can permanently affect the foetal development. However, the use is indiscriminate in the lack of ways to figure out individual requirements. The need for genetic testing before prescribing medicine is the key to the personalized medicine. Dietary folate is converted to active folate depending upon the availability and activity of the MTHFR enzyme. Individuals homozygous for the variant allele of 677C>T polymorphism are poor folate metabolizers because of only 30% enzyme activity available as compared to the wild-type enzyme and heterozygotes have 65% of enzyme activity [75]. The individuals with 1298A>C homozygous substitution have about 68% of enzyme activity [76]. The presence of both these polymorphisms would further reduce enzyme activity. Thus, these polymorphisms dictate the level of active folate. A decreased enzyme activity of MTHFR can reduce the transition of homocysteine to methionine, resulting in the deposition of homocysteine in the blood vessels [77]. Hyperhomocysteinemia can cause endothelial vascular damage and lead to blood clots during pregnancy [10, 78]. Inadequate foetal blood flow and villous necrosis due to placental artery embolization may ultimately lead to pregnancy loss [79].
The availability of complete human genome sequence has boosted genetic diagnosis of a number of health conditions. In a previous meta-analysis, we reported a significant association of 1298A>C polymorphism with RPL risk, suggesting it to be a good candidate for genetic analysis in RPL cases. But MTHFR polymorphisms have not yet been adopted in clinical testing of RPL. A number of recent studies have further suggested its importance in RPL. The present meta-analysis on 5888 cases and 8401 controls established 1298A>C to be a significant risk factor for RPL in an ethnic-specific manner. This meta-analysis suggested 1298A>C to be a significant risk factors in the Caucasians, but not in East Asians. The latter is in agreement with previous studies on the East Asian populations [19, 20]. In contrast, 677C>T polymorphism affects RPL risk in East Asians only [19,20,21, 49]. There were only two studies on Brazilian populations and only one on Sinhalese, which forced their exclusion from sub-group analysis. Since both these polymorphisms affect MTHFR significantly, some studies have also analysed both the polymorphisms, showing a significant association of compound heterozygote combination (677C>T/1298A>C) with RPL [51, 55, 58, 66]. Variations in the association across ethnic populations could be partly due to the dietary variations which affect folate availability.
To date, there have been a few meta-analyses for association between MTHFR 1298 A>C polymorphism and the risk of recurrent pregnancy loss. Most of the meta-analyses till date have been conducted on Asian populations, showing no association of 1298A>C substitution with RPL [19, 49, 80]. Similarly, another meta-analysis also showed no significant association of 1298A>C with RPL in Asians [81]. Later on, a Chinese group conducted a meta-analysis on 2924 maternal cases, 375 foetal cases and 327 paternal cases and found that the maternal and paternal MTHFR 677C>T and 1298A>C polymorphisms are associated with RPL [23]. Previously, we performed a meta-analysis on 1,080 maternal cases and 709 controls and 375 case and 384 control samples of spontaneously aborted embryos, which suggested a significant association of this polymorphism with increased risk of pregnancy loss in the carriers of AC and CC genotypes [22]. Similarly, in the case of Iranian populations, 1298A>C showed a significant association with RPL in all genetic models [82]. Most of these studies suggest a significant association of MTHFR 1298A>C substitution with an increased risk of RPL in populations other than Asians.
There is evidence that MTHFR genotyping can help in the management of RPL. The individuals with MTHFR mutations have low enzyme activity to convert folate into its active form, thus slowing the whole pathway. A few recent studies have undertaken MTHFR genotyping in the course of RPL management. Servy et al. (2018) prescribed folate (5 mg/day) to women with MTHFR mutations, but without any improvement; however, administration of the active form of folate, i.e. 5-MTHF (600mcg/day), resulted in spontaneous pregnancies [83]. Some other studies have tried different combinations of medicines for RPL management in consultation with MTHFR genotyping. For example, Merviel et al. (2017) recruited MTHFR 677C>T mutant females to study their response to treatment. Folate (5 mg/day) and aspirin with or without enoxaparin (0.4 mg/day) were administered. The authors observed a much higher delivery rate in the group receiving anti-coagulant (79.7%) in comparison to those receiving only folate and aspirin (46.3%) [84]. Though the exact reason for better efficacy of the treatment regimen incorporating the anti-coagulant could be complex, this may be related to blood clots in fine vasculature as a result of MTHFR mutations and hyper-homocysteinemia [85]. Similarly, another case of RPL benefitted from MTHFR testing. A patient with a history of several miscarriages and hypothyroidism was treated with low dose aspirin, enoxaparin, LT4 (levothyroxine) and folic acid (5 mg/day), but she still faced two miscarriages at the 14th week of gestation. MTHFR testing identified the patient to be 1298A>C homozygous, and a change from folate to 5-MTHF and cobalamin in this patient resulted in a successful birth [86]. From these evidences, it is clear that MTHFR genotyping can help in the management of RPL and also in ensuring successful birth in pregnancy loss patients.
Heterogeneity across the studies and small sample size in some of the studies could be considered as the limitation of this meta-analysis. We tried to overcome the limitations of heterogeneity by selecting random effects model wherever applicable, and the issue of small sample size was taken care by undertaking a sensitivity analysis. Overall, the present analysis suggests that MTHFR 1298A>C has a significant association with the risk of recurrent pregnancy loss. Similarly, MTHFR 677C>T has already been established to be a significant risk factor for RPL. This suggests the advantage of genotyping of these two polymorphisms before planning a pregnancy or in infertility clinics for better management of infertility and pregnancy loss. Since genetic polymorphisms show a population-specific penetration, population-wise analysis is the key to the personalized medicine. 1298A>C is a significant risk factor only in the Caucasian populations, while 677C>T polymorphism is a significant risk factor only in the East Asian populations. This meta-analysis in view of previous studies on MTHFR gene polymorphisms emphasizes that MTHFR 1298A>C and 677C>T analysis could be included in the clinical workup during pregnancy, if not before. This can be of great help in reducing the burden of pregnancy loss and offer higher success rate in infertility patients who had difficulty in conceiving. MTHFR genotyping in clinical management of pregnancy loss is in infancy. Identification of other similar risk factors would pave the way to the development of a genetic screening panel for pregnancy loss to guide its management.
Data Availability
Not applicable
References
Abu-Asab NS, Ayesh SK, Ateeq RO, Nassar SM, El-Sharif WA. Association of inherited thrombophilia with recurrent pregnancy loss in palestinian women. Obstet Gynecol Int Hindawi. 2011;2011.
Greenberg JA, Bell SJ, Guan Y, Yu Y. Folic acid supplementation and pregnancy: more than just neural tube defect prevention. Rev Obstet Gynecol. MedReviews, LLC. 2011;4:52.
Christiansen OB. Evidence-based investigations and treatments of recurrent pregnancy loss. Curr Opin Obstet Gynecol. LWW. 2006;18:304–12.
Sugiura-Ogasawara M. Recurrent pregnancy loss and obesity. Best Pract Res Clin Obstet Gynaecol. Elsevier. 2015;29:489–97.
Kolte AM, Bernardi LA, Christiansen OB, Quenby S, Farquharson RG, Goddijn M, et al. Terminology for pregnancy loss prior to viability: a consensus statement from the ESHRE early pregnancy special interest group. Hum Reprod. Oxford University Press. 2015;30:495–8.
Farren J, Jalmbrant M, Ameye L, Joash K, Mitchell-Jones N, Tapp S, Timmerman D, Bourne T. Post-traumatic stress, anxiety and depression following miscarriage or ectopic pregnancy: a prospective cohort study. BMJ Open. 2016;6:e011864.
Boynton P. Miscarriage: you don’t have to be strong for me. Lancet. Elsevier. 2015;385:222–3.
Laanpere M, Altmäe S, Stavreus-Evers A, Nilsson TK, Yngve A, Salumets A. Folate-mediated one-carbon metabolism and its effect on female fertility and pregnancy viability. Nutr Rev. Oxford University Press Oxford, UK. 2010;68:99–113.
Summers CM, Mitchell LE, Stanislawska-Sachadyn A, Baido SF, Blair IA, Von Feldt JM, et al. Genetic and lifestyle variables associated with homocysteine concentrations and the distribution of folate derivatives in healthy premenopausal women. Birth Defects Res A Clin Mol Teratol. Wiley Online Library. 2010;88:679–88.
Sztenc S. Hyperhomocysteinemia and pregnancy complications. Ginekol Pol. 2004;75:317–25.
Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. BioMed Central. 2015;14:1–10.
Regan L, Rai R. Epidemiology and the medical causes of miscarriage. Best Pract Res Clin Obstet Gynaecol. Elsevier. 2000;14:839–54.
Ni W, Li H, Wu A, Zhang P, Yang H, Yang X, et al. Lack of association between genetic polymorphisms in three folate-related enzyme genes and male infertility in the Chinese population. J Assist Reprod Genet. Springer. 2015;32:369–74.
Wu X, Yang K, Tang X, Sa Y, Zhou R, Liu J, et al. Folate metabolism gene polymorphisms MTHFR C677T and A1298C and risk for preeclampsia: a meta-analysis. J Assist Reprod Genet. Springer. 2015;32:797–805.
Sohda S, Arinami T, Hamada H, Yamada N, Hamaguchi H, Kubo T. Methylenetetrahydrofolate reductase polymorphism and pre-eclampsia. J Med Genet. BMJ Publishing Group Ltd. 1997;34:525–6.
Wang J, Trudinger BJ, Duarte N, Wilcken DE, Li WX. Elevated circulating homocyst (e) ine levels in placental vascular disease and associated pre-eclampsia. BJOG. Wiley Online Library. 2000;107:935–8.
Chaudhry SH, Taljaard M, MacFarlane AJ, Gaudet LM, Smith GN, Rodger M, et al. The role of maternal homocysteine concentration in placenta-mediated complications: findings from the Ottawa and Kingston birth cohort. BMC Pregnancy Childbirth. Springer. 2019;19:75.
Ren A, Wang J. Methylenetetrahydrofolate reductase C677T polymorphism and the risk of unexplained recurrent pregnancy loss: a meta-analysis. Fertil Steril. Elsevier. 2006;86:1716–22.
Chen H, Yang X, Lu M. Methylenetetrahydrofolate reductase gene polymorphisms and recurrent pregnancy loss in China: a systematic review and meta-analysis. Arch Gynecol Obstet. Springer. 2016;293:283–90.
Wu X, Zhao L, Zhu H, He D, Tang W, Luo Y. Association between the MTHFR C677T polymorphism and recurrent pregnancy loss: a meta-analysis. Genet Test Mol Biomarkers. Mary Ann Liebert, Inc. 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA. 2012;16:806–11.
Rai V. Methylenetetrahydrofolate reductase C677T polymorphism and recurrent pregnancy loss risk in Asian population: a meta-analysis. Indian J Clin Biochem. Springer. 2016;31:402–13.
Nair RR, Khanna A, Singh R, Singh K. Association of maternal and fetal MTHFR A1298C polymorphism with the risk of pregnancy loss: a study of an Indian population and a meta-analysis. Fertil Steril. Elsevier. 2013;99:1311–8.
Yang Y, Luo Y, Yuan J, Tang Y, Xiong L, Xu M, et al. Association between maternal, fetal and paternal MTHFR gene C677T and A1298C polymorphisms and risk of recurrent pregnancy loss: a comprehensive evaluation. Arch Gynecol Obstet. Springer. 2016;293:1197–211.
Popescu F, Jaslow CR, Kutteh WH. Recurrent pregnancy loss evaluation combined with 24-chromosome microarray of miscarriage tissue provides a probable or definite cause of pregnancy loss in over 90% of patients. Hum Reprod. Oxford University Press. 2018;33:579–87.
Sato T, Sugiura-Ogasawara M, Ozawa F, Yamamoto T, Kato T, Kurahashi H, et al. Preimplantation genetic testing for aneuploidy: a comparison of live birth rates in patients with recurrent pregnancy loss due to embryonic aneuploidy or recurrent implantation failure. Hum Reprod. Oxford University Press. 2019;34:2340–8.
Rull K, Nagirnaja L, Laan M. Genetics of recurrent miscarriage: challenges, current knowledge, future directions. Front Genet. Frontiers. 2012;3:34.
Hyde KJ, Schust DJ. Genetic considerations in recurrent pregnancy loss. Cold Spring Harb Perspect Med. 2015;5:a023119.
Quintero-Ronderos P, Mercier E, Fukuda M, González R, Suárez CF, Patarroyo MA, et al. Novel genes and mutations in patients affected by recurrent pregnancy loss. PLoS One. Public Library of Science San Francisco, CA USA. 2017;12:e0186149.
Hohlagschwandtner M, Unfried G, Heinze G, Huber JC, Nagele F, Tempfer C. Combined thrombophilic polymorphisms in women with idiopathic recurrent miscarriage. Fertil Steril. Elsevier. 2003;79:1141–8.
Li XM, Zhang YZ, Xu YX, Jiang S. Study on the relationship of MTHFR polymorphisms with unexplained recurrent spontaneous abortion. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2004;21:39–42.
Mtiraoui N, Zammiti W, Ghazouani L, Braham NJ, Saidi S, Finan RR, et al. Methylenetetrahydrofolate reductase C677T and A1298C polymorphism and changes in homocysteine concentrations in women with idiopathic recurrent pregnancy losses. Reproduction. Society for Reproduction and Fertility. 2006;131:395–401.
Wang X, Ma Z, Lin Q. Inherited thrombophilia in recurrent spontaneous abortion among Chinese women. Int J Gynaecol Obstet. 2006;92:264–5.
Callejon G, Mayor-Olea A, Jimenez AJ, Gaitán MJ, Palomares AR, Martínez F, et al. Genotypes of the C677T and A1298C polymorphisms of the MTHFR gene as a cause of human spontaneous embryo loss. Hum Reprod. Oxford University Press. 2007;22:3249–54.
Ren J, Han X, Liu X. Methylenetetrahydrofolate reductase gene polymorphism in women with recurrent pregnancy loss. Chin J Perinat Med. 2007;10:80–4.
Bae J, Choi DH, Kang MS, Cha SH, Oh D, Kim NK. Effect of methylenetetrahydrofolate reductase and thymidylate synthase enhancer region polymorphisms on the risk of idiopathic recurrent spontaneous abortion in a Korean population. Fertil Steril. Elsevier. 2009;91:1560–2.
Ciacci C, Tortora R, Scudiero O, Di Fiore R, Salvatore F, Castaldo G. Early pregnancy loss in celiac women: the role of genetic markers of thrombophilia. Dig Liver Dis. Elsevier. 2009;41:717–20.
Rodríguez-Guillén M d R, Torres-Sánchez L, Chen J, Galván-Portillo M, Blanco-Muñoz J, Anaya MA, et al. Maternal MTHFR polymorphisms and risk of spontaneous abortion. Salud Publica Mex. SciELO Public Health. 2009;51:19–25.
Bagheri M, Abdirad I, Omrani MD, NAN BF. C677T and A1298C Mutations in the Methylenetetrahydrofolate reductase gene in patients with recurrent abortion from the Iranian Azeri Turkish. Int J Fertil Steril. 2010;4:134–9.
Kim SY, Park SY, Choi JW, Kim DJ, Lee SY, Lim JH, Han JY, Ryu HM, Kim MH. Association between MTHFR 1298A>C polymorphism and spontaneous abortion with fetal chromosomal aneuploidy. Am J Reprod Immunol. 2011;66:252–8.
Settin A, Elshazli R, Salama A, ElBaz R. Methylenetetrahydrofolate reductase gene polymorphisms in Egyptian women with unexplained recurrent pregnancy loss. Genet Test Mol Biomarkers. Mary Ann Liebert, Inc. 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA. 2011;15:887–92.
Jeddi-Tehrani M, Torabi R, Zarnani AH, Mohammadzadeh A, Arefi S, Zeraati H, et al. Analysis of plasminogen activator inhibitor-1, integrin beta3, beta fibrinogen, and methylenetetrahydrofolate reductase polymorphisms in Iranian women with recurrent pregnancy loss. Am J Reprod Immunol. Wiley Online Library. 2011;66:149–56.
Dissanayake VHW, Sirisena ND, Weerasekera LY, Gammulla CG, Seneviratne HR, Jayasekara RW. Candidate gene study of genetic thrombophilic polymorphisms in pre-eclampsia and recurrent pregnancy loss in Sinhalese women. J Obstet Gynaecol Res. Wiley Online Library. 2012;38:1168–76.
Idali F, Zareii S, Mohammad-Zadeh A, Reihany-Sabet F, Akbarzadeh-Pasha Z, Khorram-Khorshid H, et al. Plasminogen activator inhibitor 1 and methylenetetrahydrofolate reductase gene mutations in iranian women with polycystic ovary syndrome. Am J Reprod Immunol. Wiley Online Library. 2012;68:400–7.
Ozdemir O, Yenicesu GI, Silan F, Köksal B, Atik S, Ozen F, et al. Recurrent pregnancy loss and its relation to combined parental thrombophilic gene mutations. Genet Test Mol Biomarkers. Mary Ann Liebert, Inc. 140 Huguenot Street, 3rd Floor New Rochelle, NY 10801 USA. 2012;16:279–86.
Poursadegh Zonouzi A, Chaparzadeh N, Asghari Estiar M, Mehrzad Sadaghiani M, Farzadi L, Ghasemzadeh A, Sakhinia M, Sakhinia E. Methylenetetrahydrofolate Reductase C677T and A1298C Mutations in Women with Recurrent Spontaneous Abortions in the Northwest of Iran. ISRN Obstet Gynecol. 2012;2012:945486.
Sheikhha MH, Kalantar SM, Ghasemi N, Soleimanian S. Association between MTHFR 1298A> C polymorphism with RSA and IVF Failure. Iran J Ped Hematol Oncol. 2012;2:109–15.
Parveen F, Tuteja M, Agrawal S. Polymorphisms in MTHFR, MTHFD, and PAI-1 and recurrent miscarriage among North Indian women. Arch Gynecol Obstet. Springer. 2013;288:1171–7.
Boas WV, Gonçalves RO, Costa OLN, Goncalves MS. Metabolism and gene polymorphisms of the folate pathway in Brazilian women with history of recurrent abortion. Rev Bras Ginecol Obstet. SciELO Brasil. 2015;37:71–6.
Cao Y, Xu J, Zhang Z, Huang X, Zhang A, Wang J, et al. Association study between methylenetetrahydrofolate reductase polymorphisms and unexplained recurrent pregnancy loss: a meta-analysis. Gene. Elsevier. 2013;514:105–11.
Hu X, Liang P, Diao L. The association of methylenetetrahydrofolate reductase gene mutation with unexplained recurrent miscarriage. Chin J Birth Health Hered. 2014;11:87–9.
Hubacek JA, Rynekrova J, Kasparova D, Adamkova V, Holmes MV, Fait T. Association of MTHFR genetic variants C677T and A1298C on predisposition to spontaneous abortion in Slavonic population. Clin Chim Acta. Elsevier. 2015;440:104–7.
Khaleghparast A, Khaleghparast S, Khaleghparast H. Association between the A1298C polymorphism of the methylenetetrahydrofolate reductase gene and recurrent spontaneous abortion. Iran J Neonatol. Mashhad University of Medical Sciences. 2014;5:7–11.
Luo L, Chen Y, Wang L, Zhuo G, Qiu C, Tu Q, et al. Polymorphisms of genes involved in the folate metabolic pathway impact the occurrence of unexplained recurrent pregnancy loss. Reprod Sci. Sage Publications Sage CA: Los Angeles, CA. 2015;22:845–51.
Yousefian E, Kardi MT, Allahveisi A. Methylenetetrahydrofolate Reductase C677T and A1298C Polymorphism in Iranian Women With Idiopathic Recurrent Pregnancy Losses. Iran Red Crescent Med J. 2014;16:e16763.
Farahmand K, Totonchi M, Hashemi M, Reyhani Sabet F, Kalantari H, Gourabi H, et al. Thrombophilic genes alterations as risk factor for recurrent pregnancy loss. J Matern Fetal Neonatal Med. Taylor & Francis. 2016;29:1269–73.
Lino FL, Traina É, Barreto JA, Moron AF, Mattar R. Thrombophilic mutations and polymorphisms, alone or in combination, and recurrent spontaneous abortion. Clin Appl Thromb Hemost. SAGE Publications Sage CA: Los Angeles, CA. 2015;21:365–72.
Zhu L. Polymorphisms in the methylene tetrahydrofolate reductase and methionine synthase reductase genes and their correlation with unexplained recurrent spontaneous abortion susceptibility. Genet Mol Res. 2015;14:8500–8.
Al-Achkar W, Wafa A, Ammar S, Moassass F, Jarjour RA. Association of methylenetetrahydrofolate reductase C677T and A1298C gene polymorphisms with recurrent pregnancy loss in Syrian women. Reprod Sci. Sage Publications Sage CA: Los Angeles, CA. 2017;24:1275–9.
López-Jiménez JJ, Porras-Dorantes Á, Juárez-Vázquez CI, García-Ortiz JE, Fuentes-Chávez CA, Lara-Navarro IJ, Jaloma-Cruz AR. Molecular thrombophilic profile in Mexican patients with idiopathic recurrent pregnancy loss. Genet Mol Res. 2016;15:gmr.15048728.
Najafian M, Ahmadi EY, Asl JM, Shariati G, Ahmadi NY. Study the correlation between polymorphism of MTHFR thrombophilic genes and pregnancy loss in Ahvaz city. Biosci Biotechnol Res Asia. 2016;13:681–6.
Hwang KR, Choi YM, Kim JJ, Lee SK, Yang KM, Paik EC, et al. Methylenetetrahydrofolate reductase polymorphisms and risk of recurrent pregnancy loss: a case-control study. J Korean Med Sci. 2017;32:2029–34.
Wolski H, Barlik M, Drews K, Klejewski A, Kurzawińska G, Ożarowski M, et al. Contribution of inherited thrombophilia to recurrent miscarriage in the Polish population. Ginekol Pol. 2017;88:385–92.
Bigdeli R, Younesi MR, Panahnejad E, Asgary V, Heidarzadeh S, Mazaheri H, et al. Association between thrombophilia gene polymorphisms and recurrent pregnancy loss risk in the Iranian population. Syst Biol Reprod Med. Taylor & Francis. 2018;64:274–82.
Kim JY, Kim JW, Sung SR, Park JE, Shim SH, Cha DH. Impact of RFC1, MTHFR, and MTHFD1 polymorphism on unexplained pregnancy loss (UPL): comparative analysis of maternal and fetal components using mother–abortus paired samples. Eur J Obstet Gynecol Reprod Biol. Elsevier. 2018;231:152–7.
Wolski H, Kurzawinska G, Drews K, Barlik M, Kadziolka P, Malewski Z, et al. MTHFR genetic polymorphism and the risk of intrauterine fetal death in Polish women. Ginekol Pol. 2019;90:76–81.
Xu Y, Ban Y, Ran L, Yu Y, Zhai S, Sun Z, Zhang J, Zhang M, Hong T, Liu R, Ren L, Hu L. Relationship between unexplained recurrent pregnancy loss and 5, 10-methylenetetrahydrofolate reductase) polymorphisms. Fertil Steril. Elsevier; 2019;111:597–603.
Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. American Psychological Association. 2006;11:193.
Hibbard BM. THE ROLE OF FOLIC ACID IN PREGNANCY* with particular reference to anaemia, abruption and abortion. BJOG. Wiley Online Library. 1964;71:529–42.
Cordeiro A, Neto AP, Carvalho F, Ramalho C, Dória S. Relevance of genomic imprinting in intrauterine human growth expression of CDKN1C, H19, IGF2, KCNQ1 and PHLDA2 imprinted genes. J Assist Reprod Genet. Springer. 2014;31:1361–8.
Kang S-S, Zhou J, Wong PW, Kowalisyn J, Strokosch G. Intermediate homocysteinemia: a thermolabile variant of methylenetetrahydrofolate reductase. Am J Hum Genet. Elsevier. 1988;43:414.
Weisberg I, Tran P, Christensen B, Sibani S, Rozen R. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. Academic Press. 1998;64:169–72.
Kim Y-I. Folic acid supplementation and cancer risk: point. Cancer Epidemiol Biomarkers Prev. AACR. 2008;17:2220–5.
Zappacosta B, Romano L, Persichilli S, Cutrone LA, Graziano M, Vitrani A, et al. Genotype prevalence and allele frequencies of 5, 10-methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C polymorphisms in Italian newborns. Lab Med. Oxford University Press Oxford, UK. 2009;40:732–6.
van der Put NMJ, Gabreëls F, Stevens EMB, Smeitink JAM, Trijbels FJM, Eskes TKAB, et al. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet. Elsevier. 1998;62:1044–51.
Ulrich CM, Kampman E, Bigler J, Schwartz SM, Chen C, Bostick R, et al. Lack of association between the C677T MTHFR polymorphism and colorectal hyperplastic polyps. Cancer Epidemiol Biomarkers Prev. AACR. 2000;9:427–33.
Weisberg IS, Jacques PF, Selhub J, Bostom AG, Chen Z, Ellison RC, et al. The 1298A→ C polymorphism in methylenetetrahydrofolate reductase (MTHFR): in vitro expression and association with homocysteine. Atherosclerosis. Elsevier. 2001;156:409–15.
Nelen WLDM, Blom HJ, Thomas CMG, Steegers EAP, Boers GHJ, Eskes TKAB. Methylenetetrahydrofolate reductase polymorphism affects the change in homocysteine and folate concentrations resulting from low dose folic acid supplementation in women with unexplained recurrent miscarriages. J Nutr. Oxford University Press. 1998;128:1336–41.
Eldibany MM, Caprini JA. Hyperhomocysteinemia and thrombosis: an overview. Arch Pathol Lab Med. 2007;131:872–84.
D’Uva M, Di Micco P, Strina I, De Placido G. Recurrent pregnancy loss and thrombophilia. J Clin Med Res. Elmer Press. 2010;2:18.
Zhang Y, He X, Xiong X, Chuan J, Zhong L, Chen G, et al. The association between maternal methylenetetrahydrofolate reductase C677T and A1298C polymorphism and birth defects and adverse pregnancy outcomes. Prenat Diagn. 2019;39:3–9.
Rai V. Methylenetetrahydrofolate reductase gene A1298C polymorphism and susceptibility to recurrent pregnancy loss: a meta-analysis. Cell Mol Biol (Noisy-le-grand). 2014;60:27–34.
Kamali M, Hantoushzadeh S, Borna S, Neamatzadeh H, Mazaheri M, Noori-Shadkam M, et al. Association between thrombophilic genes polymorphisms and recurrent pregnancy loss susceptibility in the iranian population: a systematic review and meta-analysis. Iran Biomed J. 2018;22:78–89.
Servy EJ, Jacquesson-Fournols L, Cohen M, Menezo YJR. MTHFR isoform carriers. 5-MTHF (5-methyl tetrahydrofolate) vs folic acid: a key to pregnancy outcome: a case series. J Assist Reprod Genet. Springer. 2018;35:1431–5.
Merviel P, Cabry R, Lourdel E, Lanta S, Amant C, Copin H, et al. Comparison of two preventive treatments for patients with recurrent miscarriages carrying a C677T methylenetetrahydrofolate reductase mutation: 5-year experience. J Int Med Res. SAGE Publications Sage UK: London, England. 2017;45:1720–30.
Moll S, Varga EA. Homocysteine and MTHFR mutations. Circulation. Am Heart Assoc. 2015;132:e6–9.
Allam MM, El-Zawawy HT, Barakat SS, Ahmed SM, Saleh RNM. A hidden cause of infertility in hypothyroid patients. Clin Case Rep. Wiley Online Library. 2020;8:374–8.
Acknowledgements
The authors are thankful the Council of Scientific and Industrial Research (CSIR) for funding under network scheme of project (BSC0101). PM is thankful to University grants commission for financial support (Ref no. 460/CSIR-UGC NET DEC.2017).
Code Availability
Not applicable
Funding
This study was financially supported by CSIR, Govt. of India.
Author information
Authors and Affiliations
Contributions
PM and SR conceived the idea; PM and RV collected the data; KS provided intellectual inputs; PM, RV and SR wrote the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable
Consent to Participate
Not Applicable
Consent for Publication
Not applicable
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Fig. 1
(PNG 8.17 mb)
Rights and permissions
About this article
Cite this article
Mehta, P., Vishvkarma, R., Singh, K. et al. MTHFR 1298A>C Substitution is a Strong Candidate for Analysis in Recurrent Pregnancy Loss: Evidence from 14,289 Subjects. Reprod. Sci. 29, 1039–1053 (2022). https://doi.org/10.1007/s43032-021-00530-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43032-021-00530-5